Central Inflammatory Myofibroblastic Tumor of the Jawbones: Exceptional Location of an Uncommon Entity
Article Information
Alex Neronov DMD1*#, Alejandro Livoff MD2#, Oded Nahlieli DMD3, Amram Zagury DMD4, David Ben-Dor MD5, Irit Allon DMD, PhD6,7
#Contributed equally
1Resident, Department of Oral and Maxillofacial Surgery, Barzilai Medical Center, Ashkelon, Israel
2Head of the Department of Pathology, Barzilai Medical Center, Ashkelon, Israel
3Professor and Chairman, Department of Oral and Maxillofacial Surgery, Barzilai Medical Center, Ashkelon, Israel
4Senior Surgeon, Department of Oral and Maxillofacial Surgery, Barzilai Medical Center, Ashkelon, Israel
5Senior Pathologist, Department of Pathology, Barzilai Medical Center, Ashkelon, Israel
6Oral Pathologist, Department of Pathology, Barzilai Medical Center, Ashkelon, Israel
7Senior Lecturer, School of Health Sciences, Ben-Gurion University, Beer-Sheba, Israel
*Corresponding Author: Dr. Alex Neronov, Department of Oral and Maxillofacial Surgery, Barzilai Medical Center, Ashkelon, Israel
Received: 05 March 2020; Accepted: 19 March 2020; Published: 11 May 2020
Citation: Alex Neronov, Alejandro Livoff, Oded Nahlieli, Amram Zagury, David Ben-Dor, Irit Allon. Central Inflammatory Myofibroblastic Tumor of the Jawbones: Exceptional Location of an Uncommon Entity. Archives of Clinical and Medical Case Reports 4 (2020): 384-408.
View / Download Pdf Share at FacebookAbstract
Aim: Inflammatory myofibroblastic tumors (IMTs) are uncommon neoplasms of intermediate biologic behavior that typically present in the lungs of children and young adults but may occur elsewhere in the body. The head and neck is an unusual site of involvement and in the jaws these are even less common. The purpose of this study is to present a central IMT arising within the mandibular bone of a seven year old female and to analyze the available literature of intrabony/central IMTs of the jaws.
Methods: Publications in the English language literature on central IMTs arising within the jawbones were located by a PubMed and googlescholar search of case reports and series.
Results: 25 cases including the present one were included. The mean age of the patients was 34.8 ± 19.6 years. The mandible was affected in most cases. All cases were osteolytic, all but the present one were unilocular, and more than 50% were well defined. Bony expansion was evident in 42.8% and root resorption in 53.8% of the cases. The lesions were composed of spindle fibroblastic and myofibroblastic cells with a variable inflammatory infiltrate, most commonly lymphocytes (85%) and plasma cells (75%), but other inflammatory cells such as neutrophils, eosinophils and mast cells were also present. Immunohistochemically smooth muscle actin was positive in 100% and ALK1 was positive in 6 of 14 cases (42.8%). 2 cases were h-Caldesmon positive. The present case stained diffusely with an antibody against Paladin, implicated in mesenchymal transition process of metastatic breast carcinoma. Treatment options consisted mostly of surgery, but pharmacological therapy with glucocorticoids and combinations of these were also documented.
Conclusion: Central IMT of the jawbone is a rare tumor that could have an alarming clinical presentation. The differential diagnosis o
Keywords
Inflammatory myofibroblastic tumor; Jaw; ALK; h-caldesmon; Paladin
Inflammatory myofibroblastic tumor articles, Jaw articles, ALK articles, h-caldesmon articles, Paladin articles
Inflammatory myofibroblastic tumor articles Inflammatory myofibroblastic tumor Research articles Inflammatory myofibroblastic tumor review articles Inflammatory myofibroblastic tumor PubMed articles Inflammatory myofibroblastic tumor PubMed Central articles Inflammatory myofibroblastic tumor 2023 articles Inflammatory myofibroblastic tumor 2024 articles Inflammatory myofibroblastic tumor Scopus articles Inflammatory myofibroblastic tumor impact factor journals Inflammatory myofibroblastic tumor Scopus journals Inflammatory myofibroblastic tumor PubMed journals Inflammatory myofibroblastic tumor medical journals Inflammatory myofibroblastic tumor free journals Inflammatory myofibroblastic tumor best journals Inflammatory myofibroblastic tumor top journals Inflammatory myofibroblastic tumor free medical journals Inflammatory myofibroblastic tumor famous journals Inflammatory myofibroblastic tumor Google Scholar indexed journals myofibroblastic tumor articles myofibroblastic tumor Research articles myofibroblastic tumor review articles myofibroblastic tumor PubMed articles myofibroblastic tumor PubMed Central articles myofibroblastic tumor 2023 articles myofibroblastic tumor 2024 articles myofibroblastic tumor Scopus articles myofibroblastic tumor impact factor journals myofibroblastic tumor Scopus journals myofibroblastic tumor PubMed journals myofibroblastic tumor medical journals myofibroblastic tumor free journals myofibroblastic tumor best journals myofibroblastic tumor top journals myofibroblastic tumor free medical journals myofibroblastic tumor famous journals myofibroblastic tumor Google Scholar indexed journals tumor articles tumor Research articles tumor review articles tumor PubMed articles tumor PubMed Central articles tumor 2023 articles tumor 2024 articles tumor Scopus articles tumor impact factor journals tumor Scopus journals tumor PubMed journals tumor medical journals tumor free journals tumor best journals tumor top journals tumor free medical journals tumor famous journals tumor Google Scholar indexed journals Jaw articles Jaw Research articles Jaw review articles Jaw PubMed articles Jaw PubMed Central articles Jaw 2023 articles Jaw 2024 articles Jaw Scopus articles Jaw impact factor journals Jaw Scopus journals Jaw PubMed journals Jaw medical journals Jaw free journals Jaw best journals Jaw top journals Jaw free medical journals Jaw famous journals Jaw Google Scholar indexed journals ALK articles ALK Research articles ALK review articles ALK PubMed articles ALK PubMed Central articles ALK 2023 articles ALK 2024 articles ALK Scopus articles ALK impact factor journals ALK Scopus journals ALK PubMed journals ALK medical journals ALK free journals ALK best journals ALK top journals ALK free medical journals ALK famous journals ALK Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals h-caldesmon articles h-caldesmon Research articles h-caldesmon review articles h-caldesmon PubMed articles h-caldesmon PubMed Central articles h-caldesmon 2023 articles h-caldesmon 2024 articles h-caldesmon Scopus articles h-caldesmon impact factor journals h-caldesmon Scopus journals h-caldesmon PubMed journals h-caldesmon medical journals h-caldesmon free journals h-caldesmon best journals h-caldesmon top journals h-caldesmon free medical journals h-caldesmon famous journals h-caldesmon Google Scholar indexed journals CT scan articles CT scan Research articles CT scan review articles CT scan PubMed articles CT scan PubMed Central articles CT scan 2023 articles CT scan 2024 articles CT scan Scopus articles CT scan impact factor journals CT scan Scopus journals CT scan PubMed journals CT scan medical journals CT scan free journals CT scan best journals CT scan top journals CT scan free medical journals CT scan famous journals CT scan Google Scholar indexed journals Paladin articles Paladin Research articles Paladin review articles Paladin PubMed articles Paladin PubMed Central articles Paladin 2023 articles Paladin 2024 articles Paladin Scopus articles Paladin impact factor journals Paladin Scopus journals Paladin PubMed journals Paladin medical journals Paladin free journals Paladin best journals Paladin top journals Paladin free medical journals Paladin famous journals Paladin Google Scholar indexed journals Surgery articles Surgery Research articles Surgery review articles Surgery PubMed articles Surgery PubMed Central articles Surgery 2023 articles Surgery 2024 articles Surgery Scopus articles Surgery impact factor journals Surgery Scopus journals Surgery PubMed journals Surgery medical journals Surgery free journals Surgery best journals Surgery top journals Surgery free medical journals Surgery famous journals Surgery Google Scholar indexed journals
Article Details
1. Introduction
Inflammatory myofibroblastic tumor (IMT) is an uncommon mesenchymal tumor composed of myofibroblastic spindle cells variably admixed with lymphocytes, plasma cells and sometimes eosinophils [1]. It was previously described by different names such as inflammatory pseudotumor, benign myofibroblastoma, plasma cell granuloma or inflammatory fibrosarcoma, histiocytoma, xanthomatous granuloma, pseudosarcomatous myofibroblastic proliferation, and spindle cell pseudotumor [2]. In 1994 the World Health Organization (WHO) reclassified these tumors as IMTs, referring to “a tumor composed of differentiated myofibroblastic spindle cells and/or lymphocytes” [3]. IMTs are more frequent in children and young adults, and typically arise in the lung, abdominopelvic region, and retroperitoneum. Unusual sites of involvement include the head and neck, genitourinary tract, heart, extremities, and central nervous system [1-5]. While these tumors are uncommon in the head and neck, they are even rarer in the jaws. IMT is an intermediate grade, locally recurring, and rarely metastasizing neoplasm [4]. Because of its potentially aggressive clinical and radiologic features, it can be mistaken as a malignancy. The histopathological features of IMTs are often diagnostic, particularly with staining for anaplastic lymphoma kinase-1 (ALK1). The ALK gene has been implicated in the pathogenesis of IMTs, with approximately 50% of IMTs harboring rearrangements of the ALK1 gene at 2p23 [5]. The WHO delineates three primary histological patterns of IMT-a ‘‘myxoid/vascular pattern’’, a ‘‘compact spindle cell pattern’’ with fascicular architecture and a distinctive infiltrate of plasma cells, and a ‘‘hypocellular pattern’’ with heavy collagenization, bearing resemblance to fibrous scar tissue [3].
This study presents a rare case of a central IMT arising in the posterior lower jawbone of a 7-year-old female. The clinical, radiographic, and histological findings, along with the surgical procedures used, are described alongside of a comprehensive analysis of the literature of central (intrabony) IMTs of the jaws.
2. Materials and Methods
Publications in the English language on IMTs involving the jawbones were collected by a PubMed and google scholar search of case reports and series that included cases arising centrally within the jawbones. The key words used were inflammatory myofibroblastic tumor, oral, jaw, bone, mandible, maxilla, central, inflammatory pseudotumor, benign myofibroblastoma, plasma cell granuloma or inflammatory fibrosarcoma, histiocytoma, xanthomatous granuloma, pseudosarcomatous myofibroblastic proliferation, and spindle cell pseudotumor. Additional pertinent references were found from the bibliographies of identified articles. Excluded were cases that appeared to arise from soft tissues and cases involving the maxillary sinus. Remaining cases of central IMT of the jawbones were analyzed, characterizing clinico-radiologic-pathologic and immunohistochemical characteristics.
3. Case Presentation
A 7-year-old female was referred to the oral and maxillofacial unit due to a swelling of her right cheek present for two months. An intraoral examination revealed a soft tissue swelling accompanied by a buccal bony expansion of the right mandible and mobility of the first molar. No history of local infection or trauma was recorded. The initial panoramic radiograph demonstrated a fairly circumscribed multilocular radiolucent lesion 4 cm in diameter in the right posterior mandible with marked root resorption of the first molar and distal displacement of the second molar to the area of ramus (Figure 1a). Cone beam computed tomography (CBCT) of the mandible showed a circumscribed hypodense expansile lesion measuring 3.3 × 1.7 cm in the right molar and ramus area. A 3-dimensional CT reconstruction confirmed this to be an osteolytic lesion with areas of locularity in the borders (Figure 1b).
The preoperative differential diagnosis included odontogenic or non-odontogenic tumors, with a request to rule out malignancy due to the alarming clinical presentation. An incisional biopsy was performed with the extraction of both molars. Histopathological assessment revealed a cellular neoplasm composed of spindle cells admixed with chronic inflammatory cells, mostly lymphocytes and fewer plasma cells. The stroma was mostly compact with looser fibromyxoid areas (Figure 2a-c).
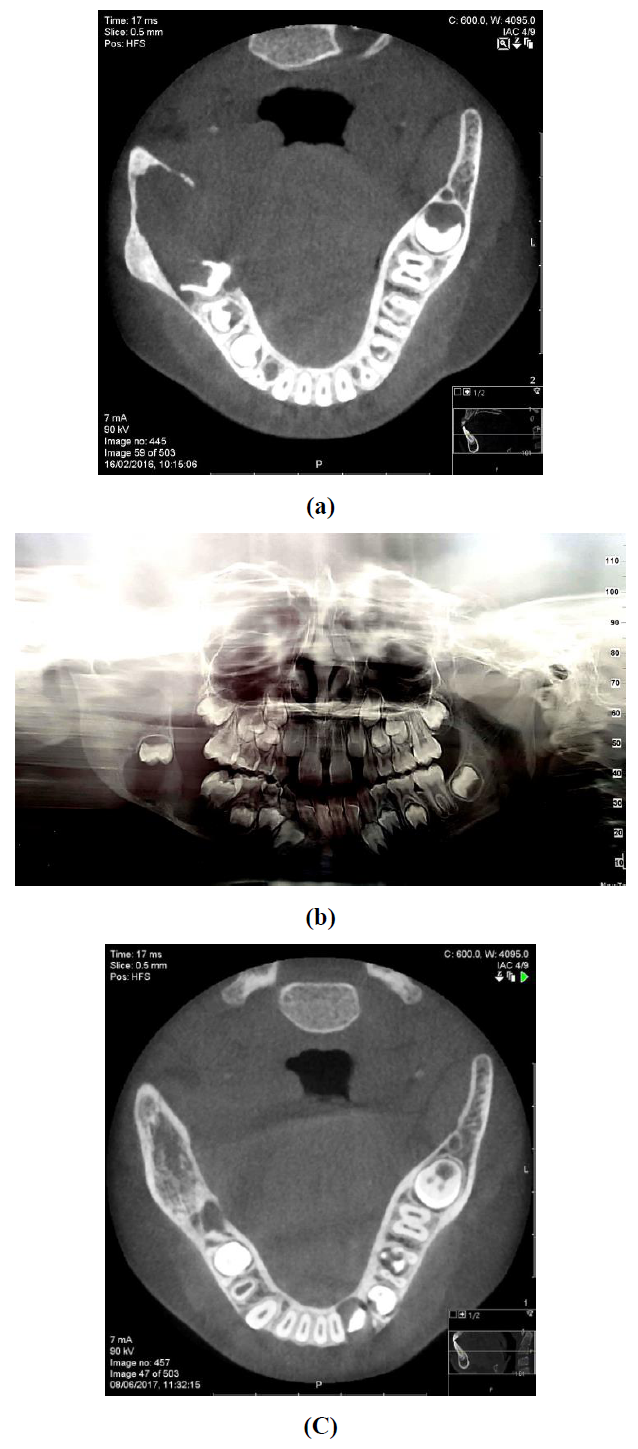
Figure 1: Preoperative imaging, a: axial CBCT showing a hypodense expansile lesion in the right posterior mandible; b: panoramic imaging demonstrating a radiolucent multilocular lesion; c: 20 months postoperative follow up, axial cone beam CT showing osseous regeneration.
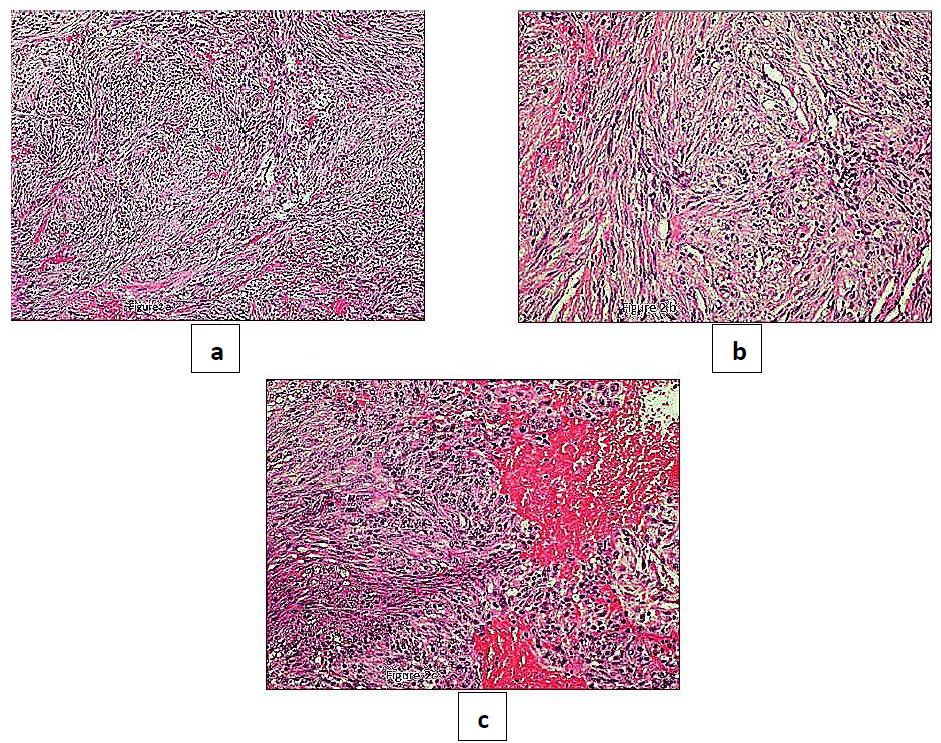
Figure 2: a-b: histopathologically, IMT presents a cellular proliferation of plump spindle cells with pale cytoplasm arranged in a zonal pattern. The zones consist of tissue culture like areas and areas of haemorrhage; c: chronic inflammatory cells, mostly lymphocytes and fewer plasma cells are present. (H&E x10, x20, x40).
Immunohistochemical stainings showed extensive positivity of the neoplastic cells to smooth muscle actin (SMA) (Figure 3), h-Caldesmon (Figure 4) and anaplastic lymphoma kinase-1 (ALK1) (Figure 5), with focal S100 positivity. ALK1 stained the cytoplasm in a granular to diffuse manner. Keratins and desmin were negative. On the basis of the above histopathologic and immunohistochemical findings, a working diagnosis of IMT was rendered and the case was confirmed by consultation. The lesion was further stained with Paladin (polyclonal, dilution 1:50, Novus Biologicals, Littleton, CO, USA), an antibody implicated in acquisition of mesenchymal phenotype in metastatic breast cell lines, and stained diffusely positive (Figure 6).
Following histopathologic evaluation the mass was excised and meticulous curettage and peripheral margin ostectomy were performed. The postoperative course was uneventful without clinical evidence of recurrence at a 36 months follow-up; a repeat CBCT at month 20 showed osseous regeneration at the previous tumor site (Figure 1c).
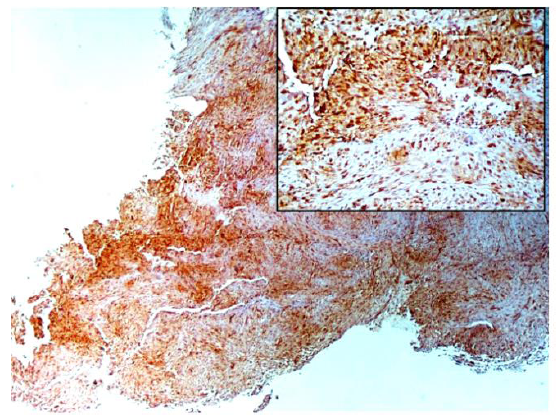
Figure 3: The tumor cells are diffusely stained with SMA, emphasizing the nodular fascicular architecture of the lesional spindle cells (x20).
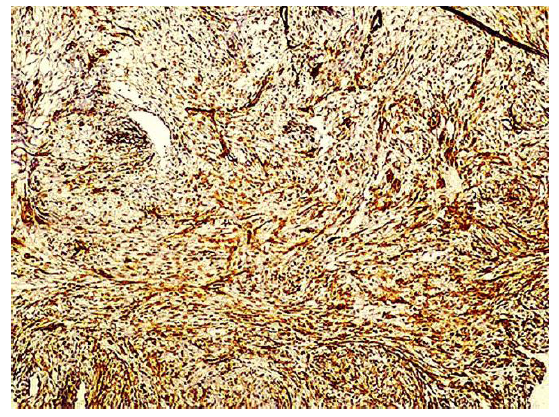
Figure 4: The lesional cells stain strongly and diffusely positive with h-caldesmon (x10).
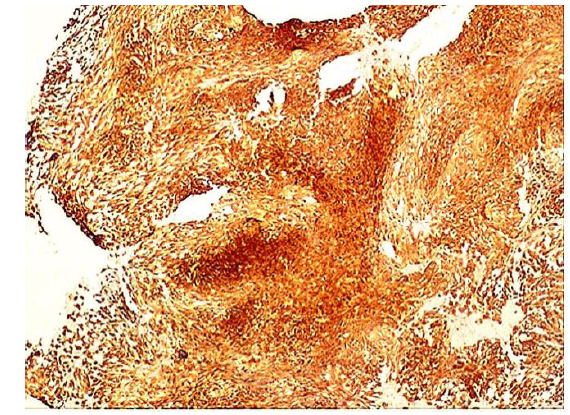
Figure 5: Positive ALK-1 staining. The ALK staining pattern is granular to diffuse (x10).
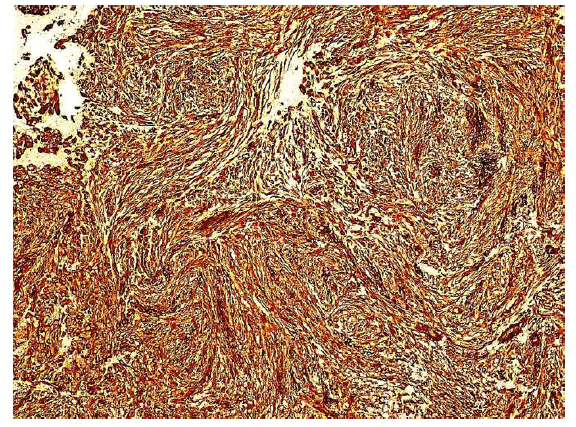
Figure 6: The tumor cells are diffusely positive for paladin antibody. The cytoplasmic staining emphasizes the spindle morphology. Endothelial cells lining the capillary lumen are also paladin positive. Inflammatory cells are paladin negative (x20, x40).
4. Literature Review
4.1 Clinical details
In total, 25 cases of central IMT, including the present one were found. The clinical information is available in Table 1. The mean age of the patients was 34.8+19.6 years, range 7-75 years. The mean size was 4.09 cm in largest dimension, range 1.0-7.5cm. The male to female ratio was almost even with 12 males and 13 females. The mandible was affected in 18 cases (72%) and the maxilla in 7 (28%). The clinico-radiologic parameters are summarized in Table 2. All lesions were osteolytic. Root resorption was documented in 7 (53.8%).
Information on demarcation of the lesion was available in 16 cases, with 8 (50%) described as well-defined. Information on bony expansion was available in 14 cases. 6 of these showed expansion (42.8%). 18 cases contained information on locularity of the lesion; 17 were unilocular and 1 was multilocular (present case). Reference to soft tissue swelling was available in 21 cases, present in 14 (67%). In 4 cases the mucosa was ulcerated (21%).
4.2 Histopathology and immunohistochemistry
The histological and immunohistochemical features of the 25 central IMTs are presented in Table 3. The lesions were described as composed of spindle fibroblastic and myofibroblastic cells. In one case the overlying epithelium was described as mildly dyplastic [11]. In one case a systemic IgG4 related disease was suspected [27], but the ratio of IgG4+/IgG+ plasma cells was less than 10% and the morphologic and immunohistochemical findings supported IMT. Information on the accompanying inflammatory infiltrate was available in 20 cases. The most prevalent cells were lymphocytes in 17 (85%), followed by plasma cells in 15 (75%), neutrophils in 9 (45%), eosinophils in 5 (25%) and mast cells in one case (5%).
Immunohistochemical features were available in 17 cases (68%). SMA was positive in 16 cases. ALK1 was reported to be positive in 6 (43%) and negative in 8 (57%) cases. The ALK1 staining was cytoplasmic granular to strongly diffuse in all the positive cases (Table 3). 8 of the cases stained with vimentin were positive.CD68 was positive in 3 cases (60%) and negative in 2 (40%). CD34 was positive in 2 cases [19, 20] and negative in 3 [10, 14, and 21]. CD31 was negative in one case [10]. h-Caldesmon was positive in one published case [27] and was diffusely positive in our case (Figure 6). Desmin was positive in one case [9] and negative in 8 cases. S100 was focally positive in our case, but in 10 published cases it was negative [7, 10, 14, 15, 19, 21, 23, 26, 28, 37]. Cytokeratin was negative in 3 cases. Each of the following: CD8, CD3, CD20 were positive in one case.
4.3 Treatment and follow-up
Information on treatment and follow up was available for all cases, except one [37], follow-up period ranged between 6-120 months, mean 21.7. In 21/24 (87.5%) there was no evidence of recurrence. The majority were treated only by surgery (20/25, 80%), two cases were treated with a combination of surgery and steroids (2/25, 8%). One of these [12] was treated by resection combined with a high dose of corticosteroids (40 mg prednisolone) with no recurrence at 11 months. The second was first treated by 30 mg/day of oral prednisolone for 2 weeks with a poor response, after which a resection including the mandibular condyle was performed [27].
One case was treated only with steroids [20], one by surgery followed by radiotherapy [21] and one by surgery followed by steroids and radiotherapy [15] , (each 1/25, 4%). The case that was treated with maxillectomy followed by radiotherapy recurred after 6 months and the patient died of the disease. The pathology report of this case mentioned a sarcomatous change [21]. The case that was treated by surgery followed by steroids and radiotherapy initially presented 4 intrabony lesions involving the cranium, mandible, ischium, and calcaneum. After surgery of the cranial and mandibular lesions, high dose corticosteroid therapy with 100 mg/d of prednisolone was given. Since five months after, magnetic resonance imaging still showed residual tumor, the patient received 20 Gy of focal irradiation for the residual lesions parietal and mandibular lesions. After 18 months, no tumor regrowth was observed [15].
|
Reference |
Age |
Gender |
Site |
Size mm |
Clinical appearance |
Imaging |
|
|
1 |
Zegarelli et al., 1974 |
56 |
F |
Rt posterior man |
50 |
Firm, non-tender swelling. Pink-red, ulcerated mucosa. |
Lateral: RL area, destruction of the alveolar ridge. |
|
2 |
Shek et al., 1996 |
36 |
F |
Lt max |
- |
Painless welling in the left infraorbital region and left upper buccal sulcus. No ulceration. |
CT: cystic lesion, erosion of the anterior wall of the sinus. |
|
3 |
Araki et al., 2002 |
22 |
M |
Rt posterior man |
19 |
Swelling and pain. Movable tender masses in right submandibular region. Mushroom-like tender dark red mass with white spots and irregular surface in gingiva of vital teeth 44-46. |
Panoramic: ill-defined RL, ground glass. Occlusal: no expansion of bucco-lingual cortical bone, no periostal reaction. Periapical: invasive bone resorption extending to the tooth root. CT: disappearance of buccal cortical bone. Large semi-circular mass in the buccal side along the destroyed bone. Tc99m scintigraphy: high accumulation in the right mandibular molar region, no foci in skull. Ga67 scintigraphy: strong positive uptake with a clear margin in the right mandible. |
|
4 |
Fang & Dym, 2004 |
23 |
M |
Lt posterior man |
50 |
Soft friable, necrotic, tender and painful mass, no paresthesia. |
Panoramic: ill-defined RL of left posterior mandible. CT: osteolytic lesion of left posterior mandible. The superior border of the mandibular canal intact. |
|
5 |
Poh & Dahlman, 2005 |
42 |
F |
Rt posterior man |
30 |
None. |
Panoramic: well demarcated RL, root resorption of molar, no tooth displacement. No swelling, no expansion. |
|
6 |
Oh et al., 2008 |
20 |
F |
Rt posterior man |
- |
Gingival swelling, mandibular first premolar mobile. |
Panoramic: ill-defined osteolysis, destruction of the buccal and lingual cortical plates. |
|
7 |
Wales et al., 2008 |
61 |
F |
Rt max |
- |
Nasal obstruction, pain, firm mass. |
CT: mass eroding the right nasal bone and filling the nasal vestibule, right maxilla and superior alveolus. |
|
8 |
Eley & Watt-Smith, 2010 |
29 |
M |
Rt alveolar max |
50 |
Soft tissue mass. |
Panoramic: absence of upper right second premolar with associated soft tissue mass. |
|
9 |
Satomi et al., 2010 |
14 |
F |
Lt man molar gingiva |
30 |
Tender, firm, ulcerative tumor. |
Panoramic: soft tissue mass with resorptive defect along the superior aspect of the mandible. |
|
10 |
Sasagawa et al., 2011 |
60 |
M |
Lt man |
- |
Pain in the right vertex, left mandible. |
CT: osteolytic lesions in the right parietal bone and the left mandible. MRI: lesion in right parietal bone isointense on T1-weighted imaging and hypointense on T2-weighted imaging homogeneously enhanced after gadolinium administration. Thallium-201 scintigraphy: abnormal in the right parietal bone. Technetium-99m scintigraphy: not only cranial lesions, but also 2 other lesions in the ischium and calcaneum. |
|
11 |
Date et al. 2012 |
70 |
M |
Rt posterior man (gingiva) |
35 |
Hemorrhagic mass. |
CT: mild bone resorption in front of the ramus. MRI: T1-mass with low signal intensity, STIR- mass of high signal intensity. |
|
12 |
Garcia et al., 2012 |
40 |
M |
Rt man |
- |
Facial pain radiating from the upper jaw burning of the right side of the face. |
CT: osteolytic lesion of the right mandible. MRI: enhancing mass abutting the brainstem in the middle intracranial fossa, involvement of the right cavernous sinus and orbital fissures. |
|
13 |
Gawande et al., 2012 |
20 |
M |
Rt posterior man |
50 |
Firm diffuse swelling. Mobile and vital mandibular right premolars and molars. |
Panoramic: irregularly defined RL. CT: soft tissue density lesion with erosion of the outer mandibular cortex. |
|
14 |
Rautava et al., 2013 |
11 |
F |
Lt max |
10 |
Complicated eruption of teeth 23, 24. |
Panoramic: resorption of the root 24. 12 weeks after extraction 24: Panoramic: resorption of the roots 22, 23. CBCT: osteolytic lesion in the palatal side of the roots 22-24, palatal cortex perforated. |
|
15 |
Sah et al., 2013 |
30 |
M |
Rt posterior man |
70 |
Tender reddish-pink mass, irregular surface. Rapid growth, mobility of all the posterior teeth. |
CT: hypodense area in premolar and molar region, breach in the continuity of the lingual cortical plate. |
|
16 |
Biniraj & Janardhanan, 2014 |
38 |
F |
Lt max |
40 |
Painless diffuse swelling of left maxillary region. Lacrimation, difficulty in breathing. Swelling on the alveolar ridge at the site of extraction 27, normal color, smooth surface. |
Panoramic: ill-defined RL. CT: irregular soft tissue lesion in the left maxillary sinus, expansion and thinning of medial wall and erosion of anterior, inferior and superior walls. Intraorbital extension with the involvement of inferior rectus muscle and extension into the ethmoid sinus, nasal cavity, oral cavity, pterygopalatine fossa and infratemporal fossa. MRI: lesion extended into the left alveolus causing erosion and expansion of alveolus. |
|
17 |
Lazaridou et al, 2014 |
75 |
F |
Lt max and sinus |
Painless progressively enlarging mass. Epistaxis, nasal obstruction, hypoesthesia of the cheek and the upper lip. No cervical lymphadenopathy. |
CT: lesion occupied the left maxillary antrum and buccal area and extended into the oral cavity, infiltrating the left malar bone and the orbital floor, extended to the nasal septum. Non-homogenous mass, moderate enhancement. No enlarged cervical lymph nodes. Radionuclide bone scan: increased uptake of the left maxilla and mandible and the head and the body of the humerus. |
|
|
18 |
Rahman et al., 2014 |
36 |
F |
Lt max |
70 |
Painless, firm, large, lobulated, pink swelling on adjacent hard palate. |
CT: heterogeneously enhancing soft tissue mass with lytic destruction. |
|
19 |
Stringer et al., 2014 |
16 |
M |
Lt posterior man |
10 |
None. |
Panoramic: well-circumscribed RL lesion resorpting roots of teeth 36-37. |
|
20 |
Adachi et al., 2015 |
42 |
M |
Rt posterior man |
18 |
Spontaneous pain and percussion pain in teeth 34-36. |
Panoramic: ill-defined RL at teeth 35-36. CBCT: osteolytic lesion with destruction of the lingual and buccal cortical plates, cortical expansion. MRI: mass with low intensity on an enhanced T1-weighted image and an enhanced margin of the lesion on a gadolinium-enhanced T1-weighted image, high signal intensity on a T1 normal. |
|
21 |
Alkindi et al., 2015 |
27 |
F |
Lt man |
60 |
Hard mass in left mandible, buccolingual expansion, teeth mobility and roots resorption. |
MRI: solid homogeneous mass with mandibular bone destruction and multi-enlarged neck lymph nodes. |
|
22 |
Sato et al., 2015 |
61 |
M |
Lt posterior man |
75 |
Swelling, trismus, pain, warmth of left cheek. Hemorrhagic soft mass in left mandibular molar and buccal mucosa region. |
Panoramic: RL around tooth 38. CT: well-defined osteolytic lesion, extending to the surrounding of left mandibular condyle. MRI: hypertrophy at the left masseter. PET: strong accumulation with maximum standardized uptake value of 11.3 around the left mandibular bone and 6.5 around the rectum. |
|
23 |
Tateishi et al., 2016 |
11 |
F |
Rt man |
30 |
Delayed eruption of tooth 43 and mental nerve palsy. |
Panoramic: unilocular RL lesion resorbing the roots of teeth 42-44. CT: well-circumscribed non-expansile osteolytic lesion, destroying the buccal cortical plate and resorbing the roots of teeth 42-44. |
|
24 |
Korlepara et al., 2017 |
22 |
M |
Lt man |
Painless enlarging mass in left posterior mandible. |
Panoramic: irregular RL at the left ramus region. CT: Perforation at left ramus region with extensions as tht of OPG. |
|
|
25 |
Present case |
7 |
F |
Rt posterior man |
40 |
Swelling of right cheek. Buccal bony expansion. Mobility of tooth 46. |
Panoramic: well-circumscribed RL, root resorption of the tooth 46 and distal displacement of the tooth 47 to the area of ramus. CBCT: well-circumscribed hypodense expansile osteolytic lesion. |
Table 1: Clinical information of central IMTs of the jaws.
|
Radiolucency |
17 RL |
8 no information |
|
|
Demarcation |
8 well defined |
9 ill defined |
8 no information |
|
Bony expansion |
8 no |
6 yes |
11 no information |
|
Bone destruction |
25 yes |
||
|
Locularity |
17 unilocular |
1 multilocular |
7 no information |
|
Tooth resorption |
7 yes |
6 no |
12 no information |
|
Soft tissue swelling |
14 yes |
7 no |
4 no information |
|
Ulcerated mucosa |
15 no |
4 yes |
6 no information |
Table 2: Clinicoradiologic features of central IMTs of the jaws.
|
Reference |
Treatment |
Follow up |
Histopathology |
Markers |
|
|
1 |
Zegarelli et al., 1974 |
Surgical excision with a rib graft |
12m NED |
Collagenous tissue with inflammatory infiltrate: neutrophils, lymphocytes, granular histiocytes, plasma cells (most abundant). |
Negative: PAS Fite-Faraco stain |
|
2 |
Shek et al., 1996 |
Resection |
13m NED |
Plump spindle cells with abundant plasma cells and lymphocytes in the background. |
Positive: SMA CD8 Vimentin ________________________ Negative: S-100 MAK-6 CD21 Ber-MAC-DRC Ki-M4 |
|
3 |
Araki et al., 2002 |
Enucleation |
- NED |
Non-specific granuloma with evidence of marked plasma cell proliferation. |
|
|
4 |
Fang & Dym, 2004 |
Commando procedure with a modified supraomohyoid neck dissection. |
6m NED |
Spindle cell proliferation in background of extensive inflammation, ulceration and a granulation tissue. |
Positive: Desmin P53 – 10% Ki-67 – 40-50% ________________________ Negative: CM 5.2 34 BE12 AE1-AE3 HMB45 Epithelial membrane antigen Myogenin |
|
5 |
Poh & Dahlman, 2005 |
Enucleation of the lesion and extraction of 46. |
14m LR |
Spindle cells, inflammatory cell infiltrate: lymphocytes, mast cells. No atypia, rare mitotic activity. |
Positive: SMA Vimentin ALK (cytoplasmic granular to diffuse) ________________________ Negative: S-100 Desmin CD31, CD34, CD68 |
|
6 |
Oh et al., 2008 |
Marginal mandibulectomy, reconstruction with iliac corticocancellous graft and bone substitute |
22m NED |
Dense nodular infiltration of plasma cells with admixture of neutrophils, eosinophils, and histiocytes. No atypical or pleomorphic features. Mild dysplasia at the epithelium. |
- |
|
7 |
Wales et al., 2008 |
Surgical resection combined with high dose corticosteroids |
11m NED |
Connective tissue densely infiltrated with medium sized atypical lymphoid like cells, plasma cells, neutrophils and some atypical plasma like cells. |
Kappa and lambda suggestive of polyclonality. |
|
8 |
Eley & Watt-Smith, 2010 |
Surgical excision |
72m NED |
Myofibroblastic proliferation in an edematous, myxoid stroma with focal fibrin deposition. Stellate and bipolar spindle cells. No atypia. |
- |
|
9 |
Satomi et al., 2010 |
Surgical excision |
120m NED |
Proliferation of fibroblastic or myofibroblastic spindle cells in a background of collagen fibers and granulation tissue-like components. Inflammatory infiltrate, mainly plasma cells and neutrophils, no atypia. |
Positive: Vimentin SMA Ki-67 – index 5% ________________________ Negative: Desmin ALK-1 S-100 CD68 CD34 CD99 P53 |
|
10 |
Sasagawa et al., 2011 |
Right frontoparietal craniectomy, left mandibular excision. Corticosteroids 20Gy of focal irradiation against for residual lesions (parietal bone and mandible). |
12m no regrowth |
Spindle cells in interlacing fascicles. Numerous inflammatory cells, mainly plasma cells and lymphocytes. |
Positive: SMA Vimentin ________________________ Negative: S-100 Epithelial membrane antigen ALK Minimal nuclear pleomorphism and low mitotic activity (MIB-1 index 4.0%) |
|
11 |
Date et al. 2012 |
Marginal resection |
12m NED |
Fascicular spindle cells, moderate amount of mitosis, no atypia. Dense chronic inflammatory cell infiltrate: neutrophils, eosinophils, lymphocytes. |
Positive: Vimentin SMA CD68- focally positive Ki-67 index 30% ________________________ Negative: ALK |
|
12 |
Garcia et al., 2012 |
Mandibular resection with titanium plate reconstruction |
36m NED |
Spindle cell proliferation with fusiform nuclei. Mixed chronic inflammatory cell infiltrate consisting of plasma cells and lymphocytes with intercellular collagen, no atypia. |
- |
|
13 |
Gawande et al., 2012 |
Surgical excision |
18m NED |
Connective tissue with diffuse and focal inflammatory infiltrate: plasma cells and lymphocytes. Focal areas of inflammation around blood vessels. |
- |
|
14 |
Rautava et al., 2013 |
Enucleation and curettage |
36m NED |
Spindle cell proliferation in collagenous stroma with storiform architecture. Mild nuclear pleomorphism, low mitotic activity. Small groups of lymphocytes scattered around the spindle cell proliferation. |
Positive: SMA Vimentin ALK (cytoplasmic granular to diffuse) Calponin Myoglobin HHF35 CD34 ________________________ Negative: S-100 Pancytokeratin Desmin Myf4 |
|
15 |
Sah et al., 2013 |
Course of steroids following which the lesion resolved |
7m NED |
Spindle cells in a fascicular growth pattern, admixed with a dense inflammatory cell infiltrate of plasma cells, eosinophils, neutrophils and lymphocytes. Cellular connective tissue stroma, overlying epithelium showing erosion. |
Positive: SMA CD34 CD138 CD3 CD20 ________________________ Negative: ALK CD23 |
|
16 |
Biniraj & Janardhanan, 2014 |
Resection RT |
6m LR Finally DOD |
Proliferating atypical spindle cells admixed with numerous inflammatory cells mainly neutrophils, lymphocytes and plasma cells. Cytological atypia with nuclear pleomorphism and increased mitotic activity. |
Positive: Vimentin SMA CD68 (focally) Negative: CD 1a S100 Desmin CD34 ALK-1 MPO Ki-67 index 8-10% |
|
17 |
Lazaridou et al, 2014 |
Extended hemimaxillectomy |
12m NED |
Spindle cells, storiform to fascicular pattern, variable component of inflammatory cells: lymphocytes, histiocytes, plasma cells, eosinophils and neutrophils. Stroma highly vascularized, edematous to fibromyxoid to collagenous. Vascular component varies from widely dilated medium-sized vascular channels to narrow, slit-like blood vessels obscured by the myofibroblasts and inflammatory cells. |
- |
|
18 |
Rahman et al., 2014 |
Surgical excision |
18m NED |
Low grade collagenous and focally myxoid spindle cell neoplasm. |
Positive: SMA ________________________ Negative: S-100 CD30 Ki-67 Cytokeratin |
|
19 |
Stringer et al., 2014 |
Surgical excision |
6m NED |
Spindle cells with an appearance suggestive of myofibroblasts with scattered lymphocytes. |
Positive: SMA ALK-1 (cytoplasmatic granular to diffuse) ________________________ Negative: Desmin |
|
20 |
Adachi et al., 2015 |
En bloc resection (including teeth 34-36 and alveolar bone with adequate margins) |
12m NED |
Spindle cells in a fascicular growth pattern with dense inflammatory cell infiltrate of plasma cells, eosinophils, neutrophils, and lymphocytes. Highly cellular connective tissue stroma. |
Positive: SMA Negative: ALK |
|
21 |
Alkindi et al., 2015 |
Resection with a 5-mm margin |
12m NED |
Non-encapsulated myofibroblastic proliferation with chronic inflammatory infiltrate, spindle and polygonal cells with inconspicuous mitotic activity. |
Positive: CD68 SMA Calponin Negative: ALK-1 S-100 Desmin. |
|
22 |
Sato et al., 2015 |
Corticosteroids- poor response. Resection with the disarticulation of the mandible including condyle. |
18m NED |
Fibrotic lesion with a wide infiltration of plasma cells and lymphocytes. |
Positive: SMA Caldesmon
Negative: Desmin ALK |
|
23 |
Tateishi et al., 2016 |
Curettage |
18m NED |
Plump spindle cell proliferation with a storiform architecture and scattered lymphocytes. Stroma included a focal myxoid matrix and small islands of an odontogenic epithelium. No mitosis or necrosis. |
Positive: SMA ALK (cytoplasmatic granular to diffuse) CD10 glial fibrillary acidic protein (GFAP) ________________________ Negative: Cytokeratin AE1/AE3, S100 proteins, p63 Ki-67 index 3%. |
|
24 |
Korlepara et al., 2017 |
Surgical excision |
- |
Nonencapsulated mass with fascicles of elongated spindle cells in fibrous stroma with intense chronic inflammatory cell infiltrate, predominantly lymphocytes. Spindle cells plump ovoid to tapering hyperchromatic nuclei with indistinct cell boundaries and mild nuclear atypia. No mitosis. |
Positive: Vimentin SMA ALK _________________________ Negative: S-100 |
|
25 |
Present case |
Surgical excision with peripheral ostectomy |
6m NED |
Cellular myofibroblastic neoplasm with spindle cells and numerous chronic inflammatory cells, mostly lymphocytes and fewer plasma cells. |
Positive: SMA ALK (cytoplasmatic granular to diffuse) Caldesmon S-100 (focally positive) ________________________ Negative: Desmin MNF |
Table 3: Pathological features of central IMTs of the jaws.
|
Childhood/ young adults |
Combination of spindle cells and inflammation |
Ancillary |
|
|
Myofibroma |
Yes |
Spindle cells but not inflammation |
|
|
Desmoplastic fibroma |
Yes |
Yes, collagenous stroma |
ß catenin positive |
|
Nodular fasciitis |
Young adults |
Yes, feather or tissue culture like |
ALK1 negative |
|
Follicular dendritic cell sarcoma |
Less characteristic |
Yes |
dendritic cell markers positive: clusterin, CD21, CD23, and CD35 and EGFR |
|
Low grade fibromyxoid sarcoma |
Young adults |
Yes, whorls of alternating fibrous and myxoid areas |
MUC4 , EMA positive |
|
Myofibrosarcoma |
Yes |
Yes, with atypia |
|
|
Infantile fibrosarcoma |
Yes |
Yes, herring bone intersecting spindle cell fascicles, variable collagenous stroma |
t (12; 15) (p13; q25 resulting in fusion gene ETV6-NTRK3, actins variably positive |
Table 4: The histopathological differential diagnosis of lesions composed of spindle cells and inflammatory infiltrate in a young patient.
5. Discussion
IMT is a mesenchymal tumor composed of fibroblastic and myofibroblastic spindle cells in a myxoid or collagenous stroma accompanied by an inflammatory infiltrate, mainly composed of plasma cells and lymphocytes. Its occurrence in the head and neck is uncommon [29] and central lesions occurring in the jawbones are even rarer [30].
IMT is considered a lesion of intermediate biological behavior, due to its tendency to locally recur, but metastases are rare and have been documented in <5% of cases [31].
This study shows that clinically, almost all the intrabony central IMTs of the jawbones presented as unilocular radiolucencies. An exception was the current case, which was multilocular. The differential diagnosis of a central unilocular radiolucent expansile lesion in a child includes odontogenic cysts and tumors such as odontogenic keratocyst, unicystic ameloblastoma, ameloblastic fibroma, or reactive/reparative lesions such as giant cell granuloma. At times, the aggressive course appearing in a child could be alarming raising suspicion towards malignancy.
Immunohistochemically, IMTs variably express SMA (80-90%), muscle-specific actin (HHF-35) and desmin (up to 60%), and about one third are focally positive to cytokeratins AE1/AE3, and CAM 5.2. Approximately 50% of IMTs show clonal rearrangements of the ALK1 gene in chromosome 2 at band 2p23 with corresponding overexpression at the protein level. ALK1, a receptor tyrosine kinase, is aberrantly expressed in anaplastic large cell lymphomas that harbor a (2;5) translocation. As a result, various gene partners can be fused to ALK1 in IMT such as tropomyosin 3, tropomyosin 4, CLTC, RANBP2, CARS, ATIC and SEC31A (35). The pattern of ALK1 immunohistochemical expression may reflect the fusion pattern: Diffuse cytoplasmic staining may be seen when the fusion involves the cytoplasmic proteins TPM3, TPM4, CARs, ATIC and SEC31A and granular cytoplasmic staining is seen when the fusion partner is CLTC which is a main component of the coated vesicles involved in selective protein transport. Nuclear membrane staining is seen with RANBP2, a large protein located in the nuclear pores [35]. In the performed review, all the 6 ALK1 positive cases were found to exhibit a granular to strongly diffused cytoplasmic staining.
The tumor was positively stained with Paladin, implicated in acquision of mesenchymal phenotype in metastatic breast carcinoma [36]. The neoplastic cells were diffusely positive. Paladin, a product of the PALD-1 gene, has roles in tissue development, angiogenesis and motility, features that could overlap with the role of myofibroblastic cells. To the best of our knowledge, this is the first report of paladin positivity in IMT. This finding should be further confirmed in additional IMT series before suggesting its use as a myofibroblastic marker.
In the published cases, different antibodies are used to confirm the diagnosis of IMT. The most common were SMA, ALK1, CD68 and vimentin. ALK1 was found to be positive in 6 and negative in 8 cases of central IMT which is consistent with other areas of the body. CD88 could be attributed to the inflammatory component. Focal S100 positivity was reported only in the present case, while in 10 cases it was reported to be negative. While "heavy", h-Caldesmon expression is considered specific for smooth muscle differentiation and according to some authors excludes myofibroblasts as cell of origin [32], 2 central IMTs were found positive. There are two isoforms, one predominantly in smooth muscle and another in other cell types with partial myogenic differentiation. High molecular weight isoforms are capable of binding actin, tropomyosin, calmodulin and myosin. As a matter of fact, h-Caldesmon expression is not entirely specific for smooth muscle as it is observed in the majority of GISTs, glomus tumors and myopericytomas. This study shows that its positivity in neoplastic cells does not exclude IMT. Although cytokeratins may be expressed in up to one third of the IMTs [33], none of the central intrabony IMTs of the jaws were positive, but this could also be explained statistically by the low number of cases.
The histopathological differential diagnosis of lesions composed of fibroblastic/ myofibroblastic spindle cells and inflammatory infiltrate in a young patient is diverse and may include: myofibroma, desmoplastic fibroma, nodular fasciitis, follicular dendritic cell sarcoma, low grade fibromyxoid sarcoma, myofibrosarcoma, myxofibrosarcoma and infantile fibrosarcoma (Table 4).
Myofibroma is a tumor of childhood that can present centrally in the jaw [34], but inflammation is not expected. Desmoplastic fibroma is composed of long fascicles of SMA positive fibroblasts / myofibroblasts in a collagenous stroma and is ß catenin positive [35]. Nodular fasciitis is a benign nodular proliferation of fibroblasts and myofibroblasts, may present in young adults and be mistaken for a sarcoma due to clinically rapid growth and that can spontaneously regress. It is characteristically described as "tissue culture like", "feathery" infiltrated by inflammatory cells of both acute and chronic lineages. Although positivity to SMA and muscle-specific actin (HHF-35) is expected, ALK1will be negative.
Follicular dendritic cell sarcoma is composed of spindle shaped cells with inflammatory cells, but is less characteristic of children. Immunohistochemically they are positive to dendritic cell markers such as clusterin, CD21, CD23, and CD35 and for epidermal growth factor receptor. Low grade fibromyxoid sarcoma is a spindle cell tumor of young adults that presents bland cytomorphology but characterized by local recurrences and late distant metastases. It is composed of whorls of alternating fibrous and myxoid areas and MUC4 is overexpressed in the cytoplasm and EMA is positive. MUC4 staining was not performed in our case.
Myofibrosarcoma is a low or high grade malignant tumor of myofibroblasts, which can arise in soft tissue or bone in adults or children. However, it should have evidence of atypia. The same applies to infantile fibrosarcoma, characterized by the herring bone pattern of intersecting spindle cell fascicles and variably collagenous stroma. It typically presents in the first year of life as a rapidly growing mass with a reciprocal translocation t (12; 15) (p13; q25) that results in the fusion gene ETV6-NTRK3, with variable positivity to actins. Although central intrabony IMTs are rare, the current study suggests that most of the patients were treated by conservative surgery, with no recurrence. A minority underwent additional treatments with corticosteroids or radiation.
In conclusion, central intrabony IMT of the jawbones is a rare tumor that could be clinically alarming, especially in the context of pediatric population. Almost all the central IMTs appear radiolucent unilocular lesions on imaging with the present case the first multilocular. The differential diagnosis of spindle cell lesions accompanied by inflammatory infiltrate in children is diverse. ALK1 positivity with granular to strongly diffuse staining is present in about half of the cases. h-Caldesmon positivity does not exclude myofibroblastic differentiation and Paladin is shown, for the first time, to be diffusely positive in the neoplastic cells. Treatment is mostly surgical with no evidence of recurrence in the majority of the cases.
Acknowledgment
The authors would like to thank Prof. C.D. Fletcher for his kind assistance in consultation confirming the diagnosis of this case.
Funding
No funding was received.
Conflict of Interest
Alex Neronov declares that he has no conflict of interest. Oded Nahlieli declares that he has no conflict of interest. David Ben-Dor declares that he has no conflict of interest. Amram Zagury declares that he has no conflict of interest. Alejandro Livoff declares that he has no conflict of interest. Irit Allon declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Weiss SW.Histological Typing of Soft Tissue Tumors. 2nd Ed. Berlin: Springer-Verlag (1994):
- Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 19 (1995): 859-872.
- Classification of Soft Tissue Tumors. New York, NY: Springer; World Health Organization International Classification of Tumours (1994).
- Coffin CM, Fletcher CD. Inflammatory myofibroblastic tumour. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. World Health Organization classification of tumours of soft tissue and bone. Lyon: IARC Press (2013): 83-8
- Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31 (2007): 509-5
- Zegarelli DJ, Rankov RM, Zegarelli EV. A large dental granuloma (? inflammatory pseudotumor) with unusual features: report of a case. J Am Dent Assoc 89 (1974): 891-894.
- Shek AW, Wu PC, Samman N. Inflammatory pseudotumour of the mouth and maxilla. J Clin Pathol 49 (1996): 164-167.
- Araki M, Hashimoto K, Shinoda K, et al. Plasma cell granuloma with severe cortical bone destruction of the mandible appearing as a malignant lesion: a case report. Oral Radiol 18 (2002): 45-51.
- Fang JC, Dym H. Myofibroblastic tumor of the oral cavity. A rare clinical entity. NY State Dent J 70 (2004): 28-30.
- Poh CF, Priddy RW, Dahlman DM. Intramandibular inflammatory myofibroblastic tumor-a true neoplasm or reactive lesion?. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100 (2005): 460-466.
- Oh JH, Yim JH, Yoon BW, et al. Inflammatory pseudotumor in the mandible. J Craniofac Surg 19 (2008): 1552-1553.
- Wales CJ, Carter LM, Whitfield PH. Facial pseudotumours. A case report and review of their management. Br J Oral Maxillofac Surg 46 (2008): 57-58.
- Eley KA, Watt-Smith SR. Intraoral presentation of inflammatory myofibroblastic tumor (pseudotumor) at the site of dental extraction: report of a case and review of the literature. J Oral Maxillofac Surg 68 (2010): 2016-2020.
- Satomi T, Watanabe M, Matsubayashi J, et al. A successfully treatedinflammatory myofibroblastic tumor of the mandible with long-term follow-up and review of the Med Mol Morphol 43 (2010): 185-191.
- Sasagawa Y, Akai T, Itou S, et al. Multiple intraosseous inflammatory myofibroblastic tumors presenting with an aggressive clinical course: case report. Neurosurgery 69 (2011): E1010-E101
- Date A, Yamagata K, Onizawa K, et al. Inflammatory pseudotumor:report of a case in the Oral Maxillofac Surg 16 (2012): 65-68.
- Garcia BA, Tinsley S, Schellenberger T, et al. Recurrentinflammatory pseudotumor of the jaw with perineural intracranial invasion demonstrating sustained response to Rituximab. Med Oncol 29 (2012): 2452-2455.
- Gawande PD, Sambhus M, Garde JB, et al. Aggressiveinflammatory pseudotumor of the J Craniofac Surg 23 (2012): 1101-1103.
- Rautava J, Soukka T, Peltonen E, et al. Unusualcase of inflammatory myofibroblastic tumor in Case Rep Dent 2013 (2013): 876503.
- Sah P, Byatnal AA, Rao L, et al. Inflammatory myofibroblastic tumor: arapidly growing soft tissue mass in the posterior mandible. Head Neck Pathol 7 (2013): 393-397.
- Biniraj KR, Janardhanan M. Inflammatory myofibroblastic tumor of maxilla showing sarcomatous change in an edentulous site with a history of tooth extraction following periodontitis: A case report with discussion. J Indian Soc Periodontol 18 (2014): 375-378.
- Lazaridou M, Dimitrakopoulos I, Tilaveridis I, et al. Inflammatory myofibroblastic tumourof the maxillary sinus and the oral cavity. Oral Maxillofac Surg 18 (2014): 111-114.
- Rahman T, Sharma JD, Krishnatreya M, et al. Inflammatory myofibroblastic tumor of the upper alveolus: A rare entity presenting as a jaw swelling. Ann Maxillofac Surg 4 (2014): 227-229.
- Stringer DE, Allen CN, Nguyen K, et al. Intraosseousinflammatory myofibroblastic tumor in the mandible: a rare pathologic case report. Case Rep Surg 2014 (2014): 565478.
- Adachi M, Kiho K, Sekine G, et al. Inflammatory Myofibroblastic TumorMimicking Apical J Endod 41 (2015): 2079-2082.
- Alkindi M, Alotaibi N, Alshiddi M. Inflammatory myofibroblastic tumour of the mandible – case report. Int J Oral Maxillofac Surg 44 (2015): e184.
- Sato T, Suenaga H, Sugiyama M, et al. Rare case report of huge inflammatory pseudotumor of the mandible.J Oral Maxillofac Surg Med Pathol 28 (2016): 222-227.
- Tateishi Y, Okudela K, Kawai S, et al. Intraosseousinflammatory myofibroblastic tumor of the mandible with a novel ATIC-ALK fusion mutation: a case report. Diagn Pathol 11 (2016): 132.
- Ong HS, Ji T, Zhang CP, et al. Head and neck inflammatory myofibroblastic tumor (IMT): evaluation of clinicopathologic and prognostic features.Oral oncol 48 (2012): 141-148.
- Park SB, Lee JH, Weon YC. Imaging findings of head and neck inflammatory pseudotumor. AJR Am J Roentgenol 193 (2009): 1180-118
- Singer S, Neilsen T, Antonescu CR. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer Principles & Practice of Oncology. 9th International edition. Philadelphia, PA: Lippincott Williams and Wilkins (2012): 1540.
- Ceballos KM, Nielsen GP, Selig MK, et al. Is anti-h- caldesmon useful for distinguishing smooth muscle and myofibroblastic tumors? An immunohistochemical study. Am J Clin Pathol 114 (2000): 746-753.
- Sukov WR, Cheville JC, Carlson AW, et al. Utility of ALK-1 protein expression and ALK rearrangements in distinguishing inflammatory myofibroblastic tumor from malignant spindle cell lesions of the urinary bladder. Mod Pathol 20 (2007): 592-603.
- Allon I, Vered M, Buchner A, et al. Central (intraosseous) myofibroma of the mandible: clinical, radiologic, and histopathologicfeatures of a rare lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103 (2007): e45-53.
- Dabbs DJ. Diagnostic immunohistochemistry. Theranostic and Genomic Applications. Elsevier Health Sciences (2013).
- Gilam A, Conde J, Weissglas-Volkov D, et al. Local microRNA delivery targets paladin and prevents metastatic breast cancer. Nat. Commun (2016).
- Korlepara R, Guttikonda VR, Madala J, et al. Inflammatory myofibroblastic tumor of mandible: A rare case report and review of literature. J Oral Maxillofac Pathol 21 (2017): 136-139.
