Cefathiamidine-Induced Toxic Epidermal Necrolysis in a Child
Article Information
Wang QY, Zou N, Zhu J, Zhang MM, Liu ZJ*
Department of Pediatrics, the Second Affiliated Hospital of Dalian Medical University, Shahekou, Dalian, China
*Corresponding Author: Dr. Zheng Juan Liu, Department of Pediatrics, the Second Affiliated Hospital of Dalian Medical University, 467 Zhongshan Road, Shahekou District, Dalian 116027, China
Received: 04 November 2019; Accepted: 14 November 2019; Published: 08 January 2020
Citation: Wang QY, Zou N, Zhu J, Zhang MM, Liu ZJ. Cefathiamidine-Induced Toxic Epidermal Necrolysis in a Child. Archives of Clinical and Medical Case Reports 4 (2020): 051-054.
View / Download Pdf Share at FacebookKeywords
Toxic Epidermal Necrolysis; Drug eruption; Mucosal damage; Mucosal damage nodes
Toxic Epidermal Necrolysis articles, Drug eruption articles, Mucosal damage articles, Mucosal damage nodes articles
Toxic Epidermal Necrolysis articles Toxic Epidermal Necrolysis Research articles Toxic Epidermal Necrolysis review articles Toxic Epidermal Necrolysis PubMed articles Toxic Epidermal Necrolysis PubMed Central articles Toxic Epidermal Necrolysis 2023 articles Toxic Epidermal Necrolysis 2024 articles Toxic Epidermal Necrolysis Scopus articles Toxic Epidermal Necrolysis impact factor journals Toxic Epidermal Necrolysis Scopus journals Toxic Epidermal Necrolysis PubMed journals Toxic Epidermal Necrolysis medical journals Toxic Epidermal Necrolysis free journals Toxic Epidermal Necrolysis best journals Toxic Epidermal Necrolysis top journals Toxic Epidermal Necrolysis free medical journals Toxic Epidermal Necrolysis famous journals Toxic Epidermal Necrolysis Google Scholar indexed journals Toxic articles Toxic Research articles Toxic review articles Toxic PubMed articles Toxic PubMed Central articles Toxic 2023 articles Toxic 2024 articles Toxic Scopus articles Toxic impact factor journals Toxic Scopus journals Toxic PubMed journals Toxic medical journals Toxic free journals Toxic best journals Toxic top journals Toxic free medical journals Toxic famous journals Toxic Google Scholar indexed journals Necrolysis articles Necrolysis Research articles Necrolysis review articles Necrolysis PubMed articles Necrolysis PubMed Central articles Necrolysis 2023 articles Necrolysis 2024 articles Necrolysis Scopus articles Necrolysis impact factor journals Necrolysis Scopus journals Necrolysis PubMed journals Necrolysis medical journals Necrolysis free journals Necrolysis best journals Necrolysis top journals Necrolysis free medical journals Necrolysis famous journals Necrolysis Google Scholar indexed journals Drug eruption articles Drug eruption Research articles Drug eruption review articles Drug eruption PubMed articles Drug eruption PubMed Central articles Drug eruption 2023 articles Drug eruption 2024 articles Drug eruption Scopus articles Drug eruption impact factor journals Drug eruption Scopus journals Drug eruption PubMed journals Drug eruption medical journals Drug eruption free journals Drug eruption best journals Drug eruption top journals Drug eruption free medical journals Drug eruption famous journals Drug eruption Google Scholar indexed journals Mucosal damage articles Mucosal damage Research articles Mucosal damage review articles Mucosal damage PubMed articles Mucosal damage PubMed Central articles Mucosal damage 2023 articles Mucosal damage 2024 articles Mucosal damage Scopus articles Mucosal damage impact factor journals Mucosal damage Scopus journals Mucosal damage PubMed journals Mucosal damage medical journals Mucosal damage free journals Mucosal damage best journals Mucosal damage top journals Mucosal damage free medical journals Mucosal damage famous journals Mucosal damage Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Mucosal damage nodes articles Mucosal damage nodes Research articles Mucosal damage nodes review articles Mucosal damage nodes PubMed articles Mucosal damage nodes PubMed Central articles Mucosal damage nodes 2023 articles Mucosal damage nodes 2024 articles Mucosal damage nodes Scopus articles Mucosal damage nodes impact factor journals Mucosal damage nodes Scopus journals Mucosal damage nodes PubMed journals Mucosal damage nodes medical journals Mucosal damage nodes free journals Mucosal damage nodes best journals Mucosal damage nodes top journals Mucosal damage nodes free medical journals Mucosal damage nodes famous journals Mucosal damage nodes Google Scholar indexed journals pregnancy articles pregnancy Research articles pregnancy review articles pregnancy PubMed articles pregnancy PubMed Central articles pregnancy 2023 articles pregnancy 2024 articles pregnancy Scopus articles pregnancy impact factor journals pregnancy Scopus journals pregnancy PubMed journals pregnancy medical journals pregnancy free journals pregnancy best journals pregnancy top journals pregnancy free medical journals pregnancy famous journals pregnancy Google Scholar indexed journals cervical articles cervical Research articles cervical review articles cervical PubMed articles cervical PubMed Central articles cervical 2023 articles cervical 2024 articles cervical Scopus articles cervical impact factor journals cervical Scopus journals cervical PubMed journals cervical medical journals cervical free journals cervical best journals cervical top journals cervical free medical journals cervical famous journals cervical Google Scholar indexed journals methylprednisolone articles methylprednisolone Research articles methylprednisolone review articles methylprednisolone PubMed articles methylprednisolone PubMed Central articles methylprednisolone 2023 articles methylprednisolone 2024 articles methylprednisolone Scopus articles methylprednisolone impact factor journals methylprednisolone Scopus journals methylprednisolone PubMed journals methylprednisolone medical journals methylprednisolone free journals methylprednisolone best journals methylprednisolone top journals methylprednisolone free medical journals methylprednisolone famous journals methylprednisolone Google Scholar indexed journals
Article Details
1. Clinical Information
The ten-year-old girl was admitted into our ward of the Second Hospital of Dalian Medical University due to fever 9 days with rashes 3 days. Because of low fever with cervical lymph nodes enlargement, she was infused cefathiamidine for 6 days in the local hospital, but the effect was not satisfactory. Three days later, the illness was aggravating, the girl had a high fever with skin rashes, which was distributed in the face, trunk and limbs with itch. She had no history of special drug or food allergies. T 39.4?, P 98 /min, R 25/min, Bp118/72 mmhg, BW: 47kg. She had purple skin rashes all over her body with itch, mainly on face and trunk. There were bilsters on oral lips and jaw. The conjunctiva of both her eyes were congested without secretions, and she had enlarged right cervical lymph node of size 2 × 2 cm2, which were tough, not painful. She had no positive signs of heart, lung and abdomen. Blood routine test: WBC 4.88 × 109/L, N 38%, L 52%, E 1%, Hb 127g/L, PLT 162 × 109/L, CRP 2.48 mg/L; Liver biochemistry: ALT 85.92 U/L, AST 64.79U/L, LDH 519.92U/L; Inflammatory factors: IL-2R 1348 U/ml, IL-6 7.37 u /ml, IL-10 25.20pg/ml, TNF-α 22.60 pg/ml; Etiology examination: EB-IgM (+). She was diagnosed with TNE and the skin rashes quickly spread to the whole body, congestive rashes appeared at first (Figure 1A), later evolved into the purple spotted, part of rashes appeared to form blisters and exudations. She was given a intravenous infusion of methylprednisolone (30 mg Q12h 5 days, 20 mg Q12h 3 days, 15 mg Q12h 3 days, 10 mg Q12h. 3 days), combined with IGIV (15 g/d, 3 days) in order to inhibit inflammation reaction. Simultaneously the girl had a symptomatic and supportive treatment. On the second day of treatment, the fever subsided, rashes and itching were alleviated, and the body condition was improved. Some blisters were found on the part of pigmentation after rashes faded. The blisters gradually dried up, scabbed and broke their molt (Figure 1B). After intravenous infusion of methylprednisolone for 14 days, the girl was given methylprednisolone oral treatment (20mg/d) for 3 days, the rashes were fade away except hands and feet (Figure 1C), and the inflammatory factor levels were return to normal. The patient was discharged from hospital. During outpatient visits, methylprednisolone was gradually decreased and stopped within 2 weeks, and the rashes did not recur (Figure 1D).
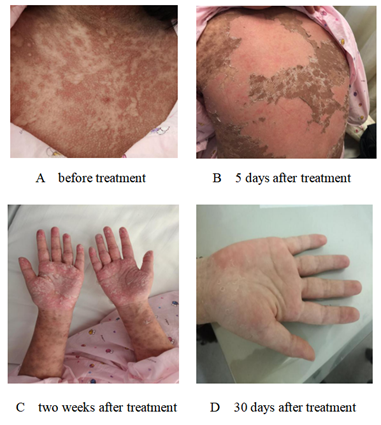
Figure 1A-1D: The rashes change before and after treatment.
2. Discussion
There are various kinds of drugs causing TEN. Some studies have shown that the main drugs causing TEN are antibiotics, among which cephalosporins are the most [3]. Other studies reported that the main drugs causing TEN were carbamazepine, allopurinol, etc. [4]. Our patient was given cefathiamidine infusion for 6 days, three days later, the patient had a high fever with itchy rashes, which quickly spread to the whole body, small rice-sized blisters gradually appeared in the pigmentation area after the rash faded. Blisters first appeared in the neck, then face, limbs, and finally palm, arch. Therefore, we diagnosed the girl suffered from TEN induced by cefathiamidine.
At present, the pathogenesis of TEN is not completely clear. The results of clinical and histopathological studies suggest that TEN is a specific hypersensitive immune response induced by drugs. Cytotoxic T lymphocyte is the main effector cells, cytotoxic proteins such as cell membrane receptor molecules Fas and its ligand Fasl, perforin and cytokine TNF-α play an important role [5-6]. Our patient had a high levels of IL-2R, IL-6, IL-10 and TNF-α, which supported above studies.
Typical early manifestations of TEN are fever, itchy or painful skin rashes, macules mostly appear in the face, and occasionally appear in the palm and plantar, with dark red center and red margin, presenting atypical target change and positive Nikolsdy sign. Macules rapidly form vesicles and gradually expand and fuse into giant relaxant bullae, ultimately epidermal peels and appears necrosis [7]. At present, the treatment of TEN includes stopping to use sensitizing drugs, symptomatic support, and the early treatment of sufficient amount of gamma globulin combined with glucocorticoid [8], which can rapidly improve the patient's condition, reduce the mortality, and achieve a good therapeutic effect.
In conclusion, TEN in children is rare and severely, glucocorticoid with gamma globulin therapy as early as possible to help control the condition, improve the prognosis.
Conllict of Interest
The authors declare that they have no conflicts of interest.
References
- Parrillo SJ. Stevens -Johnson syndrome and toxic epidermal necrolysis. Curr Allergy Asthma Rep 7 (2007): 243-247.
- Mockenhaupt M, Viboud C, Dunant A, et al. StStevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs.The Euro SCAR-study. J Invest Dermatol 128 (2008): 35-44.
- XIE Xiaoju, SHANG Jingjing. Analysis of 71patients with bullous epidermal necrolysis type of drug eruption. Evaluation and Analysis of Drug-Use in Hosptials of China 15 (2015): 1673-1675.
- Lonjou C, Borot N, Sekula P, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics18 (2008): 99-107.
- Abe R. Immunological response in Stevens-Johnson sydrome and toxic epidermal necrolysis. J Dermatol 42 (2015): 42-48.
- Saeed HN, Chodosh J. Immunologic medistors in Stevens-Johnson syndrome and toxic epidermal necrolysis. Semin Ophthalmol 31 (2016): 85-90.
- Sun Wei, Min Dinghong, Guo Guanghua. Advancement in the diagnosis and management of toxic epidermal necrolysis. Chinese Journal of Burns 32 (2016): 341-343.
- Yang Xiaolei, Deng Danqi, XieHong, et al. Clinical analysis of 33 patients with bullous epidermal necrolysis type of drug eruption. Journal of Clinical Dermatology 40 (2011): 408-409.
