Case studies on RetroMAD1™, an orally administered recombinant chimeric protein to treat naturally infected Feline Leukemia Virus (FeLV) cats
Article Information
Ung Eng Huan1*, Patricia Mafei F. Da Silva2 , Aline Petrolini Floriano3
1Biovalence Sdn Bhd, C-12-03, 3 Two Square, No. 2, Jalan 19/1 Selangor, 46300 Petaling Jaya, Selangor, Malaysia
2Royal Canin Brazil, Av. OL3, 200, Galpao 1, Parque Duque, 25085-375, Duque de Caxias, Rio de Janeiro, Brazil
3Av. Gen. Francisco Glicério, 651-José Menino, Santos – SP, 11015-120, Brasil
*Corresponding Author: Ung Eng Huan, Biovalence Sdn Bhd, C-12-03, 3 Two Square, No. 2, Jalan 19/1 Selangor, 46300 Petaling Jaya, Selangor, Malaysia
Received: 05 November 2019; Accepted: 21 November 2019; Published: 27 November 2019
Citation: Ung Eng Huan, Patricia Mafei F. Da Silva, Aline Petrolini Floriano. Case studies on RetroMAD1™, an orally administered recombinant chimeric protein to treat naturally infected FelineLeukemia Virus (FeLV) cats. Archives of Veterinary Science and Medicine 2 (2019): 041-057.
View / Download Pdf Share at FacebookAbstract
Treatment options for Feline leukimia are presently very limited due to lack of efficacy and drug toxicity. A recombinant chimeric protein RetroMAD1 is described for the first time in the treatment of FeLV. The chimera has an A-B-C configuration comprising of multiple documented antiviral domains that can potentially overcome single point mutation driven resistance by multidomain action. Oral administration of RetroMAD1 was given daily at 0.4mg/kg/day divided equally into two feeds roughly 12 hours apart for one month to highly symptomatic FeLV cats in Brazil. RetroMAD1 treated cats were monitored for survival until day 433 and untreated cats were monitored until day 173. A significant different in survival rate of 73% (treated N=55) versus 33% (untreated N=21) existed. Gingivitis, F.U.R.D. and anaemia were shown to improve significantly as signs indicating improvement in quality of life. Some of the RetroMAD1 treated cats were given interferon treatment by their respective vets as supportive treatment (N=14) that gave a slightly improved survival outcome compared to those given only RetroMAD1 and other supportive medication. Being orally administered, this protein may be easily administered by cat owners. In addition, biochemistry and CBC data also indicated that RetroMAD1 is safe and well tolerated. A safe, efficacious oral delivery antiviral chimera will be very useful for veterinary treatment especially if it is also broad-spectrum in nature.
Keywords
Feline Leukimia Virus; RetroMAD1; Antiviral; FeLV treatment; Oral administration; Chimeric protein
Feline Leukimia Virus articles, RetroMAD1 articles, Antiviral articles, FeLV treatment articles, Oral administration articles, Chimeric protein articles
Article Details
1. Introduction
Feline Leukimia (FeLV) is responsible for more illness in cats globally than any other virus [1]. Three subgroups exist as FeLV-A being the most common, FeLV-B being the recombinant of FeLV-A and endogenous genomic FeLV with higher morbidity and mortality and FeLV-T that induces T-cell tropic leukemia [2-4]. High sequence heterogeneity and variable endogenous enFeLV copy number suggests high potential for new variants evolving [4]. Almost three-quarters of Brazilian FeLV symptomatic cats were FFV+ while less than a quarter of asymptomatic animals were FFV+ suggesting a linkage [5].
Infections where viruses replicate in the oropharyngeal lymphoid tissue and cats having strong immune responses that never become viremic are termed abortive infections. Regressive infections follow an effective immune response where viremia stops within weeks or months and although proviral DNA is present within the cellular genome, viruses are undetected and hence, non-infective. Progressive infections are uncontained, spreading to the marrow and mucosal/glandular epithelium with persistent viremia and death within a few years. Focal infections are rare and occur only within mammary glands, bladder or eyes [1].
Except for Feline interferon-ὠ, controlled in vivo studies using Zidovudine (AZT), Zalcitabine (ddC), Adefovir (PMEA), Human interferon-α (IFN-α) were either ineffective or highly toxic [6], [7] with many owners opting for euthanasia or palliative care or complete isolation that may stress both the owner and the animal [8]. Vaccines are the first line of defence in FeLV management and its widespread use has contributed significantly to lessening prevalence despite none of the current vaccines offering 100% protection [9]. One reason why some owners avoid vaccination is fear of Vaccine-assisted Sarcoma (VAS), an aggressive malignant tumour requiring surgery and chemotherapy and in fact, the American Association of Feline Practitioners reserves vaccination for high-risk populations such as indoor-outdoor cats and catteries [10-12]. AZT is the only drug routinely used but exhibits significant side effects. Tenofovir and raltegravir are thought to be antiretroviral candidates for their successful action on HIV-1 [8]. However, resistance against antiviral drugs may have been developed through single-point mutations. Around 2007, the H274Y mutation made Influenza-A virus (IAV) resistant to oseltamivir treatment. Resistance to several earlier HIV-1 drugs led to multi-drug regimens becoming standard HIV-1 treatment today [13].
A recombinant chimera (RetroMAD1) in an A-B-C configuration comprising of multiple documented antiviral domains is a way to design a novel FeLV drug and also mitigate future antiviral resistance [14]. Expressed as Inclusion Bodies using E.coli BL21(DE3) with a pET-22b(+) vector, it comprises of Retrocyclin, MAP30 and Dermaseptin. Retrocyclins are active in Old-world primates possessing high affinity for HIV-1 glycoproteins gp120/gp41 and with inhibition of the 6-helix bundle formation together prevent viral entry [15]. Passaging in cells beyond 140 days did not result in HIV1 resistant strains [16]. Its non-inflammatory, non-haemolytic and non-cytotoxic attributes make retrocyclin an ideal anti-retroviral agent [15], [16]. MAP30 from Bitter Melon is an HIV1 integrase inhibitor and type 1 RIP (ribosomal inactivating protein) that contain an RNA glycosidase domain [17]. It cleaves the terminal adenine of the α-SRL (sarcin-ricin loop) of the ribosomal large subunit that affects interaction between the elongation factor and the ribosome. MAP30 has a broad-spectrum antiviral activity that suggests that inhibiting the elongation factor causes host ribosomes inactivation in virally infected cells [18]. Recombinant MAP30 has been shown to be capable of relaxing HIV1 supercoiled DNA and catalyzing double-strand breakage to form topologically inactive products [19]. Dermaseptins are cationic antimicrobial peptides (CAP) from tree frogs that play a key defensive role against enveloped viruses including HIV1, HSV and HPV whose ability to destabilize membranes due to their amphiphilic are thought to contribute to block viral entry [20]. Dermaseptin S4 has been demonstrated to inhibit HIV1 infectivity against human primary T-lymphocytes by affecting virion integrity [21].
By targeting different viral entry steps, bAVPs (bifunctional antiviral proteins) inhibit viral entry better [22]. Both tenofovir and raltegravir have anti-HIV-1 mechanisms thought to be similar for FeLV being chain elongation inhibition at the ribosomal level (for tenofovir) and integration inhibition (for raltegravir) both of which have been described for MAP30 not to mention relaxation of supercoiled DNA giving rise to double-strand breakage [17-19]. Coupled with retrocyclin being a glycoprotein blocking entry inhibitor the stage is set for RetroMAD1 to being a potentially multifunctional antiviral chimeric protein [15], [16]. Additionally, a team from Cambridge University has shown using a VLP (viral like particle) model containing fluorescent dye fusing with liposomes that RetroMAD1 may also have fusion-inhibition characteristics (Y Modis, 2018, personal communication). Delivery of bAVPs is by injection, infusion or GMO Lactobacilli probiotics [22]. Conventional opinion is that protein drugs cannot be orally administered. In 2010, a moribund terminal stage FIV cat named Orange that had ceased feeding or movement was orally administered RetroMAD1 based on a biomass conversion ratio that was efficacious in shrimp. Within a fortnight, Orange began feeding and behaving normally and survived another 3+ years (Dr. Benny Tan, 2012, personal communication). This was documented in the leading local newspaper (New Sunday Times- April 29th 2012).
Most protein pharmaceutics exhibit a short half-life measured in minutes due to high glomerular clearance in the kidneys because they are generally smaller than 30 kDa. Small Molecule drugs are eliminated mainly by drug metabolism enzymes in the liver and other tissue and by urinary excretion. Proteins less than 40-50 kDa are cleared by renal filtration [23]. The mechanism of RetroMAD1 clearance is therefore probably by renal filtration as it is approximately 40 kDa. Rodent and monkey pK indicate a half-life of 2 hours (UEH, unpublished). Dermaseptins are used as Cell-Penetrating Peptides for intracellular delivery of protein drugs based on their 3-dimensional structure, spatial occupation and hydrophilic or hydrophobic nature. Studies show that CPPs as fusion constructs (such as RetroMAD1) mediate the delivery of proteins into a wide variety of cells both in vitro and in vivo confirming our unpublished immunoflorescence-tagged cell-imaging showing intracellular delivery is possible [24]. Recombinant chimeric anti-HIV1 proteins blocking two separate steps of viral entry, that are highly efficacious has been produced and is said to be relatively inexpensive and easy to produce [25]. Given the existing developmental landscape, we feel it is pertinent to discuss the observations derived from this trial despite not having a totally complete understanding of RetroMAD1 yet.
2. Materials and Methods
2.1. Choice of Trial Location
Trial location influences the successful recruitment of meaningful sample sizes and for completion within a certain timeframe. A 2009 review of FIV and FeLV indicated that FeLV prevalence of sick cats ranged from a low of 2.2% in Norway and 2% in Australia, to a median of 13% in Switzerland and 18% in Italy, to a high of 30.4% in Spain and 38% in Israel [9]. More recently after this trial was conducted, low FeLV+ prevalence of 2.6% was also noted in New Zealand and 4% in Australia [26], [27].
Before this 2013-2015 study, Brazilian studies based on nested-PCR reported that within urban domestic cats, 47.2% of sick cats had FeLV proviral DNA which was no different from 47.4% of healthy cats. What was more important to us was that all FeLV-B cats were clinically symptomatic while all FeLV-A cats were healthy without any clinical signs! All FeLV-B cats were FeLV-A co-infected and FeLV-C was undetected [28]. Therefore, holding a trial in Brazil would allow selective recruitment of FeLV-B symptomatic animals as conventional p27 antigen detection kits cannot distinguish FeLV-A from FeLV-B. A recent review using three FeLV antigen-based point-of-care kits gave similar results being 100% specific (to p27) but only 82% sensitive [29]. The transmembrane protein p15E may be a future alternative [30].
2.2. Recruitment Criteria
A limitation of most studies is the inability to distinguish between FeLV subtypes [31]. By recruiting in Brazil, based on the published 2008 findings [28], we can select for FeLV-B symptomatic cats that were FeLV-A co-infected. We excluded cats demonstrating neurological or psychotic behaviour to exclude FeLV-C. A double-armed multi-centric field trial was carried out in Brazil from September 2013 to March 2015 with recruitment using Facebook with consent forms signed for ethical reasons. Recruits were tested using the p27 IDEXX SNAP FIV/FeLV Combo and tested twice at least 3 months apart to rule out regressive infection (formerly transient viremia). Only symptomatic FeLV cats were recruited with approval from their vets. Asymptomatic, pregnant, terminally-ill, renally compromised, tumour-presenting and FIV/FIPV co-infections were not disqualified. Supportive treatments specified by their vets were continued, in particular interferon.
Written owner consent for blood collection and for the use of blood samples and all data for scientific purposes is routinely given at the admission examination each time a patient visits the clinic during routine visits prior to blood donation. Therefore, based on the regulations, formal ethical approval for this study was not needed.
2.3. Treatment Regime
Oral daily administration of RetroMAD1 given at 0.4mg/kg/day (divided equally into two feeds roughly 12 hours apart). RetroMAD1 is an almost colourless liquid without negative taste response in cats. RetroMAD1 was contained in clear autoclaved sterile borosilicate glass bottles of 50 ml. Each owner was instructed to store RetroMAD1 at 4°C and equilibrate to ambient temperature (20-30°C) prior to dispensing using plastic droppers.
2.4. Clinical Scoring and Complete Blood Count (CBC)
Criteria selected for clinical scoring as tabulated in Table 1 were agreed by consensus among some participating vets based on their FeLV experience. Scoring sheets were filled in Portuguese and translated to English retrospectively. Meetings were held among interested participating vets and owners before and after the trial. Scoring was done by the attending vets on day 0,7, and months 1,2,3,6. A total of 5 parameters were graded from 0 to 1 to 2 in order of increasing severity. Owners were contacted by the trial coordinator by phone on an irregular basis to determine the cat’s status and if applicable, date of death. With client consent, CBC was conducted by a reputable diagnostic laboratory before and 2 months after the end of the month-long regime.
|
Clinical Sign |
Clinical Score |
||
|
0 |
1 |
2 |
|
|
Anorexia |
Ideal weight |
Skinny |
Very Skinny |
|
Inappetance |
Good appetite |
Moderate appetite |
Poor appetite |
|
Gingivitis |
None |
Mild |
Severe |
|
F.U.R.D. |
None |
Mild |
Severe |
|
Anaemia |
None |
Mild |
Severe |
Table 1: Scoring sheet for clinical sign
2.5. RetroMAD1 Description
RetroMAD1 is a clear slightly yellowish odourless fully miscible liquid at pH 11 containing the target protein around 37-40 kDa. Stability at tropical room temperature (26-34°C) is >99% viral reduction of HSV2 on vero cell (ATCC CC-81) over 4 years. The Batch used for this trial was #51 where QC gave an MNTD of 100-120 µg/ml and an antiviral concentration of 100 µg/ml with a 4-log reduction of virus using RT-PCR.
2.6. Malaysian RT-PCR Dataset
After the Brazilian trial, 6 highly symptomatic Malaysian cats were treated with RetroMAD1 with owner’s consent and blood samples taken before and after 3 months of oral RetroMAD1 treatment for qPCR analysis. RNA was extracted from processed clinical samples (200µl) using High Pure viral nucleic acid kit (Roche Applied Science, Meylan, France) according to manufacturer’s instructions. The extracted RNA (5µl) was added to a reaction mixture (final volume of 20µl) then subjected to qPCR using iTaq Universal SYBR supermix (Bio-Rad) in a CFX96 real-time PCR detection system (Bio-Rad, USA). The qPCR analysis was performed with forward primer 5’-AACAGCAGAAGTTTCAAGGCC-3’; reverse primer, 5’-TTATAGCAGAAAGCGCGCG-3’. Positive and negative controls were included in each experiment to ensure reproducible results. A standard curve was established with a serially-diluted in vitro transcribed viral RNA from a positive control for absolute quantification of FeLV viral RNA. The limit of detection was 1000 copies/rx.
2.7. Statistical Analysis
Data analysed using GraphPad Prism version 5.02 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Survival curves for cats were estimated by the Kaplan-Meier Product Limit Estimator which is a non-parametric statistic used to estimate the survival rate from lifetime data. The log-rank test for censored data was used to compare curves between Control vs RetroMAD1-treated group. A two-tailed paired t-test was used to determine significant differences for vet score between before treatment, 1 month after treatment and 3 months after treatment. Box plots were used to show median and IQR. Probability (p) values were calculated on the basis of two-tailed tests and p value of less than 0.05 was considered for statistical significance.
3. Results
3.1. Survival
|
Sample size* for Kaplan-Meier |
Survival rate at |
|
Control group (n=21) |
33% |
|
RetroMAD1-treated group (n=55) |
73% |
*Total sample size (n=76)
Table 2: Survival rate based on Kaplan-Meier curve
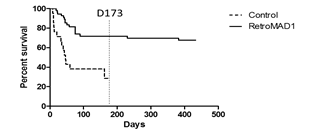
RetroMAD1 treated cats were monitored for survival until day 433. Untreated cats were monitored until day 173 and monitoring stopped after it was evident a marked difference in survival of 73% (treated) versus 33% (untreated) existed as shown in Table 2. Initially, it was planned to recruit N=25 (control) and N=75 (treated) but unfortunately, certain cases were disqualified due to non-compliance. All recruited cats were symptomatic for FeLV according to their respective vets. Symptomatic Brazilian cats were all shown to be FeLV-B/FeLV-A co-infections while FeLV-A+ cats were non-symptomatic [28] and so may account for the large gap in survival observed as FeLV-B is deadlier. Comparing with another study on Interferon, we observe the same rapid decline around day 60 followed by a gradual flattening out of the curves. However, at around day 173, we note about a 15% difference in survival in the Interferon study while in our case the difference was 40% as displayed in 1 [32]. Their study was conducted in France where FeLV-A/FeLV-B co-infections may be different from Brazil. Detection was p27 based and unable to differentiate FeLV-A from FeLV-B. In the Interferon study, the difference in survival was about 10% on day 360 while for this study, even if the untreated curve were to totally flatten with no increase in deaths post day-173, the difference at day 433 would be at least 35%. Kaplan-Meier curves depicting survival for FeLV+ versus FeLV- cats also show an initial very rapid decrease (before day 60) in survival before a gradual flattening out all the way to day 2000+ [9]. Some cats in this study were also interferon/RetroMAD1 treated as some vets used this as supportive therapy. This provides insight on possible synergism discussed later.
3.2. Vet Scores
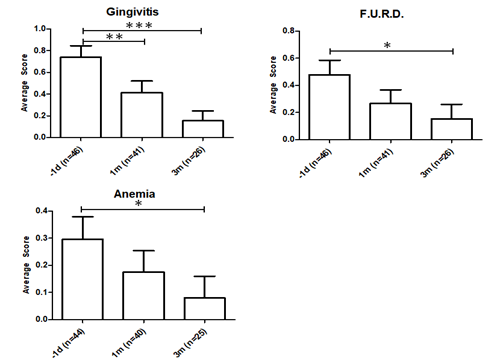
The average vet score that was obtained for gingivitis, feline upper respiratory disease (F.U.R.D) and anemia was displayed in 2. Gingivitis evaluation is based on the oral examination by experienced vets with over 5 years experience. Scoring for gingivitis indicated a very significant improvement at P ≤ 0.001. Gingivitis is a major symptom of progressive FeLV and the decrease is indicative that the FeLV-A/FeLV-B co-infections are less pathologically damaging after RetroMAD1 treatment [1], [33]. Severe gingivitis is stressful for both the cat and owners who sometimes bottle-feed cats unable to chew pellets. Gingivitis scores improved significantly after a month at P ≤ 0.01. The recurrent feedback from the vets we met was that alleviation of gingivitis was almost always the first improvement owners noted.
Feline upper respiratory disease (F.U.R.D.) also known as Feline Viral Respiratory Complex or Upper Respiratory Tract Diseases (URTD) is the most frequent clinical presentation of progressive FeLV cats [1], [9], [33]. The dataset is significant at P ≤ 0.05 comparing pre-treatment and the 3rd month scores (Table 3). Since immunosuppression is a characteristic of FeLV various accompanying opportunistic infections give rise to F.U.R.D [1], [3]. Decreasing F.U.R.D. scores indicate improvement in the cat’s natural immune system as RetroMAD1 has no antibacterial activity while many secondary pathogens of F.U.R.D. are bacterial or fungal in nature. Signs of URTD/F.U.R.D. is present in 93% of FeLV symptomatic cats [34].
Anaemia is a major non-neoplastic complication common in symptomatic FeLV cats. Most FeLV anaemia is caused by bone marrow suppressive effects of the virus resulting from primary infection of hematopoietic stem cells and infection of stroma cells that constitute the supporting environment for hematopoietic cells [33]. Anaemia was judged by oral examination of the gums on a 0 = none, 1 = mild and 2 = severe clinical score. Reduction of anaemia was significant (P ≤ 0.05). RetroMAD1 was shown to be able to enter the bone marrow of rodents (UEH, unpublished data) and if applicable to cats, reduction of viral infection of the hematopoietic and stroma cells may alleviate anaemia.
|
Clinical Symptoms |
p-value summary after treatment start |
|||
|
7 days |
1 month |
2 months |
3 months |
|
|
Gingivitis |
ns |
** |
* |
*** |
|
F.U.R.D. |
ns |
ns |
ns |
* |
|
Anaemia |
ns |
ns |
ns |
* |
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001: paired t-test comparison test
Table 3: Summary for p-value for 7 days, 1 month, 2 months and 3 months after the treatment start
3.3. Age Of Cat And Survival Rate
|
Age |
Number of cats |
Survival rate |
|
1 year |
13 |
64% |
|
2 year |
19 |
78% |
|
3 year |
5 |
60% |
|
4 year |
2 |
100% |
|
5 year |
4 |
50% |
|
6 year |
5 |
60% |
|
7 year |
4 |
50% |
|
more than 8 year |
3 |
66% |
|
48 months and below |
39 |
74% |
|
More than 48 months |
16 |
56% |
Table 4: Survival rate for cats treated with RetroMAD1 based on their age
Recruitment was highly skewed towards younger cats because in another study in Brazil, younger cats had a 5.19 times higher likelihood to be FeLV+ than elderly cats [31]. As displayed in Table 4, among treated cats, younger animals <4 years old had 74% (N = 39) survival compared to 56% in cats that were 5-8+ years old. Younger symptomatic FeLV cats had a better chance of surviving the next 433 days than older cats. A study of longevity in 4009 cats in the UK revealed a median life expectancy of 14 years [35]. Thus, lower survival in older treated cats were probably not primarily due to old age.
3.4. Treatment Combination with Interferon
|
Treatment |
No. of Cats |
Survival Rate |
|
RetroMAD1 only (day 433) |
41 |
68% |
|
Interferon only (day 173) |
12 |
25% |
|
RetroMAD1 + Interferon (day 433) |
14 |
71% |
At day 173, 73% of RetroMAD1 treated cats were still alive compared to 43% of those untreated. At a study conducted in France, 61% interferon treated cats were still alive compared to 41% of the untreated on day 270. At the 360-day study end point, survival for interferon treated cats were 53% versus 41% for the untreated [32]. Interferon has been suggested as FIV supportive treatment, and for FIV/FeLV, for acting on innate immunity by reduction of pro-inflammatory stimuli, for induction of innate immune-modulation and as a potential treatment along with glucocorticoid for FIPV [36-40]. The survival rate of RetroMAD1 treated cats at day 433 was 68% while we note a slight improvement of 71% with cats treated with RetroMAD1 + interferon. The cats treated with only interferon had only 25% survival on day 173 (Table 5).
|
Parameters |
N |
Mean ± SD |
p-value |
Reference range |
|
|
Before |
After |
||||
|
Haematocrit (%) |
31 |
36.39 ± 1.225 |
37.09 ± 1.089 |
0.0534 |
24 – 451 |
|
MCV (fL) |
33 |
49.01 ± 0.9456 |
47.78 ± 1.085 |
0.2962 |
39 – 551 |
|
MCH (uu3) |
10 |
16.22 ± 0.5272 |
16.46 ± 0.5059 |
0.6074 |
12.5 – 17.52 |
|
Leukocyte (cells/uL) |
33 |
13902 ± 1255 |
11051 ± 1095 |
0.1079 |
5500 – 195001 |
|
Lymphocyte (%) |
33 |
27.27 ± 2.353 |
27.79 ± 2.656 |
0.8892 |
20 – 552 |
|
Platelets (cells/uL) |
32 |
326959 ± 27470 |
325818 ± 29522 |
0.5347 |
200,000 – 600,0001 |
|
ALT (U/L) |
32 |
75.83 ± 8.192 |
63.27 ± 3.749 |
0.0233 |
6 – 831 |
|
AST (U/L) |
30 |
55.65 ± 5.897 |
48.16 ± 5.849 |
0.2644 |
26 – 431 |
|
Creatinine (mg/dL) |
33 |
1.278 ± 0.07087 |
1.347 ± 0.09619 |
0.1044 |
0.8 – 1.81 |
|
Urea (mg/dL) |
33 |
57.18 ± 3.201 |
50.05 ± 3.351 |
0.0883 |
43 – 741 |
- Vet Análises – R. Ipiranga, 53 - Laranjeiras, Rio de Janeiro - RJ, 22231-120, Brazil
- TECSA Laboratórios – Av. do Contorno , 6226 - Savassi 30.110-042 - Belo Horizonte- MG/Brasil
Table 6: Comparison of haematological and serum biochemistry parameters for before and after the treatment
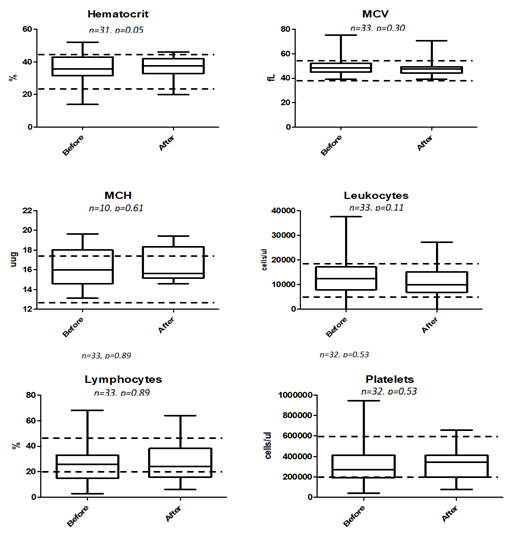
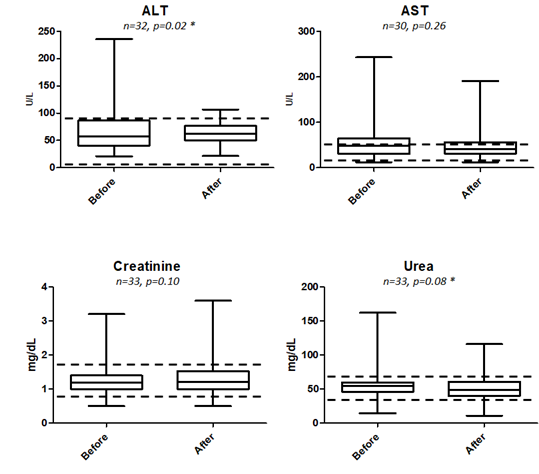
Of all the blood parameters studied as tabulated in Table 6 as well as box plot analysis displayed in 3 and 4, prior to treatment and approximately a month after treatment, only ALT being lowered from 75.83 +/- 8.192 to 63.27 +/- 3.749 U/L was found to be statistically significant at P = 0.0233. Elevated ALT is indicative of liver injury and abnormality and our box-plot shows that there is no indication of such after being fed with RetroMAD1 for a month (Figure 4). This was based upon 32 data pairs before and after the 1-month treatment and although both values are within the reference range of 6-83 given by the analytical lab in Rio de Janeiro, Brazil that handled the analysis. From the ALT results, treatment with RetroMAD1 did not cause any liver damage and was in fact bringing the values more towards the median normal value. AST was reduced from a median of 55.65+/- 5.897 to 48.16 +/- 5.849 U/L and although not statistically significant, both results were still outside the reference range of 26-43 U/L. Thus, it represented a value 129% beyond the reference maxima coming down to 112% which is reflective of generalized tissue recovery from damage. Haematocrit increased slightly by 2% with a probability value of P = 0.0534 that was just approaching the limit of significance at P ≤ 0.05. Both values are within the reference range. All other parameters were also not statistically different before and after treatment and were within the given reference range. Only the MCH paired dataset of N=10 was much less than the others due to the fact that only one lab carried out this particular analysis.
3.5. PCR result
Six symptomatic progressive FeLV cats in Kuala Lumpur, Malaysia were treated with RetroMAD1 to retrospectively study its effect on viral titre. Table 7 showed that viral copy number reduction ranged from a low of 53% to a high of 100% (undetectable by RT-PCR). A plasma viremia loads greater than 1 x 103 copies per ml is considered high [3]. Although this is a preliminary uncontrolled study, a 1-3 log reduction was noted suggesting that RetroMAD1 is acting as an antiviral.
|
Pet |
Sample type |
Viral copy number/ul |
Viral RNA reduction (%) |
|
|
Before treatment |
3 months after treatment |
|||
|
Awang |
Serum |
6.77 X 104 |
5.52 X 103 |
91.8 |
|
Blood |
2.24 X 107 |
6.77 X 104 |
99.7 |
|
|
Black |
Blood |
8.52 X 108 |
6.65 X 106 |
99.2 |
|
Blacky |
Serum |
3.72 X 108 |
1.57 X 104 |
99.9 |
|
Chingster |
Blood |
1.86 X 107 |
1.37 X 106 |
92.63 |
|
Tam |
Serum |
2.30 X 105 |
1.08 X 105 |
53.0 |
|
Blood |
3.06 X 106 |
2.86 X 105 |
90.7 |
|
|
Yen Yen Chan |
Serum |
8.81 X 107 |
8.09 X 104 |
99.9 |
Table 7: Viral reduction for before and 3 months after the treatment start
4. Discussion
Current FeLV studies are limited by the inability to differentiate between FeLV sub-types [31]. The fact that an earlier 2008 Brazilian nested-PCR study reported that every symptomatic cats tested had FeLV-B while all of those with FeLV-A alone but were asymptomatic [28] agrees with the concept that FeLV-B requires FeLV-A as a helper virus for transmission and recombination [1], [41]. This study influenced our decision to conduct this trial in Brazil so that FeLV-A + FeLV-B progressive FeLV cats are used where the normal outcome would be one of high morbidity and mortality. A study in a confined cat colony of domestic cat/ leopard cat hybrids showed that FeLV-B was detected in 68% of FeLV-A cats that suggests FeLV-B infection may also in some cases be independent of any helper mechanisms from FeLV-A [3]. Nonetheless, the clinical outcome of FeLV-A now appears to be influenced by FeLV-B+ and probably FFV+ [3]. This study shows that orally administered RetroMAD1 improves the survivability and quality of life in symptomatic Brazilian cats presumably of the FeLV-A/FeLV-B progressive infection category where morbidity and mortality without treatment is high. After a month of treatment through orally administered of RetroMAD1, a survival rate of 68% (N=41) and 71% (N=14) of RetroMAD1 + interferon treated cats to survive till day 433 was recorded. Preliminary qPCR results showed promising reduction of viral copy number. Untreated FeLV+ cats in New Zealand in a 2010-2016 study had a survival of 17-18% at the day 433 equivalent time point to our study while healthy uninfected cats had a survival rate of 78% [26]. A total of 71% survival rate after treated with RetroMAD1 + interferon survival was actually quite near to those of healthy uninfected cats suggesting that such combinatorial treatments might be useful in the future. In countries such as New Zealand where FeLV vaccines are no longer commercially available, there may be a gradual recurrence of FeLV and such treatments may be useful [26].
Anaemia is a major clinical preoccupation associated with progressive FeLV infection [1] and defined by decrease in haematocrit, RBC and haemoglobin below the normal reference range. Although common in FeLV+ cats, it is only reported for 3.6% of cat blood samples taken from around Vienna between 2003 - 2011 retrospectively. (N= 30,503 samples) [1], [42]. In another retrospective study involving 493 cats conducted from 2010 – 2014 in Brazil, 31% were FeLV positive and of these, 30.7% presented with anaemia, 14.7% had leukopenia, 11.6% had neutropenia while 42.2% had thrombocytopenia [31]. Our results indicate that RetroMAD1 treatment significantly reduced anaemia (P= 0.05) in haematocrit values as well as the reduction of observable anaemia indicated by gum coloration (P ≤ 0.05). In recent New Zealand study involving 572 cats showed anaemia in 39.4% of FeLV+ cats (P ≤ 0.001) and inflammatory oral disease in 10.6% (P ≤ 0.001) [26]. These clinical outcomes resulted in a low survival of 17-18% by the day 433 equivalent time point with the end of this present study. Although anaemia accounted for 30.7% in the Brazil study and 39.4% in the New Zealand study, it alone cannot account for the low survivorship in progressive FeLV cats and many contributory interactive factors exist.
Gingivitis and F.U.R.D both involve opportunistic secondary pathogens. Feline chronic gingivostomatitis (FCGS) is a painful long-term oral inflammation and is differentiated from gingivitis when the inflammation crosses the mucogingivial junction to the buccal and caudal oral mucosa [42]. In this trial’s vet scoring, both gingivitis and FCGS were grouped together. Management options for FGCS range from tooth extraction to cyclosporins to thalidomide and interferon with varying reported success rates. Recombinant feline interferon omega was reported to give a 100% remission but the sample size was only N = 2 [43]. Since FCGS, gingivitis and F.U.R.D. involve opportunistic pathogens exploiting general virally driven immunosuppression, RetroMAD1 might be useful in reduction of viral load as shown in our qPCR results and reduce immunosuppression if less B-cells and T-cells are killed off. Alleviation of gingivitis/FCGS and F.U.R.D. was evident and vets consistently reported during meetings that such reductions were often the first signs of clinical efficacy.
Most of our trial cats were recruited from Rio de Janeiro and a recent study there found 78% of FeLV progressive (symptomatic) cats were FFV+ from buccal swabs while only 22% of FeLV regressive (asymptomatic) were FFV+ suggesting that FFV-FeLV co-infection may worsen the exacerbate co-infected cats [5]. FFV is also and RetroMAD1’s broad spectrum antiviral activities [14], it may be possibly anti-FFV also. If so, use of RetroMAD1 may reduce FFV and decrease the synergistic virulence of these viruses. There is a likelihood based on studies done in Brazil that a majority of symptomatic FeLV cats may be actually FeLV-A + FeLV-B + FFV co-infected [5], [28].
5. Conclusion
RetroMAD1 increases survivorship to cats that are likely to have FeLV-A + FeLV-B + FFV or FeLV-A + FeLV-B that are highly symptomatic. Quality of life as in alleviation of symptom common to FeLV cats, is also improved. Being orally delivered, administration is simple and easily performed by cat owners. CBC and biochemistry data infer that the protein is safe and well tolerated. These studies show that RetroMAD1 can be an additional treatment option for Feline Leukemia and may be used as a stand-alone treatment or together with interferon treatment.
Acknowledgment
The authors thank Caren Soares and Dina Puccini for trial coordination, Professor Maria Helena Da Silva for lab facilities at University Federal Rio de Janeiro, former Biovalence staff who were involved in data processing and lab analysis especially Daniel Lu, Tiffany Ung and Audrey Hooi. We wish to thank Suhail Shoaib for the formatting help and Thamil Vaani for qPCR analysis.
Conflict of interest
Ung Eng Huan is the co-inventor of RetroMAD1 and CTO of Biovalence S/B. Patricia Mafei was not in the employment of Royal Canin at the time of the trial.
Funding
Funding for the study came from Biovalence S/B. We wish to thank IDEXX for providing free SNAP FIV/FeLV combo kits for testing all the prospective cats for recruitment into the trial.
References
- Hartmann K. Clinical aspects of feline retroviruses: a review. Viruses 4 (2012): 2684-2710.
- Anderson MM, Lauring AS, Burns CC, et al. Identification of a cellular cofactor required for infection by feline leukemia virus. Science 287 (2000): 1828-1830.
- Powers JA, Chiu ES, Kraberger SJ, et al. Feline leukemia virus (FELV) disease outcomes in a domestic cat breeding colony: Relationship to endogenous felv and other chronic viral infections. Journal of Virology 92 (2018): e00649-618.
- Chiu E, Hoover E, Vande Woude S. A retrospective examination of feline leukemia subgroup characterization: viral interference assays to deep sequencing. Viruses 10 (2018): 29.
- Cavalcante L, Muniz C, Jia H, et al. Clinical and molecular features of feline foamy virus and feline leukemia virus co-infection in naturally-infected cats. Viruses 10 (2018): 702.
- Hartmann K. Efficacy of antiviral chemotherapy for retrovirus-infected cats: What does the current literature tell us? Journal of Feline Medicine Surgery 17 (2015): 925-939.
- Stuetzer B, Brunner K, Lutz H, et al. A trial with 3′-azido-2′, 3′-dideoxythymidine and human interferon-α in cats naturally infected with feline leukaemia virus. Journal of Feline Medicine Surgery 15 (2013): 667-671.
- Greggs III WM, Clouser CL, Patterson SE, et al. Discovery of drugs that possess activity against feline leukemia virus. Journal of General Virology 93 (2012): 900-905.
- Gleich SE, Krieger S, Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. Journal of Feline Medicine Surgery 11 (2009): 985-992.
- Greggs III WM, Clouser CL, Patterson SE, et al. Broadening the use of antiretroviral therapy: the case for feline leukemia virus. Therapeutics and Clinical Risk Management 7 (2011): 115-122.
- Saba CF. Vaccine-associated feline sarcoma: current perspectives. Veterinary Medicine: Research and Reports 8 (2017): 13-20.
- Gobar GM, Kass PH. World Wide Web-based survey of vaccination practices, postvaccinal reactions, and vaccine site-associated sarcomas in cats. Journal of the American Veterinary Medical Association 220 (2002): 1477-1482.
- Irwin KK, Renzette N, Kowalik TF, et al. Antiviral drug resistance as an adaptive process. Virus Evolution 2 (2016): vew 014.
- Bakar AM, Ung EH. Antimicrobial fusion compounds and uses thereof. Patent 9155800, USA, 2015.
- Venkataraman N, Cole AL, Ruchala P, et al. Reawakening retrocyclins: ancestral human defensins active against HIV-1. PLOS Biology 7 (2009): e95.
- Penberthy WT, Chari S, Cole AL, et al. Retrocyclins and their activity against HIV-1. Cellular and Molecular Life Sciences 68 (2011): 2231-2242.
- Lee-Huang S, Huang PL, Bourinbaiar AS, et al. Inhibition of the integrase of human immunodeficiency virus (HIV) type 1 by anti-HIV plant proteins MAP30 and GAP31. Proceedings of the National Academy of Sciences of the United States of America 92 (1995): 8818-8822.
- Puri M, Kaur I, Kanwar RK, et al. Ribosome inactivating proteins (RIPs) from Momordica charantia for anti viral therapy. Current Molecular Medicine 9 (2009): 1080-1094.
- Lee-Huang S, Huang PL, Chen HC, et al. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene 161 (1995): 151-156.
- Lorin C, Saidi H, Belaid A, et al. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology 334 (2005): 264-275.
- Bergaoui I, Zaire A, Tangy F, et al. In vitro antiviral activity of dermaseptin S4 and derivatives from amphibian skin against herpes simplex virus type 2. Journal of Medical Virology 85 (2013): 272-281.
- Falkenhagen A, Joshi S. HIV entry and its inhibition by bifunctional antiviral proteins. Molecular Therapy - Nucleic Acids 13 (2018): 347-364.
- Ho RJ, Gibaldi M. Biotechnology and Biopharmaceuticals: Transforming Proteins and Genes into Drugs. 1st ed. Hoboken, New Jersey: John Wiley and Sons (2003): 97-123.
- Trabulo S, Cardoso AL, Mano M, et al. Cell-penetrating peptides—mechanisms of cellular uptake and generation of delivery systems. Pharmaceuticals 3 (2010): 961-993.
- Zhao B, Mankowski MK, Snyder BA, et al. Highly potent chimeric inhibitors targeting two steps of HIV cell entry. Journal of Biological Chemistry 286 (2011): 28370-28381.
- Luckman C, Gates MC. Epidemiology and clinical outcomes of feline immunodeficiency virus and feline leukaemia virus in client-owned cats in New Zealand. Journal of Feline Medicine and Surgery Open Reports 3 (2017): 2055116917729311.
- Westman ME, Paul A, Malik R, et al. Seroprevalence of feline immunodeficiency virus and feline leukaemia virus in Australia: risk factors for infection and geographical influences (2011–2013). Journal of Feline Medicine and Surgery Open Reports 2 (2016): 2055116916646388.
- Coelho FM, Bomfim MR, de Andrade Caxito F, et al. Naturally occurring feline leukemia virus subgroup A and B infections in urban domestic cats. Journal of General Virology 89 (2008): 2799-2805.
- Westman ME, Malik R, Norris JM. Diagnosing feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) infection: an update for clinicians. Australian Veterinary Journal 97 (2019): 47-55.
- Boenzli E, Hadorn M, Hartnack S, et al. Detection of antibodies to the feline leukemia virus (FeLV) transmembrane protein p15E: an alternative approach for serological FeLV detection based on antibodies to p15E. Journal of Clinical Microbiology 52 (2014): 2046-2052.
- Da Costa FV, Valle SD, Machado G, et al. Hematological findings and factors associated with feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) positivity in cats from southern Brazil. Pesquisa Veterinária Brasileira 37 (2017): 1531-1536.
- De Mari K, Maynard L, Sanquer A, et al. Therapeutic effects of recombinant feline interferon-co on feline leukemia virus (FeLV)-Infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic cats. Journal of Veterinary Internal Medicine 18 (2004): 477-482.
- Hartmann K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Veterinary Immunology and Immunopathology 143 (2011): 190-201.
- Najafi H, Madadgar O, Jamshidi S, et al. Molecular and clinical study on prevalence of feline herpesvirus type 1 and calicivirus in correlation with feline leukemia and immunodeficiency viruses. Veterinary Research Forum 5 (2014): 255-261.
- O’Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of cats attending primary care veterinary practices in England. Journal of Feline Medicine and Surgery 17 (2015): 125-133.
- Gil S, Leal RO, McGahie D, et al. Oral recombinant feline interferon-omega as an alternative immune modulation therapy in FIV positive cats: clinical and laboratory evaluation. Research in Veterinary Science 96 (2014): 79-85.
- Gil S, Leal RO, Duarte A, et al. Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Research in Veterinary Science 94 (2013): 753-763.
- Leal RO, Gil S, Duarte A, et al. Evaluation of viremia, proviral load and cytokine profile in naturally feline immunodeficiency virus infected cats treated with two different protocols of recombinant feline interferon omega. Research in Veterinary Science 99 (2015): 87-95.
- Leal R, Gil S. The use of recombinant feline interferon omega therapy as an immune-modulator in cats naturally infected with feline immunodeficiency virus: new perspectives. Veterinary Sciences 3 (2016): 32.
- Ishida T, Shibanai A, Tanaka S, et al. Use of recombinant feline interferon and glucocorticoid in the treatment of feline infectious peritonitis. Journal of Feline Medicine and Surgery 6 (2004): 107-109.
- Stewart MA, Warnock M, Wheeler A, et al. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. Journal of Virology 58 (1986): 825-834.
- Furman E, Leidinger E, Hooijberg EH, Bauer N, et al. A retrospective study of 1,098 blood samples with anemia from adult cats: frequency, classification, and association with serum creatinine concentration. Journal of Veterinary Internal Medicine 28 (2014): 1391-1397.
- Winer JN, Arzi B, Verstraete FJ. Therapeutic management of feline chronic gingivostomatitis: a systematic review of the literature. Frontiers in Veterinary Science 3 (2016): 54.