Cancer Cell Plasticity in Urothelial Carcinoma: When it Give Rise to Melanoma
Article Information
Alix Fontaine1, Denis Roblet2, Racha Benmeziani3, Gaelle Fromont1,4*
1Department of Pathology, Bretonneau Hospital, University Hospital - University of Tours, 37000 Tours, France
2Department of Pathology, Saint Michel Hospital, 16959 Angouleme, France
3Department of Urology, Saint Michel, 16959 Angouleme, France
4Inserm UMR U1069, 37000 Tours, France
*Corresponding Author: Gaelle Fromont, Department of Pathology, Bretonneau Hospital-CHU de Tours, 2 Bd Tonnellé, 37044 Tours Cedex 9, France.
Received: 21 March 2022; Accepted: 09 May 2022; Published: 30 May 2022
Citation: Alix Fontaine, Denis Roblet, Racha Benmeziani, Gaelle Fromont. Cancer Cell Plasticity in Urothelial Carcinoma: When it Give Rise to Melanoma. Archives of Clinical and Medical Case Reports 6 (2022): 459-465.
View / Download Pdf Share at FacebookAbstract
Primary bladder melanoma is very rare, and the association with urothelial carcinoma has never been described to date. We report a case of a biphasic tumor of the bladder, composed of high grade urothelial carcinoma adjacent to melanoma, characterized using immunohistochemistry and DNA sequencing. The urothelial component was positive for pan-cytokeratin and GATA3, and negative for melanoma markers HMB45, Melan A, and Sox10. In contrast, the melanocytic component was negative for pancytokeratin and positive for HMB45, Melan A, and Sox10. Moreover, some nuclei were weakly stained for GATA3, a transcription factor of the urothelial lineage. Molecular genetic analysis showed that both components shared a never described identical serine/threonine kinase 11 (STK11) D365G molecular alteration, and a c.-124C>T Telomerase Reverse Transcriptase (TERT) promoter mutation, an early event in urothelial carcinogenesis. Our report underlines the phenotypic plasticity of urothelial carcinomas, and raises the question of an urothelial origin of urinary tract melanomas.
Keywords
Cancer cell plasticity; Cancer stem cell; Melanoma; Urothelial carcinoma; Bladder
Cancer cell plasticity articles; Cancer stem cell articles; Melanoma articles; Urothelial carcinoma articles; Bladder articles
Cancer cell plasticity articles Cancer cell plasticity Research articles Cancer cell plasticity review articles Cancer cell plasticity PubMed articles Cancer cell plasticity PubMed Central articles Cancer cell plasticity 2023 articles Cancer cell plasticity 2024 articles Cancer cell plasticity Scopus articles Cancer cell plasticity impact factor journals Cancer cell plasticity Scopus journals Cancer cell plasticity PubMed journals Cancer cell plasticity medical journals Cancer cell plasticity free journals Cancer cell plasticity best journals Cancer cell plasticity top journals Cancer cell plasticity free medical journals Cancer cell plasticity famous journals Cancer cell plasticity Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals Melanoma articles Melanoma Research articles Melanoma review articles Melanoma PubMed articles Melanoma PubMed Central articles Melanoma 2023 articles Melanoma 2024 articles Melanoma Scopus articles Melanoma impact factor journals Melanoma Scopus journals Melanoma PubMed journals Melanoma medical journals Melanoma free journals Melanoma best journals Melanoma top journals Melanoma free medical journals Melanoma famous journals Melanoma Google Scholar indexed journals Urothelial carcinoma articles Urothelial carcinoma Research articles Urothelial carcinoma review articles Urothelial carcinoma PubMed articles Urothelial carcinoma PubMed Central articles Urothelial carcinoma 2023 articles Urothelial carcinoma 2024 articles Urothelial carcinoma Scopus articles Urothelial carcinoma impact factor journals Urothelial carcinoma Scopus journals Urothelial carcinoma PubMed journals Urothelial carcinoma medical journals Urothelial carcinoma free journals Urothelial carcinoma best journals Urothelial carcinoma top journals Urothelial carcinoma free medical journals Urothelial carcinoma famous journals Urothelial carcinoma Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Bladder articles Bladder Research articles Bladder review articles Bladder PubMed articles Bladder PubMed Central articles Bladder 2023 articles Bladder 2024 articles Bladder Scopus articles Bladder impact factor journals Bladder Scopus journals Bladder PubMed journals Bladder medical journals Bladder free journals Bladder best journals Bladder top journals Bladder free medical journals Bladder famous journals Bladder Google Scholar indexed journals SARS-CoV2 articles SARS-CoV2 Research articles SARS-CoV2 review articles SARS-CoV2 PubMed articles SARS-CoV2 PubMed Central articles SARS-CoV2 2023 articles SARS-CoV2 2024 articles SARS-CoV2 Scopus articles SARS-CoV2 impact factor journals SARS-CoV2 Scopus journals SARS-CoV2 PubMed journals SARS-CoV2 medical journals SARS-CoV2 free journals SARS-CoV2 best journals SARS-CoV2 top journals SARS-CoV2 free medical journals SARS-CoV2 famous journals SARS-CoV2 Google Scholar indexed journals
Article Details
1. Introduction
The juxtaposition of two tumors with distinct morphology and immunophenotype is relatively rare, and could be induced by either collision tumors or transdifferentiation of a malignant component into a different phenotype. Collision tumors are two independent neoplasms, with different cells of origin, that occur in the same anatomic site. In contrast, transdifferentiation of one component to another or divergent differentiation from a cancer stem cell population implies a common origin. We described herein a case of bladder melanoma associated with high grade urothelial carcinoma, and evidenced a filiation between the two components using molecular genetic analysis.
2. Case Report
A 80-years-old man without previous history of urothelial tumor or melanoma, underwent a transuretral resection of the bladder for gross hematuria. Both clinical examination and Positron Emission Tomography (PET) 18F-fluorodeoxyglucose(18F-FDG) performed after the diagnosis revealed no other cancer localization. Microscopic analysis showed a biphasic tumor invading the bladder wall, with adjacent components. The most representative component (A) was composed of large size cells, with eosinophilic cytoplasm, irregular nuclei, and numerous mitoses. Cells were arranged in clusters or nests within a fibrous stroma (Figure 1A). The second component (B) was composed of sheets of smaller cells (Figure 1B), without pigment deposition. Cells of the component A were positive after staining for pan-cytokeratin, GATA3 (Figure 1C), and were negative for HMB45 (Figure 1E), Melan A, and Sox10 (Figure 1G). In contrast, component B was negative for pan-cytokeratin, and was diffusely positive for HMB45 (Figure 1F), Melan A, and Sox10 (Figure 1H). In addition, few nuclei were weakly stained for GATA3 (Figure 1D). No expression was found for the neuroendocrine markers Chromogranin A and Synaptophysin. Moreover, we observed an overexpression of P53 in both components (Figure I and J). Samples of both components were macrodissected from the paraffin blocks, and analyzed with Next Generation Sequencing (NGS). NGS analysis identified similar genetic alterations in both components for serine/threonine kinase 11 (STK11) and telomerase reverse transcriptase (TERT). Moreover, a mutation of phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) was observed in the urothelial component (Table 1). No anomalies were found in the following genes: AKT1, ALK, BRAF, CTNNB1, DDR2, EGFR, ERBB2, ERBB4, FGFR2, FGFR3, HRAS, KIT, KRAS, MAP2K1, MET, NRAS, PDGFRA, POLE, and RAC1. These findings led us to propose the final diagnosis of high-grade urothelial carcinoma with focal melanocytic transdifferentiation. Due to his age and comorbidities, the patient was planned to be treated by radiation therapy using 36 Gy in 6 fractions. The patient died 2 months after diagnosis.
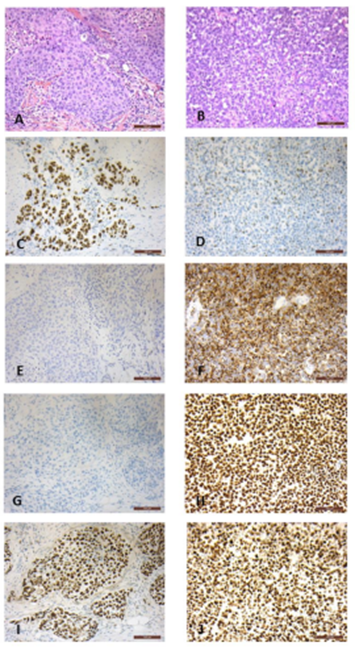
Figure 1: HES staining and immunohistochemical findings in both components. The most representative component (A) is composed of large or intermediate size cells, with eosinophilic cytoplasm, enlarged and irregular nuclei, and numerous mitoses. Cells are arranged in clusters or nests within a fibrous stroma (HES, A). The second component (B) is composed of sheets of smaller cells (HES, B). Cells of the component A are positive after immunohistochemical staining for GATA3 (C), and negative for HMB45 (E), and Sox10 (G). In contrast, component B is diffusely positive for HMB45 (F) and Sox10 (H), with few nuclei weakly stained for GATA3 (D). P53 protein was overexpressed in both components (I, J).
|
Component |
Gene |
Variant |
Protein alteration |
Significance |
|
Melanoma |
STK 11 exon 8 |
c.1094A>G |
p.(Asp365Gly) |
Uncertain significance |
|
TERT promoter |
c.-124C>T |
Pathogenic |
||
|
High-grade urothelial carcinoma |
STK 11 exon 8 |
c.1094A>G |
p.(Asp365Gly) |
Uncertain significance |
|
TERT promoter |
c.-124C>T |
Pathogenic |
||
|
PIK3CA exon 9 |
c.1624G>A |
p.(Glu542Lys) |
Pathogenic |
Table 1: Genetic alterations in the melanoma and high-grade urothelial carcinoma components.
3. Methods
Immunohistochemistry was performed with the automated BenchMark XT stainer (Ventana Medical Systems Inc., Oro Valley, AZ) using OptiView Detection Kit (Ventana Medical Systems Inc.). Slides were deparaffinized, rehydrated, and heated in citrate buffer pH 6 for antigenic retrieval. The primary antibodies included CK AE1/AE3 (AE1/AE3 clone, 1/200 dilution, 32 min; Dako, Carpintera, CA), GATA3 (L50-823 clone, 1/1 dilution, 32 min at 37°C; Ventana, Rotkreuz, Switzerland), HMB45 (HMB45 clone, 1/50 dilution, 16 min at 37°C; Dako), Melan A (A103 clone, 1/1 dilution, 16 min at 37°C; Ventana), Sox10 (SP267 clone, 1/1 dilution, 32 min at 37°C; Ventana), Chromogranin A (CK2H10 clone, 1/200 dilution, 60 min; Zytomed, Berlin, Germany), Synaptophysin (DAK-SYNAP clone, 1/50 dilution, 32 min; Dako), and P53 (D07 clone, 1/50 dilution; 32 min, Dako). Tumor DNA was extracted after macrodissection of formalin-fixed paraffin-embedded tissue sections for each contingent, using a Maxwell® 16 FFPE Tissue LEV DNA Purification Kit (Promega®, Madison, WI), according to the manufacturer's instructions, and a Maxwell® 16 instrument (Promega®). DNA concentration was assayed using a Qubit 4 fluorometer (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. The sequencing libraries were prepared using a QIAseq Targeted DNA Custom Panel kit (amplicon technology, Qiagen®, Germantown, MD), following the supplier's recommendations. Sequencing was performed on an Illumina Miseq Platform. Bioinformatic analysis was performed with an internal pipeline, generated using CLC Genomics Workbench software (Qiagen®).
Sequence alignment was performed using the GRCh37 (hg19) genome. List of targeted genes, exons and mRNA RefSeq used (NM) : AKT1 (exon 3, NM_001014431.1), ALK (exons 20, 21, 22 23,24 and 25, NM_004304.4), BRAF (exons 11 and 15, NM_004333.4), CTNNB1 (exons 2 to 4 and 6 to 8, NM_001904), EGFR (exons 18, 19, 20 and 21, NM_005228.4), ERBB2 (HER2)(exon 20, NM_004448.3), ERBB4 (exons 10 and 12, NM_005235.2), FGFR2 (exons 7, 12 and 14, NM_000141.4), FGFR3 (exons 7, 9 and 14, NM_000142.4), HRAS (exons 2, 3 and 4, NM_005343.3), KIT (exons 8, 9, 11, 13, 17 and 18, NM_000222.2), KRAS (exons 2, 3 and 4, NM_004985.4), MAP2K1 (exon 2, NM_002755.3), MET (exons 2 and 14 à 20, NM_001127500.2), NRAS (exons 2, 3 and 4, NM_002524.4), PDGFRA (exons 12, 14 and 18, NM_006206.5), PIK3CA (exons 9 and 20, NM_006218.3), POLE (exons 9 to 14, NM_006231) RAC1 (exon 2, NM_018890), STK11 (exons 1 to 9, NM_000455), TERT (promoter).
4. Discussion
This association of two components in a patient without any cancer history suggests either a collision of primary bladder melanoma with urothelial carcinoma, or a filiation between the two components. To date, less than 50 primary bladder melanomas have been reported, whereas urothelial carcinoma is the most frequent bladder cancer [1]. The second hypothesis is supported by both immunohistochemical and genetic findings. The focal expression of GATA3 in the melanocytic component of the tumor is in favor of a filiation between the urothelial origin and the melanocytic differentiation. Moreover, NGS analysis identified similar genetic alterations in both components for STK11 and TERT. The tumor suppressor STK11 encodes a member of the serine/threonine kinase family that activates AMPK and negatively regulates mTOR signaling pathway. STK11 point variations are common in urothelial carcinoma, particularly in high grade cancer, where it has been shown to activate mTOR pathway [2]. In skin metastatic melanoma, STK11 is mutated in around 15% of cases [3]. However, STK11 p.(Asp365Gly) alteration has never been described in either urothelial carcinoma or melanoma. Its significance remains uncertain, but the finding of this specific genetic alteration in both the urothelial and melanocytic component of the present case is in favor of a filiation. Reactivation of telomerase activity drives human cell immortality and cancer [4]. In urothelial carcinoma, telomerase activity level correlates with pathologic grade and clinical stage [5], and has been suggested to be an early event in bladder carcinogenesis [6]. Point variations of the TERT promoter occur in more than 50% of urothelial carcinoma, with the c.-124 nucleotide involved in the majority of cases, but none of them was a c.-124C>T variation as in our case [7]. Around 80% of metastatic melanomas have TERT promoter variations, and one of the hot spots is the c.-124C>T [2,7]. Although TERT promoter variations have been shown to occur often together with BRAF or NRAS variations, none of these genes was mutated in the present case. One previous report has analyzed the genetic alterations in melanomas of the urinary tract, and evidenced in only 2 of 8 cases BRAF p.(Val600Xxx) variations [8]. We observed in the urothelial component a variation of PIK3CA that was not found in the melanocytic component. PIK3CA is involved in the PI3K signaling pathway, as STK11. PIK3CA variations have been described in around 10% of urothelial cancers [9], and some of them led to alteration of the protein p.(Glu542Lys), as in our case. Similar genetic alterations have been used to evidence a filiation between two cancer components. Rearrangements involving ERG and the androgen regulated gene TMPRSS2, specific of prostatic adenocarcinoma, have also been evidenced in small cell neuroendocrine prostate cancers, suggesting that neuroendocrine cancer cells in this location are derived from adenocarcinoma cells [10]. In small cell neuroendocrine carcinoma of the bladder, the urothelial origin is supported by the finding of TERT promoter variations, common in urothelial carcinomas, but not observed in small cell neuroendocrine carcinomas from other locations [11]. The transformation of a cancer component into another component with a different phenotype and different cell lineage characteristics is called phenotypic plasticity. It could be due to transdifferentiation, i.e. conversion of a differentiated cell into another differentiated cell of distinct lineage, or to divergent differentiation from a Cancer Stem Cell (CSC) population. Transdifferentiation and divergent differentiation shares common mechanisms, since it has been shown that differentiated cancer cells can acquire CSCs properties, allowing the tumor to adapt quickly to its environment [12]. These mechanisms are those involved in the Epithelial-to-Mesenchymal Transition process, that contributes to tumor progression and aggressiveness [12]. Both urothelial carcinoma and melanoma have been shown to have propensity for divergent differentiation. The range of divergent differentiation in melanoma include most often mesenchymal or neuroendocrine, and rarely epithelial differentiation [13]. Several types of variants have been described in urothelial carcinomas, including squamous, glandular or neuroendocrine differentiation [14,15]. Due to the rarity of primary bladder melanoma, it is likely that in the present case the cancer component of origin is urothelial carcinoma. In conclusion, the present report provides pieces of evidence for a filiation between high grade urothelial cancer and bladder melanoma, that has never been described to date, and underlines the phenotypic plasticity of urothelial carcinomas.
Compliance with Ethical Standards
All experiments have been performed for diagnostic purpose. Studies are conducted according the guidelines of the local ethical committee.
Source of Funding
None
Conflict of Interest
The authors have no conflict of interest to declare
Contributions
Alix Fontaine(junior pathologist), data analysis, wrote the manuscript
Denis Roblet (local pathologist), provided biological ressources
Racha Benmeziani (Urologist) in charge of the patient, performed the resection
Gaëlle Fromont (senior pathologist): design of the project, data analysis, wrote the manuscript
References
- Venyo AKG. Melanoma of the Urinary Bladder: A Review of the Literature. Surg Res Pract 2014 (2014): 605802.
- Tigli H, Seven D, Tunc M, et al. LKB1 mutations and their correlation with LKB1 and Rheb expression in bladder cancer. Mol Carcinog 52 (2013): 660-665.
- Tomczak K, Czerwinska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol 19 (2015): A68-77.
- Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun 4 (2013): 2185.
- Lin Y, Miyamoto H, Fujinami K, et al. Telomerase activity in human bladder cancer. Clin Cancer Res Off J Am Assoc Cancer Res 2 (1996): 929-932.
- Lancelin F, Anidjar M, Villette JM, et al. Telomerase activity as a potential marker in preneoplastic bladder lesions. BJU Int 85 (2000): 526-531.
- Ekedahl H, Lauss M, Olsson H, et al. High TERT promoter mutation frequency in non-acral cutaneous metastatic melanoma. Pigment Cell Melanoma Res 29 (2016): 598-600.
- Acikalin A, Bagir E, Karim S, et al. Primary melanoma of the urinary tract; Clinicopathologic and molecular review of a case series. Pathology Research and Practice 216 (2020): 153095.
- Platt FM, Hurst CD, Taylor CF, et al. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res 15 (2009): 6008-6017.
- Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 1(2011): 487-495.
- Zheng X, Zhuge J, Bezerra SM, et al. High frequency of TERT promoter mutation in small cell carcinoma of bladder, but not in small cell carcinoma of other origins. J Hematol OncolJ Hematol Oncol 7 (2014): 47.
- Gupta PB, Pastushenko I, Skibinski A, et al. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 24(2019): 65-78.
- Jalas JR, Vemula S, Bezrookove V, et al. Metastatic melanoma with striking adenocarcinomatous differentiation illustrating phenotypic plasticity in melanoma. Am J Surg Pathol 35 (2011): 1413-1418.
- Shanks JH, Iczkowski KA. Divergent differentiation in urothelial carcinoma and other bladder cancer subtypes with selected mimics. Histopathology 54 (2009): 885-900.
- Humphrey PA, Moch H, Cubilla AL et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 70 (2016): 106-119.
