Botulinum Toxin in Management of Benign Essential Blepharospasm
Article Information
Farzana Afzal1, Nazmul Haque Robi2, Sadia Sultana3, Riffat Rashid4, Dr. Nasrin Habib5 Dr. Mamunur Rashid6
1Department of Oculoplasty, Ispahani Islamia Eye Institute and Hospital, Dhaka.
2Department of Oculoplasty, Ispahani Islamia Eye Institute and Hospital, Dhaka.
3Department of Oculoplasty, Ispahani Islamia Eye Institute and Hospital, Dhaka.
4Department of Oculoplasty, Ispahani Islamia Eye Institute and Hospital, Dhaka.
5Dept. of Physiology, Faculty of Medicine, Quest International University Perak, Malaysia.
6Department of Medicine, Faculty of Medicine, Quest International University Perak, Malaysia.
*Corresponding Author: Farzana Afzal, Department of Oculoplasty, Ispahani Islamia Eye Institute and Hospital, Dhaka
Received: 06 April 2021; Accepted: 15 April 2021; Published: 06 May 2021
Citation: Farzana Afzal, Nazmul Haque Robi, Sadia Sultana, Riffat Rashid, Nasrin Habib, Mamunur Rashid. Botulinum Toxin in Management of Benign Essential Blepharospasm. Journal of Ophthalmology and Research 4 (2021): 153-160.
View / Download Pdf Share at FacebookAbstract
The objective of this study is to evaluate effectiveness of botulinum toxin injection in treatment of benign essential blepharospasm. It is a prospective interventional study conducted in Ispahani Islamia Eye Institute and Hospital within the period of June 2018 to May 2020. In this study 102 patients, suffering from benign essential blepharospasm, were included. After taking standard precaution, botulinum toxin injection was injected at 5 to 6 sites of each eye with 2.5 units per site. Patients were followed up routinely on the following day, after 7 day, then after 1 month and 3 months. 65.68% patients are of 50 years or more with mean age 54.91 years. 35.29% patients are male and 64.70% are female. 62.74% patient had bilateral blepharospasm while 37.25% patient presented with unilateral symptoms. Useful effect of injection appeared within 48 hours in all patients. According to Blepharospasm disability index, 13.72% patient had minor blepharospasm (mean BSDI score 1to 2), 34.31% patient had moderate (mean BSDI score >2 to 3) and 51.96% patient had severe blepharospasm (>3 to 4). Baseline mean BSDI score before injection of 102 patients was 2.62. 7 days after injection, the score reduced to 1.46 and after 1month, score was 0.95. Minor transient side effect like ptosis, dry eye and lagophthalmos was found in very few patients. The useful effect of injection lasted for 3-4 months in majority of patients. This study explored Botulinum toxin as the first line treatment of benign essential blepharospasm,however, there are few limitations of this agent, including short-term effect, repeated application, and high cost.
Keywords
Blepharospasm; orbicularis; anticholinergic; spasmodic; Clostridium Botulinum; lagophthalmos
Article Details
1. Introduction
Blepharospasm is a focal cranial dystonia characterized by repetitive involuntary contraction of orbicularis oculi muscle resulting in forceful intermittent or complete closure of eyelids [2]. Benign essential blepharospasm (BEB) is spasmodic involuntary eyelid closure in the absence of any other ocular or adnexal cause [1]. Sometimes the blinking is so unsettling that patients may become desperate, frustrated, and angry. Blepharospasm may be influenced by environmental factors like sunlight exposure, stress, or fatigue [2].
BEB is more common in women (2-3 times) as compared to men and more so in people over 50 years of age [1]. Blepharospasm can range in severity from mild cases that do not appreciably interfere with daily activities to severe cases that render individuals functionally blind, preventing them from driving, working, reading, and walking [3]. The true pathologic basis of BEB is unknown, but abnormalities in basal ganglia and cortico-striato-pallido-thalamic loop have been considered responsible and abnormal auditory brainstem response potentials have been noticed in these patients [4,5]. Blepharospasm can be treated pharmacologically (using anticholinergic drugs, dopamine agonists and antagonists and anti-psychotic drug) or surgically by myectomy [7,8]. Though surgical procedure is effective, but they are invasive and irreversible. Similarly drugs also have side effects like dry eye, dry mouth, blurred vision, hallucination. So, introduction of botulinum toxin A provided a new horizon to ophthalmologists to treat BEB.
Botulinum toxin is derived from bacteria Clostridium Botulinum. It prevents acetylcholine release from presynaptic terminals, thus blocks neuromuscular transmission at peripheral cholinergic nerve endings [6]. The onset of effect takes usually about 24-72 hours [9,10,11]. The plateau effect is achieved in about 5-7days and usually lasts for 10 to 15 weeks [12]. Generally, the response rate to the injection is 95-98% [11].
Recovery of muscular function occurs in about three to four months’ time by axonal sprouting and formation of new neuromuscular junctions [1]. Inspite of having few minor transient side effects like dryness, ptosis, lagophthalmos, botulinum toxin is still the best choice of treatment in benign essential blepharospasm.
The objective of this study was to evaluate the effect of botulinum toxin A in patients having benign essential blepharospasm.
2. Materials and Methods
It is a prospective interventional study conducted in Ispahani Islamia Eye Institute and Hospital within the period of June 2018 to May 2020. 102 patients were enrolled in this study. All the patients were suffering from benign essential blepharospasm from several months to years. The inclusion criteria for the patients in this study were-
-Idiopathic essential blepharospasm
-No neurologic or psychiatric illness.
-No history of previous botulinum toxin injection
-Not having concomitant hemifacial spasm
Exclusion criteria included:
-Blepharospasm due to secondary cause
-Patients having hypersensitivity to botulinum toxin injection.
The severity of EBS was graded according blepharospasm disability index (BSDI). The patients had been explained about the study and informed consent was taken. The patients were explained about the effects, possible side effects and the expected duration of the useful effects of the injection.
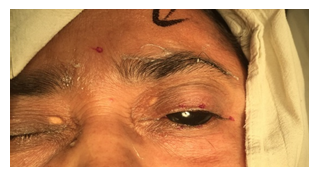
The injection was prepared by adding 4ml of distilled water to 100 units of purified dry botulinum toxin supplied in a sealed glass vial with a negative pressure inside. This would give 2.5 units of toxin per 10th division of 1 ml insulin syringe. Precautions were taken to maintain the cold chain of supply (around 4 degree Celsius). Under aseptic conditions, taking standard precautions for injection procedure, the toxin was injected subcutaneously at five selected sites in the periocular region and the starting dose in all the patients was 2.5 units per injection site. (see Figure.1).
The patients were then followed up regularly on next day, after 7 day, after 1 month, 3months and 6 months. The treatment was repeated after every 3 to 6 months.
3.Results
Total 102 patients were enrolled in this study.
3.1 Age distribution
Most of the patients in this study group were above 50 years. Mean age of the patients were 54.91 year. (See Table.1).
3.2 Gender distribution
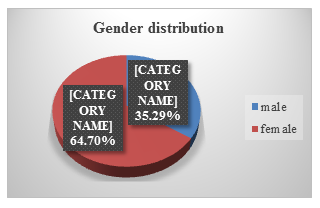
In our study, 66 patients were female, and 36 patients were male. (see Figure.2).
3.3 Laterality
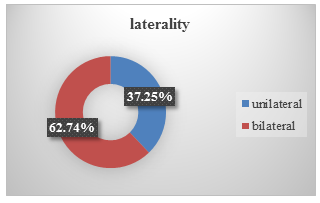
Among the patients, 38 patients had unilateral blepharospasm and 64 patients had bilateral blepharospasm. (see Figure.3).
3.4 Severity of essential blepharospasm
Severity of benign essential blepharospasm was measured by blepharospasm disability index (BSDI). If mean BSDI score 1 to 2, it is graded as mild blepharospasm, if mean score is >2 to 3 then
graded as moderate and if mean score is >3 to 4 then patient had severe blepharospasm. (see Table 2).
3.5 Effects of botulinum toxin
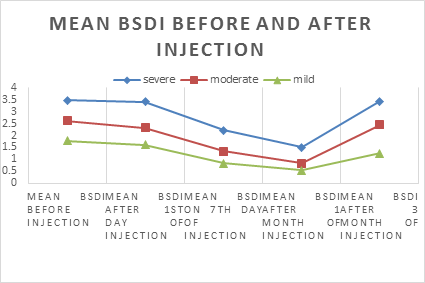
This is the chart which shows the changes of mean BSDI score before the injection and over 3 months period after injection. Mean BSDI score of 102 patients before injection was 2.62 which after 1 day of injection reduced to 2.45. after 7 day the score was 1.46 and after 1 month it was reduced to 0.95. After 3 months, the score was increased to 2.38.(see Figure.4 and Figure.5).
|
Age |
Patients (n) |
Percentage (%) |
|
<50 years |
35 |
34.31 |
|
>50 years |
67 |
65.68 |
Table1: Age Distribution
|
Severity of EBS |
Patient (n) |
Percentage (%) |
|
Mild |
14 |
13.72 |
|
Moderate |
35 |
34.31 |
|
Severe |
53 |
51.96 |
Table 2: Severity of Essential blepharospasm
3.6 Side effects of botulinum toxin
There were few minor side effects seen after injection. But they were temporary and instantaneously resolved within few days. (see Table.3)
|
Side effect |
On 1st day |
After 1 week |
After1 month |
|
Dry eye |
5 |
3 |
0 |
|
Ptosis |
3 |
2 |
0 |
|
Lagophthalmos |
2 |
1 |
0 |
|
Diplopia |
0 |
||
|
Redness of eye |
3 |
0 |
0 |
Table 3: Side effects of botulinum toxin
4. Discussion
In many cases of Benign essential blepharospasm, the patients are treated with anxiolytic or sedative agents. they are partially effective or ineffective measures in most of the cases.
Botulinum toxin A has been established as the first line treatment of choice for benign essential blepharospasm. it has been found safe and effective for long term treatment of BEB, especially if injection doses, preparation and right technique are chosen properly.
There are many publications regarding blepharospasm and its different management technique along with outcome all over the world. In our country, publication over this topic is limited.
The objective of our study is to discuss the clinical feature and distress of the patients with Benign Essential Blepharospasm, preparation and administration technique of botulinum toxin A and observe the effectiveness, side effect and over all outcome of this agent over the patients.
In our study 65.68% patient were above 50 years with mean age 54.9 years. Shakib et al showed that most of their patients were between 45 to 65 years (mean age 55 years) [1].
In most study, females are more affected. In our study 64.70 % patients were female and 35.29% patients were male. Zahid et al. had 60% female and 40% male in his study [2].
Benign essential blepharospasm usually have bilateral presentation. 62.74% of our patient were suffering from bilateral disease while 37.25% patient had unilateral presentation.
Very few studies measured the severity of blepharospasm. There are many grading systems to measure the severity of blepharospasm, but we followed a more common system which is blepharospasm disability index (BSDI). According to BSDI scale, 51.96% patients of our study have severe blepharospasm hampering the day-to-day activities, 34.44% patient had moderate, and 13.72% patient had mild blepharospasm.
Effects of botulinum toxin injection was observed on 1st day after injection, then 7th day, after 1 month and 3 months. Regardless of severity, mean BSDI score were declined from 2.62 to 2.45 on first day after injection, which further reduced to 1.46 on 7 th day and 0.95 after 1 month. The score then inclined to 2.38 after 3 months.
Zahid Mahmood highlighted that condition of 90% 0f patients were significantly improved but effects remained for 3 to 4 months [2].
Common side effects in our study group were dry eye, ptosis and lagophthalmos which were temporary, and effects were completely resolved within 1 month. Side effect of botulinum toxin in other studies are diplopia, ptosis, dry eye, lagophthalmos and red eye [2].
Although number of patients in our study groups was small but compliance of the patients regarding follow up was satisfactory.
5. Conclusion
The outcome of our study suggests that botulinum toxin type A is effective and safe and is the treatment of choice as the first line therapy for benign essential blepharospasm. Though short-term effect, high cost and repeated injection are the drawback of this agent, if proper dosage and administration technique can be followed, botulinum toxin type A significantly reduces the blepharospasm disability index with in very short period of time and improves the quality of life of the patients.
Conflicts of interest
The Authors declare that there is no conflict of interest in this study.
Funding
There are no funders to report for this submission as well as the authors did not receive any fund for this study.
References
- Anwar MS, Zafar H. Efficacy of botulinum toxin in benign essential blepharospasm: Desirable & undesirable effects. Pak J Med Sci 29 (2013): 1389-1393.
- Mahmood Z, Khan S, Saeed M. Treatment of benign essential blepharospasm with Botulinum toxin Type A. JSZMC 6 (2015): 753-756.
- Botulinum Toxin Therapy in Congenital Blepharospasm. Bettina Wabbels. Case Rep Ophthalmol 5 (2014): 435–438.
- Creel DJ, Holds JB, Anderson RL. Auditory brainstem neurophysiology.86 (1993): 138-140.
- Zhou B, Wang J, Huang Y, et al. Resting state functional magnetic resonance imaging study of patients with benign essential blepharospasm. J Neuro ophthalmol 33 (2013): 235-240.
- Rainer L. The use of botulinum toxin in head and face medicine: An interdisciplinary field. Head Face Med.4 (2008): 5
- Lang AE. High dose anticholinergic therapy in adult dystonia.Can J Neurol Sci 13 (1986): 42-46.
- Lang AE. Dopamine agonists in the treatment of dystonia. Clinical Neuropharmacol. 8 (1985): 38- 57
- Dutton JJ, Buckley EG. Botulinum toxin in the management of blepharospasm. Arch Neurol 43 (1986): 380-382.
- Ruusavaara P, Setala Long-term treatment of involuntary facial spasms using botulinum toxin. Acta Ohthalmol 68 (1990): 331-338.
- Physician’s Desk Reference 50th Ed. Montval NJ: Medical Economics Company (1996): 477-478.
- Blasi J, Chapman ER, Link E. Botulinum toxin selectively cleaves the synaptic protein SNAP25. Nature 365 (1993): 160-163.
- Gonnering RS. Pharmacology of botulinum toxin. Int Ophthalmol Clin 33 (1993): 203-227.
- Dutton JJ. Acute and chronic, local and distant effects of botulinum toxin. Surv Ophthalmol 40 (1996): 51-65.
- Georgescu D, Vagefi MR, McMullan TF, et al. Upper eyelid myectomy in blepharospasm with associated aprexia of lid opening. Am J Ophthalmol.145 (2008): 541-547.