Bladder Cancer Burden and Challenges of Management in Kano, North Western Nigeria
Article Information
Bashir Yunusa1*, Muzzammil Abdullahi1, Sharfuddeen Abbas Mashi1, Sani Ali Aji1, Auwal Sani1, Sani Abdullahi Giade2, Sani Alhassan Usman1
1Urology unit, Department of Surgery, Aminu Kano Teaching Hospital, Kano PMB 3452, Kano, Nigeria
2Department of Surgery, Federal Medical Centre, Azare, Bauchi state, Nigeria
*Corresponding Author: Dr. Bashir Yunusa, Department of Surgery, Bayero University, Kano/Aminu Kano Teaching Hospital, Kano PMB 3011, Nigeria
Received: 10 August 2020; Accepted: 08 September 2020; Published: 05 October 2020
Citation: Bashir Yunusa, Muzzammil Abdullahi, Sharfuddeen Abbas Mashi, Sani Ali Aji, Auwal Sani, Sani Abdullahi Giade, Sani Alhassan Usman. Bladder Cancer Burden and Challenges of Management in Kano, North Western Nigeria. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 424-433.
View / Download Pdf Share at FacebookAbstract
Introduction: Bladder cancer is the ninth most common cancer worldwide and second most common urologic cancer after prostate cancer. Worldwide, about ninety percent of bladder cancers are transitional cell cancers (TCC); others are squamous cell carcinoma (SCC) which comprises approximately 6-8%, and adenocarcinoma about 2%. However, in schistosomiasis endemic areas, SCC constitutes as high as 44-82% of bladder cancer. Schistosomiasis caused by S. hematobium is endemic in Africa, southern tips of Europe and parts of Japan.
Objectives: We aim to review the burden, pathology and clinical management of bladder cancers in Kano, North-Western Nigeria.
Methods: We retrospectively reviewed the records of all (98) patients seen and diagnosed to have bladder cancer and offered some form of intervention at Aminu Kano Teaching Hospital, Kano, from January 2011 to June 2016. Information obtained were risk factors, mode of presentation, histological type and intervention given and analysed by SPSS Version 16 and presented in tables and charts.
Results: There were a total of 98 patients seen with clinical diagnosis of bladder cancer. The mean age (±SD) was 51.4 +13.9 years, with male to female ratio of 10:1. Ninety one (91%) presented as an emergency, 37 (84%) had identifiable risk factors. More than half of the patients (52%) couldn’t have a representative tissue for histology because of advanced disease with necrotic tissues at presentation. About 44% has SCC while 29% was TCC.
Conclusion: Squamous cell carcinoma is still the predominant histological subtype of bladder cancer in our environment owing to endemicity of schistosomiasis and usually present in advanced stage.
Keywords
Bladder cancer; Schistosomiasis; Histological type
Bladder cancer articles Bladder cancer Research articles Bladder cancer review articles Bladder cancer PubMed articles Bladder cancer PubMed Central articles Bladder cancer 2023 articles Bladder cancer 2024 articles Bladder cancer Scopus articles Bladder cancer impact factor journals Bladder cancer Scopus journals Bladder cancer PubMed journals Bladder cancer medical journals Bladder cancer free journals Bladder cancer best journals Bladder cancer top journals Bladder cancer free medical journals Bladder cancer famous journals Bladder cancer Google Scholar indexed journals Schistosomiasis articles Schistosomiasis Research articles Schistosomiasis review articles Schistosomiasis PubMed articles Schistosomiasis PubMed Central articles Schistosomiasis 2023 articles Schistosomiasis 2024 articles Schistosomiasis Scopus articles Schistosomiasis impact factor journals Schistosomiasis Scopus journals Schistosomiasis PubMed journals Schistosomiasis medical journals Schistosomiasis free journals Schistosomiasis best journals Schistosomiasis top journals Schistosomiasis free medical journals Schistosomiasis famous journals Schistosomiasis Google Scholar indexed journals Histological type articles Histological type Research articles Histological type review articles Histological type PubMed articles Histological type PubMed Central articles Histological type 2023 articles Histological type 2024 articles Histological type Scopus articles Histological type impact factor journals Histological type Scopus journals Histological type PubMed journals Histological type medical journals Histological type free journals Histological type best journals Histological type top journals Histological type free medical journals Histological type famous journals Histological type Google Scholar indexed journals squamous cell carcinoma articles squamous cell carcinoma Research articles squamous cell carcinoma review articles squamous cell carcinoma PubMed articles squamous cell carcinoma PubMed Central articles squamous cell carcinoma 2023 articles squamous cell carcinoma 2024 articles squamous cell carcinoma Scopus articles squamous cell carcinoma impact factor journals squamous cell carcinoma Scopus journals squamous cell carcinoma PubMed journals squamous cell carcinoma medical journals squamous cell carcinoma free journals squamous cell carcinoma best journals squamous cell carcinoma top journals squamous cell carcinoma free medical journals squamous cell carcinoma famous journals squamous cell carcinoma Google Scholar indexed journals adenocarcinoma articles adenocarcinoma Research articles adenocarcinoma review articles adenocarcinoma PubMed articles adenocarcinoma PubMed Central articles adenocarcinoma 2023 articles adenocarcinoma 2024 articles adenocarcinoma Scopus articles adenocarcinoma impact factor journals adenocarcinoma Scopus journals adenocarcinoma PubMed journals adenocarcinoma medical journals adenocarcinoma free journals adenocarcinoma best journals adenocarcinoma top journals adenocarcinoma free medical journals adenocarcinoma famous journals adenocarcinoma Google Scholar indexed journals urologic cancer articles urologic cancer Research articles urologic cancer review articles urologic cancer PubMed articles urologic cancer PubMed Central articles urologic cancer 2023 articles urologic cancer 2024 articles urologic cancer Scopus articles urologic cancer impact factor journals urologic cancer Scopus journals urologic cancer PubMed journals urologic cancer medical journals urologic cancer free journals urologic cancer best journals urologic cancer top journals urologic cancer free medical journals urologic cancer famous journals urologic cancer Google Scholar indexed journals S. hematobium articles S. hematobium Research articles S. hematobium review articles S. hematobium PubMed articles S. hematobium PubMed Central articles S. hematobium 2023 articles S. hematobium 2024 articles S. hematobium Scopus articles S. hematobium impact factor journals S. hematobium Scopus journals S. hematobium PubMed journals S. hematobium medical journals S. hematobium free journals S. hematobium best journals S. hematobium top journals S. hematobium free medical journals S. hematobium famous journals S. hematobium Google Scholar indexed journals necrotic tissues articles necrotic tissues Research articles necrotic tissues review articles necrotic tissues PubMed articles necrotic tissues PubMed Central articles necrotic tissues 2023 articles necrotic tissues 2024 articles necrotic tissues Scopus articles necrotic tissues impact factor journals necrotic tissues Scopus journals necrotic tissues PubMed journals necrotic tissues medical journals necrotic tissues free journals necrotic tissues best journals necrotic tissues top journals necrotic tissues free medical journals necrotic tissues famous journals necrotic tissues Google Scholar indexed journals immune cells articles immune cells Research articles immune cells review articles immune cells PubMed articles immune cells PubMed Central articles immune cells 2023 articles immune cells 2024 articles immune cells Scopus articles immune cells impact factor journals immune cells Scopus journals immune cells PubMed journals immune cells medical journals immune cells free journals immune cells best journals immune cells top journals immune cells free medical journals immune cells famous journals immune cells Google Scholar indexed journals histological type articles histological type Research articles histological type review articles histological type PubMed articles histological type PubMed Central articles histological type 2023 articles histological type 2024 articles histological type Scopus articles histological type impact factor journals histological type Scopus journals histological type PubMed journals histological type medical journals histological type free journals histological type best journals histological type top journals histological type free medical journals histological type famous journals histological type Google Scholar indexed journals
Article Details
1. Introduction
Bladder cancer is one of the common malignancies worldwide responsible for significant morbidity and mortality and accounting for the huge cost on healthcare delivery system of many countries. It’s the ninth most common cancer worldwide [1, 2] and second most common urologic malignancy after prostate cancer [3]. Urinary bladder cancer, which is mostly a urothelial carcinoma, is one of the most frequently diagnosed neoplasm, with nearly 550,000 new cases and 200,000 deaths estimated in 2018 worldwide [1, 2]. It is more common in the industrialized communities and among Caucasians. In Europe and North America, most patients are over 50 years old with peak age between 60-70 years and a male to female ratio of 3:1 [1].
In Africa the exact prevalence of bladder cancer is not known, however, a recent analysis of several reports across the continent put the pooled incidence of bladder cancer in Africa as 7.0 per 100 000 population in men and 1.8 per 100 000 in women. The incidence of bladder cancer was consistently higher in North Africa in both sexes. Incidence rates increased significantly among men from 5.6 (95% credible interval 4.2–7.2) in the 1990s to up to 8.5 per 100 000 in 2010 [2, 3].
Worldwide, about ninety percent of bladder cancers are transitional cell cancers (TCC), reflecting their origin from the transitional cells lining the bladder, others are squamous cell carcinoma (SCC) which comprises approximately 6-8%, and adenocarcinoma about 2% [4, 5]. However, in schistosomiasis endemic areas, SCC constitutes as high as 44-82% of bladder cancer [6, 7, 8]. In much of Africa, the predominant subtype continues to be SCC [2]. Squamous cell cancer poses challenge to urologist and surgeons in Africa as its presentation and approach to treatment is often different from those of TCC.
A 10-year retrospective review of the cancer registry in Kano, north-western Nigeria (2005-2014) revealed bladder cancer as the second most common male cancer (after prostate cancer) and the 11th most common female cancer [9]. Also a previous report of histopathological review of bladder cancer in Kano (1998 – 2001) showed SCC was the predominant histological variety (53%), others were TCC - 35% and adenocarcinoma- 4%. The mean age of incidence was 48.8years and male to female ratio was 5.2:1 [10]. However, an update of histopathological review from the same centre (2001-2015) showed a switch of the most common histological variety of bladder cancer to urothelial cancer found in 49.6%; others were SCC – 44.3% and sarcomas -5%. The mean age and sex preponderance remained similar (51.2years and M: F- 7.6:1) [11].
The causal relationship between urinary schistosomiasis and bladder cancer was first reported by Fergusson [6]. There is now compelling body of evidence on the contribution of chronic urinary bladder schistosomiasis to the aetiopathogenesis of bladder cancer. Non-bilharzial cases are usually associated with chronic bladder irritations by vesical stones, chronic indwelling catheters or in bladder diverticula [6,12,13]. Different aetiopathogenic mechanisms have been suggested for bladder cancer; in schistosomiasis-induced bladder cancer no direct carcinogenic product from the parasite has been isolated. Current evidence suggests enhanced inflammatory cells and bacterial nitrosation of endogenous nitrite to carcinogenic N- nitroso compounds in schistosoma infected bladder [13]. In contrast, several compounds found in tobacco, occupational exposure to aromatic amines or polycyclic aromatic hydrocarbons in petroleum products, agricultural chemicals and industrial compounds (rubber/dye) are linked to development of TCC [4]. Diagnosis of TCC is made from urinary cytology, urinary biomarkers and urethrocystoscopy and biopsy while in SCC the diagnosis is largely made from urethrocystoscopy and biopsy.
Transurethral resection, partial cystectomy, radiation therapy and chemotherapy are the most common treatment modalities for TCC; however, these are not effective in the treatment of SCC, in which radical cystectomy and urinary diversion is the treatment of choice in selected cases. Importantly, the prognosis for bladder cancer patients remained largely unimproved, during the last few decades, in spite of considerable advances in diagnostic technologies, as well as treatment strategies. In general, SCC has a poorer prognosis [14].
2. Aim
We aim to review the burden of bladder cancer in our centre, possible risk factors, clinical presentation, treatment options offered, our limitations and to offer recommendations.
3. Methodology
We retrospectively reviewed the records of all the (98) patients seen and diagnosed to have bladder cancer and who were offered some treatment at Aminu Kano Teaching Hospital, Kano, from January 2011 to June 2016.
4. Data Collection
Data was retrieved from medical records. The charts of all patients admitted to the hospital with the clinical diagnosis of bladder cancer were reviewed. The demographic data such as age, gender, occupation and address were recorded. Others were risk factors for bladder cancer, clinical presentation, investigations done, treatment given and patient outcome were all noted and the database was rescreened and recorded.
5. Data Analysis
Patients bio-data information were recorded using a simple proforma and was entered in to Microsoft excel spread sheet, then exported in to SPSS Version 16 for windows and analysed (by University of Bristol Information Services, UK, October, 2008). Qualitative variables were summarized using frequency and percentage while quantitative variables were summarized using mean and standard deviation or median range and presented as tables and charts.
6. Results
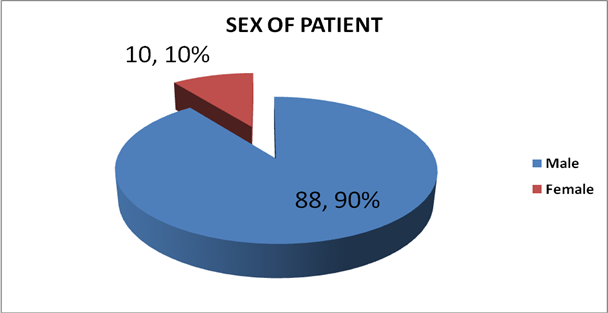
Over the period covered by the study, a total of 98 patients with clinical diagnosis of bladder cancers were seen at the facility. Their age ranges between 21-90years with mean age of 51.4+13.9. Other details are as shown below and Majority of the patients were males as shown in Figure 1.
|
Age Group(years) |
Frequency |
Percent (%) |
|
21 – 30 |
4 |
4.1 |
|
31 – 40 |
9 |
9.2 |
|
41 – 50 |
19 |
19.4 |
|
51 – 60 |
23 |
23.5 |
|
61 – 70 |
28 |
28.5 |
|
71 – 80 |
14 |
14.3 |
|
81 - 90 |
1 |
1.0 |
|
TOTAL |
98 |
100 |
Table 1: Age distribution of patients.
The patients predominantly presented in emergency situations warranting admission and resuscitative measures and only few presented with mild stable diseases as shown in Table 2.
|
Variables |
Frequency |
Percent |
|
Emergency |
89 |
91 |
|
Elective |
9 |
9 |
|
Total |
98 |
100 |
Table 2: Distributions of mode of presentation.
Similarly, risk factors for bladder cancer were established in up to 86% of the patients and these include childhood schistosomiasis, cigarette smoking and exposure to organic/industrial compounds. Majority had only one risk factor with a small percentage found to have more than one. Table 3 showed the distribution of the risk factors.
|
Risk Factor |
Frequency |
percent |
|
None |
14 |
14.3 |
|
1 |
40 |
40.8 |
|
2 |
6 |
6.1 |
|
3 |
7 |
7.2 |
|
1+2 |
10 |
10.2 |
|
1+3 |
9 |
9.2 |
|
2+3 |
7 |
7.1 |
|
1+2+3 |
5 |
5.1 |
|
Total |
98 |
100 |
KEY: 1-Childhood schistosomiasis; 2-Cigarette smoking; 3-Exposure to organic and industrial compounds
Table 3: Distribution of risk factors for Bladder Cancer.
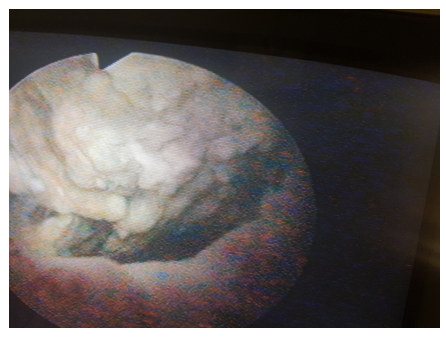
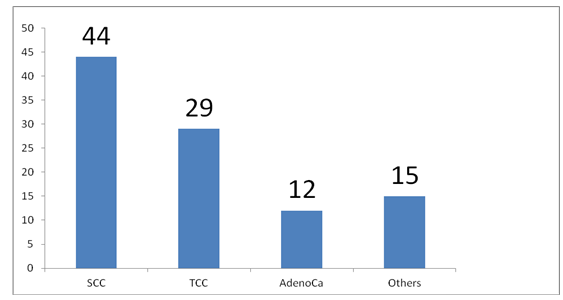
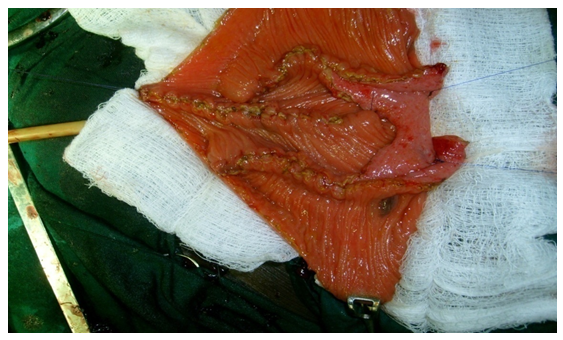
The patients had several investigations in the course of their treatment, including urethrocystoscopy with biopsy for histological diagnosis however we were only able to retrieve the urethrocystoscopic finding of 62 patients and histology of 41 patients. The summary of urethrocystoscopic findings are shown in Table 4. A common cystoscopic finding in our patients with bladder tumour is shown in Figure 2. The histological diagnosis in the 41 patients is shown in Figure 3.
|
Bladder tumour sites and number |
Frequency |
percent |
|
Occupying most of bladder lumen |
32 |
51.6 |
|
Occupying trigone and at least 2 walls |
15 |
24.2 |
|
Involving bladder neck and urethra |
10 |
16.1 |
|
Single focal tumour |
3 |
4.9 |
|
Multi focal tumour |
2 |
3.2 |
|
Total |
62 |
100 |
Table 4: Urethrocystoscopic Findings among the patients.
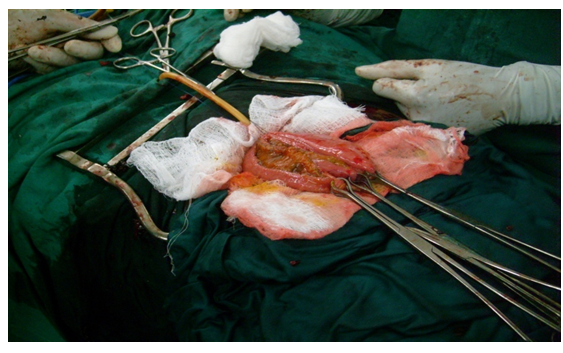
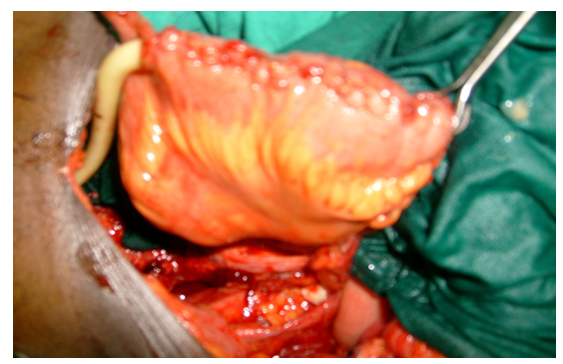
Majority of the patients had only palliative resuscitative measures as they had locally advanced/metastatic diseases at presentation while only 13 patients (13.3%) had radical cystectomy with urinary diversion (cutaneous ureterostomy or orthotopic ileoneocystoplasty). The palliative resuscitative measures include blood transfusion, continous bladder irrigation, haemodialysis and percutaneous nephrostomy. This is summarized Table 5. A typical ileoneocystoplasty after radical cystectomy is shown in Figure 3-5,
|
Intervention |
Frequency |
Percent |
|
Palliative resuscitative measures |
85 |
86.7 |
|
Cystectomy +cutaneous ureterostomy |
4 |
4.1 |
|
Cystectomy + orthotopic ileoneocystoplasty |
9 |
9.2 |
|
Total |
98 |
100 |
Table 5: Interventions for the patients with Bladder Cancer.
Out of the 13 patients who had cystectomy, 4 (30.7%) developed post operative complications (mainly tumour recurrence) of which 3 (23.7%) died within 2 years of follow up.
7. Discussion
The patients’ age ranges between 21-90years with mean age of 51+ 13.9 years [Table 1]. The highest incidence was among patients aged 61-70years, though about 30% of the patients are below the age of 51years.These findings are consistent with those reported in other studies [5, 11]. Relatively younger age group in our environment may be related to the fact that, most of the diagnosed tumours are SCC which presents at an earlier age, more aggressive and advanced at the time of diagnosis [12]. The male to female ratio was found to be 9:1 (Figure 1), this is similar to the findings by Mungadi et al. in Sokoto, Nigeria who found a ratio of 11.1:1 but higher than what was obtained in Ghana [14], and in Ibadan [15]. This could be explained by the fact that men engaged more in activities predisposing them to developing schistosomiasis (such as swimming and irrigation farming) hence more at risk in developing SCC.
Most of our patients (91%) presented as emergency with either Anemia, Shock, Clot retention, Uraemia or Urosepsis (Table 2) for which they required resuscitations with fluids, blood transfusion, antibiotics, dialysis or percutaneous kidney punctures similar to reports from Egypt [14]. The emergency presentation suggest advanced disease which could be attributed to the prevalent SCC and poor health seeking behaviour of our people who are mainly farmers from rural areas exposed to bilharzia and Agricultural chemicals with limited access to healthcare facilities.
The most common risk factor identified in our study is poorly treated childhood terminal haematuria secondary to schistosomiasis. Others were cigarette smoking and exposure to industrial compounds (Table 3). This is expected as Kano is in a region endemic of schistosomiasis. This also supports the finding of a predominantly SCC in our study (Figure 3) as a direct link has been documented with schistosomiasis by metaplasia and dysplasia [12, 13]. However we are limited by lack of resources to evaluate for the presence of other risk factors including genetic predisposition, chemical carcinogens, reactive oxygen species and other organic compounds as reported by Gul Lone and colleagues [17]. Most common cystoscopic finding is that of usually huge tumours, filling the whole of the bladder lumen with a lot of necrotic tissues in which several attempts at biopsies may only reveal a non representative necrotic tissues hence requiring repeated procedures (Table 4, Figure 2).
Squamous cell carcinoma still remain the predominant histological variety in our environment (Figure 3) in contrast to a change to TCC as predominant histological variety seen for example in Egypt that previously had predominantly SCC [4]. This difference is probably due to the fact that schistosomiasis is still endemic in our environment despite effort at eradicating it. Radical cystectomy has been describe as the goal standard for the treatment of bladder cancer, however only 13.3% of our patients were fit for and had the procedure (Table 5). This is because majority of our patients (86.7%) presented with a locally advanced or metastatic disease.
Among the 13 patients who had cystectomy, complications rate was 30.7% and mortality rate was 23.7%. Cancer related mortality of 29% was reported by Mokhtar et al. [18], which is slightly higher than our report but predicted 50% mortality in 2 years due to development of metastasis. Wayan et al. [19] and Zhu et al. [19], related early death with under estimation of tumour stage, aggressive characteristics of bladder cancer and peri-operative complications.
8. Limitations
- It is a retrospective study, with some missing data, may not reflect the exact number diagnosed over the period of study, but rather those with available records.
- Advanced nature of the disease, limited our ability to get correct biopsies on cystoscopy and unable to make histological diagnosis of those cases.
9. Recommendations
- Community based studies to get the exact prevalence.
- More effort in prevention of schistosomiasis at all levels
- Encourage patient to present with early disease through health education.
- Screening to detect early disease and surveillance for people with history of childhood haematuria.
- Better facilities for cystoscopy and biopsy and other tumour markers
Conflict of Interest
None declared.
References
- Sebastein A et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 71 (2017): 96-108.
- Davies A, et al. Estimate of the incidence of bladder cancer in Africa: A systematic review and Bayesian meta?analysis. Int J Urol 26 (2018): 102-112.
- Heyns CF, Vander Merwe A. Bladder cancer in Africa. The Canadian J of Urol 5 (2008): 3899-3908.
- Hosni KS, Soheir M. Changing Patterns (Age, Incidence, and Pathologic Types) of Schistosoma-associated Bladder Cancer in Egypt in the Past Decade. Urol 79 (2012): 379-383.
- Abdullah SA, Saleh M. Urinary Bladder Cancer in Yemen. Oman Medical Journal 28 (2013): 337-340.
- Eni U, Na'aya H, Nggada H, et al. Carcinoma Of The Urinary Bladder In Maiduguri: The Schistosomiasis Connection. The Int J of Oncol 5 (2007): 1-7.
- Bowa K, Kachimba JS, Labib MA, et al. The Pattern of Urological Cancers in Zambia. Afr J Urol 15 (2009): 84-87
- Diao B, Amath T, Fall B, et al. Bladder cancers in Senegal: epidemiological, clinical and histological features. Journal de L'Association Francaise D'urologie et de la Societe Francaise D'urologie18 (2008): 445-448.
- Ibrahim Y, Akinfenwa TA, et al. Cancer in Kano, Northwestern Nigeria: A 10-year update of the kano cancer registry. Ann Trop Pathol 8 (2017): 87-93.
- Ochicha O, Alhassan S, Muhammed AZ, et al. Bladder cancer in Kano-A histopathological review.West African Journal of Medicine 22 (2003): 202-204.
- Sule AA, Ochicha O, Ibrahim Y, et al. Update on bladder cancer in Kano, Northern Nigeria. Niger J Basic Clin Sci 14 (2017): 26-29.
- Amonkar P, Murali G, Krishnamurthy S. Schistosoma induced squamous cell carcinoma of the bladder Indian J of Pathology and Microbiology 44 (2001): 363-364.
- Mungadi AI, Malami SA. Urinary Bladder Cancer and schistosomiasis in northwestern Nigeria. WAMJ 26 (2007): 226-229.
- Mohamed I. El-Sayed, Ahmed M. Abdel-Rahim. Survival Analysis in Patients with Nonmetastatic Squamous Cell Carcinoma of the Urinary Bladder. Middle east j cancer 2 (2011): 59-64.
- Klufio GO. A review of Genitourinary cancers at the Korle-Bu Teaching Hospital, Accra, Ghana. WAMJ 23 (2004): 131-134.
- Takure AO, Odubanjo MO, Adebayo SA, et al. Histopathologic Pattern Of Bladder Cancers In Ibadan Southwest Nigeria: An Update, Journal Of The West African College Of Surgeons 5 (2015): 17-42.
- Lone WG, Makhdoom M, Khan MS, et al. Role of Genetic and Exogenous Factors in Urinary Bladder Cancer (UBC). International Journal of Genetics and Cancer Compilation 1 (2014): 43-53.
- Mokhtar A, Al Alawi MM, Al Taweel WM, et al. Al Otaibia MF Is survival after radical cystectomy for bladder cancer in Saudi patients different from that of Western patients? Ann Saudi Med 37 (2017): 194-200.
- Wayan Y, Ayu PD, Gde OAA, et al. Pathological Profile, Early Complications, Functional and Oncological Outcome after Radical Cystectomy - Ileal Conduit for Bladder Cancer Patients in Sanglah General Hospital between January 2013 and December 2016. Open Access Maced J Med Sci 6 (2018): 1647-1651.