Biomarker Analysis in A Randomized Phase 2 Study of Panitumumab Versus Cetuximab in Colorectal Cancer (WJOG6510GTR)
Article Information
Hiroya Taniguchi1,2*#, Yasuhiro Koh3#, Naotoshi Sugimono4, Tomohiro Nishina5, Takao Tamura6, Hiroki Hara7, Taito Esaki8, Tadamichi Denda9, Akitaka Makiyama10,11, Aya Sakai12, Hiroyuki Okuda13, Naoki Izawa14, Takayuki Ando15, Kentaro Yamazaki16, Shinya Tokunaga17, Toshikazu Moriwaki18, Akihito Tsuji19, Hidekazu Kuramochi20, Katsunori Shinozaki21, Yukinori Ozaki22, Hironori Yamaguchi23, Hisateru Yasui24, Satoshi Otsu25, Mio Ikeda3, Junji Kishimoto26, Taroh Satoh27, Daisuke Sakai27, and Kei Muro2
#Authors contributed equally
1Department of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, Chiba, Japan
2Department of Clinical Oncology, Aichi Cancer Center Hospital, Aichi, Japan
3Internal Medicine III, Wakayama Medical University, Wakayama, Japan
4Department of Medical Oncology, Osaka International Cancer Institute, Osaka, Japan
5Department of Gastrointestinal Medical Oncology, National Hospital Organization Shikoku Cancer Center, Ehime, Japan
6Department of Medical Oncology, Kindai University, Osaka, Japan
7Department of Gastroenterology, Saitama Cancer Center, Saitama, Japan
8Department of Gastrointestinal and Medical Oncology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
9Division of Gastroenterology, Chiba Cancer Center, Chiba, Japan
10Department of Hematology/Oncology, Japan Community Healthcare Organization Kyushu Hospital, Fukuoka, Japan
11Cancer Center, Gifu University Hospital, Gifu, Japan
12Department of Gastroenterological Oncology, Hyogo Cancer Center, Hyogo, Japan
13Department of Medical Oncology, Keiyukai Sapporo Hospital, Hokkaido, Japan
14Department of Clinical Oncology, St. Marianna University School of Medicine, Kanagawa, Japan
15Third Department of internal Medicine, University of Toyama, Toyama, Japan
16Division of Gastrointestinal Oncology, Shizuoka Cancer Center, Shizuoka, Japan
17Department of Medical Oncology, Osaka City General Hospital, Osaka, Japan
18Division of Gastroenterology, Faculty of Medicine, University of Tsukuba, Ibaraki, Japan
19Department of Clinical Oncology, Faculty of Medicine, Kagawa University, Kagawa, Japan
20Department of Chemotherapy, Tokyo Women's Medical University Yachiyo Medical Center, Tokyo, Japan
21Division of Clinical Oncology, Hiroshima Prefectural Hospital, Hiroshima, Japan
22Department of Medical Oncology, Toranomon Hospital, Tokyo, Japan
23Department of Clinical Oncology, Jichi Medical University Hospital
24Department of Medical Oncology, Kobe City Medical Center General Hospital, Hyogo, Japan
25Department of Medical Oncology and Hematology, Oita University Faculty of Medicine, Oita, Japan
26Department of Research and Development of Next Generation Medicine, Kyushu University, Fukuoka, Japan
27Department of Frontier Science for Cancer and Chemotherapy, Osaka University Graduate School of Medicine, Osaka, Japan
*Corresponding Author: Dr. Hiroya Taniguchi, Department of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, 6-5-1, Kashiwano-ha, Kashiwa, Chiba 277-8577, Japan
Received: 06 October 2020; Accepted: 16 October 2020; Published: 27 October 2020
Citation: Hiroya Taniguchi, Yasuhiro Koh, Naotoshi Sugimono, Tomohiro Nishina, Takao Tamura, Hiroki Hara, Taito Esaki, Tadamichi Denda, Akitaka Makiyama, Aya Sakai, Hiroyuki Okuda, Naoki Izawa, Takayuki Ando, Kentaro Yamazaki, Shinya Tokunaga, Toshikazu Moriwaki, Akihito Tsuji, Hidekazu Kuramochi, Katsunori Shinozaki, Yukinori Ozaki, Hironori Yamaguchi, Hisateru Yasui, Satoshi Otsu, Mio Ikeda, Junji Kishimoto, Taroh Satoh, Daisuke Sakai, Kei Muro. Biomarker Analysis in A Randomized Phase 2 Study of Panitumumab Versus Cetuximab in Colorectal Cancer (WJOG6510GTR). Journal of Cancer Science and Clinical Therapeutics 4 (2020): 538-549.
View / Download Pdf Share at FacebookAbstract
Background: The randomized phase II WJOG6510G study demonstrated the non-inferiority of panitumumab to cetuximab in terms of progression-free survival (PFS) in patients with wild-type KRAS exon 2 metastatic colorectal cancer. In this study, we performed exploratory analyses of updated survival data using the KRAS exon 2 and RAS/BRAF statuses.
Methods: Patients with wild-type KRAS exon 2 metastatic colorectal cancer who experienced progression after the failure of fluoropyrimidine, oxaliplatin, and irinotecan were randomized to receive panitumumab or cetuximab in combination with irinotecan. An independent central laboratory performed RAS/BRAF testing using a PCR-reverse sequence-specific oligonucleotide method.
Results: In the updated analysis of 121 enrolled patients after a median follow-up of 31.3 months, the median PFS was 5.4 months in the panitumumab arm, versus 4.3 months in the cetuximab arm (hazard ratio [HR]=0.68). The median overall survival (OS) times were 14.9 and 12.5 months in the panitumumab and cetuximab arms, respectively (HR=0.68). In 83 analyzed patients, RAS/BRAF testing identified 19 (23%) and 4 patients (5%) with RAS and BRAF mutations, respectively. In the wild-type RAS and BRAF population, trends of better PFS (6.5 months versus 4.6 months; HR=0.57) and OS (15.3 months versus 11.8 months; HR=0.77) were observed for panitumumab.
Conclusions: In this updated analysis, panitumumab was associated with a modest survival benefit versus cetuximab in patients with centrally confirmed wild-type KRAS exon 2 metastatic colorectal cancer as well as in the wild-type RAS and BRAF population.
Keywords
RAS; BRAF; Panitumumab; Cetuximab; Colorectal cancer
RAS articles; BRAF articles; Panitumumab articles; Cetuximab articles; Colorectal cancer articles
RAS articles RAS Research articles RAS review articles RAS PubMed articles RAS PubMed Central articles RAS 2023 articles RAS 2024 articles RAS Scopus articles RAS impact factor journals RAS Scopus journals RAS PubMed journals RAS medical journals RAS free journals RAS best journals RAS top journals RAS free medical journals RAS famous journals RAS Google Scholar indexed journals BRAF articles BRAF Research articles BRAF review articles BRAF PubMed articles BRAF PubMed Central articles BRAF 2023 articles BRAF 2024 articles BRAF Scopus articles BRAF impact factor journals BRAF Scopus journals BRAF PubMed journals BRAF medical journals BRAF free journals BRAF best journals BRAF top journals BRAF free medical journals BRAF famous journals BRAF Google Scholar indexed journals Panitumumab articles Panitumumab Research articles Panitumumab review articles Panitumumab PubMed articles Panitumumab PubMed Central articles Panitumumab 2023 articles Panitumumab 2024 articles Panitumumab Scopus articles Panitumumab impact factor journals Panitumumab Scopus journals Panitumumab PubMed journals Panitumumab medical journals Panitumumab free journals Panitumumab best journals Panitumumab top journals Panitumumab free medical journals Panitumumab famous journals Panitumumab Google Scholar indexed journals Cetuximab articles Cetuximab Research articles Cetuximab review articles Cetuximab PubMed articles Cetuximab PubMed Central articles Cetuximab 2023 articles Cetuximab 2024 articles Cetuximab Scopus articles Cetuximab impact factor journals Cetuximab Scopus journals Cetuximab PubMed journals Cetuximab medical journals Cetuximab free journals Cetuximab best journals Cetuximab top journals Cetuximab free medical journals Cetuximab famous journals Cetuximab Google Scholar indexed journals Colorectal cancer articles Colorectal cancer Research articles Colorectal cancer review articles Colorectal cancer PubMed articles Colorectal cancer PubMed Central articles Colorectal cancer 2023 articles Colorectal cancer 2024 articles Colorectal cancer Scopus articles Colorectal cancer impact factor journals Colorectal cancer Scopus journals Colorectal cancer PubMed journals Colorectal cancer medical journals Colorectal cancer free journals Colorectal cancer best journals Colorectal cancer top journals Colorectal cancer free medical journals Colorectal cancer famous journals Colorectal cancer Google Scholar indexed journals cetuximab articles cetuximab Research articles cetuximab review articles cetuximab PubMed articles cetuximab PubMed Central articles cetuximab 2023 articles cetuximab 2024 articles cetuximab Scopus articles cetuximab impact factor journals cetuximab Scopus journals cetuximab PubMed journals cetuximab medical journals cetuximab free journals cetuximab best journals cetuximab top journals cetuximab free medical journals cetuximab famous journals cetuximab Google Scholar indexed journals oligonucleotide method articles oligonucleotide method Research articles oligonucleotide method review articles oligonucleotide method PubMed articles oligonucleotide method PubMed Central articles oligonucleotide method 2023 articles oligonucleotide method 2024 articles oligonucleotide method Scopus articles oligonucleotide method impact factor journals oligonucleotide method Scopus journals oligonucleotide method PubMed journals oligonucleotide method medical journals oligonucleotide method free journals oligonucleotide method best journals oligonucleotide method top journals oligonucleotide method free medical journals oligonucleotide method famous journals oligonucleotide method Google Scholar indexed journals fluoropyrimidine articles fluoropyrimidine Research articles fluoropyrimidine review articles fluoropyrimidine PubMed articles fluoropyrimidine PubMed Central articles fluoropyrimidine 2023 articles fluoropyrimidine 2024 articles fluoropyrimidine Scopus articles fluoropyrimidine impact factor journals fluoropyrimidine Scopus journals fluoropyrimidine PubMed journals fluoropyrimidine medical journals fluoropyrimidine free journals fluoropyrimidine best journals fluoropyrimidine top journals fluoropyrimidine free medical journals fluoropyrimidine famous journals fluoropyrimidine Google Scholar indexed journals oxaliplatin articles oxaliplatin Research articles oxaliplatin review articles oxaliplatin PubMed articles oxaliplatin PubMed Central articles oxaliplatin 2023 articles oxaliplatin 2024 articles oxaliplatin Scopus articles oxaliplatin impact factor journals oxaliplatin Scopus journals oxaliplatin PubMed journals oxaliplatin medical journals oxaliplatin free journals oxaliplatin best journals oxaliplatin top journals oxaliplatin free medical journals oxaliplatin famous journals oxaliplatin Google Scholar indexed journals irinotecan articles irinotecan Research articles irinotecan review articles irinotecan PubMed articles irinotecan PubMed Central articles irinotecan 2023 articles irinotecan 2024 articles irinotecan Scopus articles irinotecan impact factor journals irinotecan Scopus journals irinotecan PubMed journals irinotecan medical journals irinotecan free journals irinotecan best journals irinotecan top journals irinotecan free medical journals irinotecan famous journals irinotecan Google Scholar indexed journals
Article Details
Abbreviations:
ADCC- Antibody dependent cellular cytotoxicity; CI- Confidence interval; CRC- Colorectal cancer; ECOG PS- Eastern Cooperative Oncology Group performance status; EGFR- Epidermal growth factor receptor; Fc- Crystalline fragment; FcγRs- Fc gamma receptors; HR- Hazard ratio; OS- Overall survival; PFS- Progression-free survival; RECIST- The Response Evaluation Criteria In Solid Tumors
1. Introduction
KRAS and NRAS (RAS) mutations are present in approximately 50–55% of patients with colorectal cancer (CRC). KRAS exon 2 mutations have been already established as a negative predictive biomarker for anti-epidermal growth factor receptor (EGFR) antibody therapies such as cetuximab or panitumumab [1, 2]. Moreover, prospective and retrospective analyses in pivotal randomized clinical trials consistently revealed that anti-EGFR antibodies are unlikely to provide benefits in patients with KRAS (exons 3 and 4) and NRAS mutations (exons 2, 3, and 4), as previously reported for patients with KRAS exon 2 mutations [3, 4]. On the basis of these results, RAS mutation testing is recommended prior to the initiation of anti-EGFR antibody therapy for patients with metastatic CRC in treatment guidelines to date [5].
BRAF is a serine-threonine kinase that is also located downstream of the EGFR pathway in the Ras/Raf/MAPK pathway. The V600E mutation is the most common BRAF mutation in CRC, being reported in 5–12% of patients with metastatic CRC, and RAS and BRAF V600E mutations are almost mutually exclusive. In addition, BRAF V600E mutations are more frequent in right-sided CRC than in left-sided CRC, and 30% of cases of BRAF-mutant CRC feature coexistent microsatellite instability [6]. Unlike RAS mutations, the predictive value of BRAF mutations regarding the efficacy of anti-EGFR therapy is less certain. Contrarily, the BRAF V600E mutation leads to a poor prognosis or rapid progression regardless of treatment in metastatic CRC [7, 8].
Antibody-dependent cellular cytotoxicity has been identified as a mechanism of cancer cell death. It is mediated by the bifunctional structure of IgG molecules. As a chimeric IgG1 monoclonal antibody, cetuximab has an antigen-binding fragment that may engage the cancer cell antigen and a crystalline fragment (Fc) that binds Fc gamma receptors (FcγRs) on effector cells. FcγRs are composed of three distinct classes, and several studies found that the efficacy of chemotherapeutic regimens including cetuximab is related to FcγR genotypes [9, 10].
Panitumumab is also an EGFR-directed antibody, but it is a fully human IgG2 monoclonal antibody, thereby differing from cetuximab. We previously reported the main results of the WJOG6510G trial, which is a head-to-head, randomized phase 2 study of panitumumab versus cetuximab, both in combination with irinotecan, for use in patients with locally evaluated wild-type KRAS exon 2 metastatic CRC. The study found that panitumumab was associated with favorable progression-free survival (PFS) and overall survival (OS) compared with cetuximab. The median PFS was 5.4 months in the panitumumab arm versus 4.3 months in the cetuximab arm (hazard ratio [HR]=0.64; 95% confidence interval [CI]=0.44–0.94; p < 0.001 for non-inferiority, p=0.06 for superiority), and the median OS times in these groups were 14.9 and 11.5 months (HR=0.68; 95% CI=0.45–1.03; p=0.05), respectively [11].
In this study, we reported biomarker analyses of the WJOG6510G study to determine whether centrally evaluated extended RAS and BRAF mutations and FcγR polymorphisms are related to the efficacy of cetuximab and panitumumab in combination with irinotecan.
2. Materials and Methods
2.1 Patients
This WJOG6510GTR trial is an accompanying biomarker study (UMIN000008786) of WJOG6510G (UMIN000006643). Concerning the WJOG6510G trial, detailed information regarding patient inclusion criteria, study design, and treatment schedules was previously reported. Briefly, the major eligible criteria were as follows: histologically confirmed unresectable metastatic CRC; lesions that are refractory or intolerant to fluorouracil-, oxaliplatin-, and irinotecan-based chemotherapy; wild-type KRAS exon 2 based on the local assessment; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2; presence of measurable disease as defined by the Response Evaluation Criteria In Solid Tumors (RECIST) v1.1; and adequate hematologic, renal, hepatic, and metabolic function. Patients previously treated with an anti-EGFR antibody were excluded. These trials were conducted according to the ethical principles of the Declaration of Helsinki, and the study protocol was approved by the institutional review board of the participating institutions. All patients provided written informed consent.
2.2 Treatment and efficacy assessment
Patients were randomized on a 1:1 basis to receive panitumumab (6 mg/kg) intravenously on day 1 of each 14-day cycle or cetuximab at an initial intravenous dose of 400 mg/m2 followed by 250 mg/m2 intravenously on day 1 of each 7-day cycle. Irinotecan at 150 mg/m2 was administered intravenously in combination with panitumumab or cetuximab every 2 weeks. The starting dose of irinotecan could be reduced to 100 or 120 mg/m2 in patients who required dose reduction during prior treatment. Treatment continued until disease progression, intolerability, or withdrawal of consent. For efficacy assessment, tumor assessments were repeated every 8 weeks from randomization to discontinuation of the protocol treatment. Responses were assessed by each investigator according to RECIST v1.1, and there was no central review of response. Laboratory assessments were assessed at screening, baseline, and then once every 2 weeks thereafter.
2.3 RAS and BRAF mutation analysis
In this biomarker analysis, RAS and BRAF mutations were centrally assessed in a central laboratory (G&G Science Co., Ltd., Fukushima, Japan). Genomic DNA was extracted from formalin-fixed, paraffin-embedded CRC tissues obtained from biopsies or surgical specimens. Extracted DNA samples were diluted to a concentration of 10–20 ng/μL using sterile TE buffer (1 mmol/L Tris–HCL [pH 8.0], 0.1 mmol/L EDTA). Assays were performed according to the manufacturer's protocol using a bead-based multiplexed immunoassay system (xMAP technology; Luminex). Mutations in KRAS and NRAS codons 12, 13, 59, 61, 117, and 146 were detected using the PCR-based MEBGEN RASKET kit (MBL, Japan) [12]. We also used the Genosearch BRAF kit to detect BRAF V600 mutations (V600E, V600K, V600R, and V600D) and mutations such as those in exon 11, including mutations in codons 464 (G464E, G464V, and G464R), 466 (G466R, G466V, and G466E), 467 (S467L), 469 (G469A, G469V, G469R, and G469E), 485 (L485F), 524 (Q524L), 525 (L525R), 581(N581S, N581I, and N581T), 594 (D594N and D594G), 596 (D596R), 597 (L597R, L597S, L597V, L597Q, and L597P), 598 (A598T), 599 (T599_600insT), and 601 (V601E and V601N) [13].
2.4 Examination of FcγR genotyping
Samples were collected in blood collection tubes containing ethylenediaminetetraacetic acid disodium salt as an anticoagulant, and genomic DNA samples were extracted using a QIAamp DNA Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's protocol. The genotyping of FcγR2A H131R (rs180127), FcγR3A V158F (rs396991), and FcγR2B I232T (rs1050501) polymorphisms was conducted using TaqMan SNP Genotyping Assays C_9077561_20 and C_25815666_10 and the custom-designed assay AHHS8M6 (Applied Biosystems, Foster City, CA, USA), respectively, according to the manufacturer's protocols with modifications.
2.5 Statistical analysis
Fisher's exact test and a trend test were used to calculate P values for the associations between genetic parameters and treatment efficacy. OS was defined as the time from registration to death from any cause. PFS was defined as the time from registration to the first appearance of progression or death from any cause. The associations of patient characteristics with OS were examined using Cox proportional hazards regression models. Data are reported as HR estimates with their 95% CIs. The associations of genetic parameters with OS and PFS were analyzed using Kaplan–Meier curves and the log-rank test. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria) [14]. More precisely, EZR is a modified version of R commander (version 1.6-3) that includes statistical functions used frequently in biostatistics. All P values were two-sided, and P < 0.05 denoted statistical significance.
3. Results
3.1 Patients and updated efficacy
In the WJOG6510G trial, a total of 121 patients were enrolled between December 2011 and September 2014, and 1 patient was excluded from the full analysis set (FAS) because of ineligibility. Therefore, the FAS included 59 patients in the cetuximab arm and 61 patients in the panitumumab arm (Supplemental Figure 1). The cutoff date for the initial primary analysis was September 2015, whereas that for the updated analysis was March 2017. The median follow-up times for the analyses were 35.1 and 32.7 months in the cetuximab and panitumumab arms, respectively. Patient and disease characteristics were well balanced between the two arms (Table 1). The median patient age was 64 years, and >85% of patients had left-sided CRC. At the time of the updated analysis, all FAS patients were evaluable for efficacy. Of these patients, 117 patients (97.5%) experienced disease progression, and 113 patients (94.2%) died. Concerning PFS, which is the primary endpoint of the WJOG6510G trial, a significant benefit in favor of panitumumab was observed (HR=0.68; 95% CI=0.46–0.98; p=0.035; Figure 1a). The median PFS was 4.3 months in the cetuximab arm, versus 5.4 months in the panitumumab arm. A similar trend was also observed for OS, as indicated by median OS times of 12.5 and 14.9 months in the panitumumab and cetuximab groups (HR=0.68; 95% CI=0.47–0.98; p=0.037; Figure 1b), respectively.
|
Whole population |
RAS/BRAF wild-type |
||||
|
Cetuximab (n=59) |
Panitumumab (n=61) |
Cetuximab (n=32) |
Panitumumab (n=30) |
||
|
Age (years), median (range) |
64 (33-79) |
64 (41-80) |
63.5 (33-75) |
66 (41-79) |
|
|
Sex |
Male |
37 (62.7%) |
42 (68.9%) |
21 (65.6%) |
19 (63.3%) |
|
Female |
22 (37.3%) |
19 (31.1%) |
11 (34.4%) |
11 (36.7%) |
|
|
ECOG PS |
0 |
32 (54.2%) |
38 (62.3%) |
17 (53.1%) |
19 (63.3%) |
|
1 |
26 (44.1%) |
22 (36.1%) |
14 (43.8%) |
11 (36.7%) |
|
|
2 |
1 (1.7%) |
1 (1.6%) |
1 (3.1%) |
0 (0%) |
|
|
Tumor locationb |
Right sided |
7 (11.9%) |
9 (14.8%) |
3 (9.4%) |
4 (13.3%) |
|
Left sided |
52 (88.1%) |
52 (85.2%) |
29 (90.6%) |
26 (86.7%) |
|
|
Primary tumor location |
Present |
10 (16.9%) |
11 (18.0%) |
5 (15.6%) |
5 (16.7%) |
|
Resected |
49 (83.1%) |
50 (82.0%) |
27 (84.4%) |
25 (83.3%) |
|
|
Histology |
Low grade |
3 (5.1%) |
6 (9.8%) |
1 (3.2%) |
2 (6.7%) |
|
High grade |
56 (94.9%) |
55 (90.2%) |
31 (96.8%) |
28 (93.3%) |
|
|
Number of metastatic sites |
1 |
20 (33.9%) |
30 (49.1%) |
9 (28.1%)a |
13 (43.3%) |
|
≥2 |
39 (66.1%) |
31 (50.9%) |
22 (67.8%) |
17 (56.7%) |
|
|
Time from starting first-line chemotherapy |
≤18 months |
28 (47.4%) |
18 (29.5%) |
18 (56.2%) |
9 (30.0%) |
|
>18 months |
31 (52.6%) |
43 (70.5%) |
14 (43.8%) |
21 (70.0%) |
|
a One patient had unresectable colorectal cancer without a metastatic site. b Primary tumors proximal to the splenic flexure were considered right-sided tumors, and those at or distal to the splenic flexure were considered left-sided tumors. ECOS PS, Eastern Cooperative Oncology Group performance status
Table 1: Patients’ backgrounds.
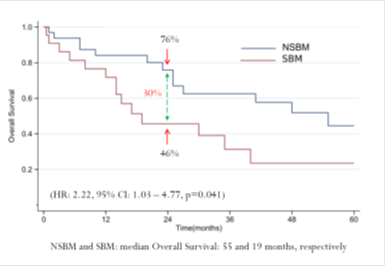
Figure 1: Updated survival outcomes in the whole population. Cmab, cetuximab; Pmab, panitumumab; IRI, irinotecan; HR, hazard ratio; CI, confidence interval; mPFS, median progression-free survival; mOS, medial overall survival; M, months
3.2 Efficacy according to the RAS and BRAF mutation status
Of the 120 patients included in the WJOG6510G study, the RAS and BRAF mutation status was evaluable in 86 patients (71.7%; Supplemental Figure 2). Samples from the remaining 34 patients were not available because of uncollectible tumors, insufficient residual tumors, or failure of the assay. Of the evaluable tumors, RAS mutations were identified in 19 tumors, including 9 tumors with KRAS exon 2 mutations. BRAF mutations were found in five patients, including the V600E mutation in three patients. All RAS and BRAF mutations were mutually exclusive in this study; therefore, 62 tumors were deemed wild-type for RAS and BRAF. In the survival analysis, clearly better survival was demonstrated in patients with wild-type RAS and BRAF tumors than in those with RAS- or BRAF-mutant tumors (Table 2). No patients with RAS-mutant or BRAF-mutant tumors responded to treatment, excluding one patient with a BRAF G466E mutation who responded to panitumumab plus irinotecan (Supplemental Table 1 and Supplemental Figure 3).
Comparing the cetuximab and panitumumab arms in the RAS and BRAF wild-type population, no differences in patient background were observed between the two arms. A clear and significant benefit associated with panitumumab treatment versus cetuximab was apparent for PFS (median, 6.5 months versus 4.6 months; HR=0.57; 95% CI=0.34–0.98; p=0.038; Figure 2a). In terms of OS, the panitumumab arm displayed numerically better survival without reaching significance (median, 15.3 months versus 11.8 months; HR=0.77; 95% CI=0.45–1.30; p=0.32; Figure 2b).
|
RAS/BRAF wild |
RAS mutant |
BRAF mutant |
p value |
||
|
(n=62) |
(n=19) |
(n=5) |
|||
|
Progression-free survival |
Number of events |
62 |
19 |
5 |
p=0.0013 |
|
Median (months) |
5.3 |
2.8 |
4.4 |
||
|
Overall survival |
Number of events |
58 |
19 |
5 |
p=0.11 |
|
Median (months) |
14 |
9.7 |
8.8 |
||
Table 2: Survival outcomes according to RAS/BRAF status.
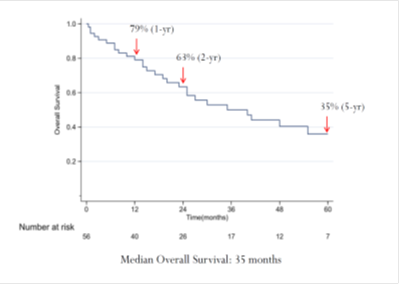
Figure 2: Updated survival outcomes in the wild-type RAS/BRAF population. Cmab, cetuximab; Pmab, panitumumab; IRI, irinotecan; HR, hazard ratio; CI, confidence interval; mPFS, median progression-free survival; mOS, medial overall survival; M, months
3.3 Analysis of FcγR polymorphisms
The analysis of FcγR polymorphisms could only be performed in 36 patients (30.0%). The FcγR2A distribution did not concur with the expected distribution (HH, 66.7%; HR, 27.8%; RR, 5.6%). The frequencies of FcγR3A polymorphisms for VV, VF, and FF were 8.3%, 38.9%, and 41.2%, respectively (Supplemental Table 2). Four patients were not categorized because of existing copy number variation for FcγR3A. In the analysis of the relationships between FcγR polymorphisms and response rate, no clear difference was noted between the panitumumab and cetuximab arms (Supplemental Table 3). FcγR2B polymorphisms for II, IT, and TT were found in 69.4%, 27.8%, and 2.8%, respectively, of patients, and no clear relationship to treatment efficacy was observed.
4. Discussion
The aim of our post hoc analysis of the WJOG6510G study was to investigate the effects of RAS and BRAF mutations on treatment efficacy. In the evaluable patients with wild-type RAS and BRAF tumors, better survival outcomes were observed in the panitumumab arm, in line with the results for the FAS population in the original report for WJOG6510G. As discussed in the original paper, surprisingly better PFS and OS were observed in the panitumumab arm. Although the mechanisms of these findings are unclear, possible explanations include prior bevacizumab exposure. For example, panitumumab has greater affinity for EGFR than cetuximab, permitting the former drug to bind the receptor under hypoxic conditions after bevacizumab therapy. A subgroup analysis of the ASPECCT trial, which was also a head-to-head comparison of panitumumab and cetuximab monotherapy [15], also reported trends of better PFS and OS in favor of panitumumab in patients who previously received bevacizumab [16]. The true mechanism remains unknown, but the RAS/BRAF analysis in this paper supports that the imbalance of patients with RAS-mutant tumors does not explain the difference in outcome in the WJOG6510G trial.
The method of RAS testing sometimes affects the results for the RAS mutation status. In fact, we identified nine patients with discordant results between the local and central assessment of the KRAS exon 2 status. For central RAS testing, we selected the PCR-based MEBGEN™ RASKET assay with a detection limit of at least 1–5%, which was better than that of the direct sequencing method used for local assessment. In total, we newly identified 19 patients with RAS-mutant tumors, none of whom responded to anti-EGFR antibody plus irinotecan therapy (data not shown). Although the significance of low-prevalence KRAS or RAS mutations in relation to the effectiveness of anti-EGFR antibody therapy in metastatic CRC remains unclear [17, 18], this PCR-based assay is appropriate for selecting patients with anti-EGFR therapy considering our results. To date, the MEBGEN™ RASKET-B kit, which can detect 48 types of RAS mutations and the BRAF V600E mutation using the same method as the RASKET kit in the present study, is approved and widely used in Japanese clinical practice [19].
The effects of BRAF mutations on the efficacy of anti-EGFR therapy are also controversial. In this study, only 3/86 (3.5%) wild-type KRAS exon 2 tumors harbored the BRAF V600E mutation. Ethnic differences in the distribution of the BRAF V600E mutation between Western countries (8–15%) and Japan (approximately 5%) have been reported [20], but the low prevalence in this study was mainly attributable to the poor prognosis of patients carrying the BRAF V600E mutation. This study included patients who previously received two or more lines of therapy; therefore, it is difficult to enroll patients with the BRAF V600E mutation and aggressive tumors in later-line clinical trials such as this study. Although clinical trials in the first-line setting demonstrated the benefits adding anti-EGFR antibody even among patients with BRAF V600E tumors [21], these tumors hardly responded to anti-EGFR therapy in later lines [22], in line with our findings that none of three patients with the BRAF V600E mutation responded to the combination of an anti-EGFR antibody plus irinotecan.
Differing from the findings in patients carrying the BRAF V600E mutation, some patients with BRAF non-V600E mutations respond to anti-EGFR therapy. Yao et al. classified BRAF mutations into three groups based on their biochemical and signaling mechanisms [23]. Among these, class 3 BRAF mutants exhibit low kinase activity or kinase death, but they activate the MAPK pathway through enhanced RAS binding and subsequent RAS-dependent CRAF activation, which could render them sensitive to anti-EGFR therapy [24]. In line with this previous report, one patient with the BRAF G469 mutation in our cohort responded to panitumumab plus irinotecan therapy. When comprehensive genomic profiling using next-generation sequencing becomes a standard of care in patients with metastatic CRC, more patients with BRAF non-V600E mutations will be observed in daily clinical practice. At present, we will continue to apply anti-EGFR therapy according to the BRAF non-V600E classification.
We found no significant association between FcγR polymorphisms and the clinical response to cetuximab and panitumumab treatment in this study. We had assumed that polymorphisms, especially those in FcγR2A and/or FcγR3A, are predictive biomarkers between cetuximab and panitumumab before starting enrollment in this study because cetuximab, which is a chimeric IgG1 antibody, has greater potential to induce antibody dependent cellular cytotoxicity (ADCC) than panitumumab, which is a fully human IgG2 antibody. Some previous studies found that the FcγR2A 131H/H and FcγR3A 158F/F genotypes are associated with longer PFS, although another study found no association of FcγR2A or FcγR3A polymorphisms with survival, in line with our study [25]. Although we could analyze FcγR polymorphisms in only 38 patients, all 3 patients with FcγR2A 131H/R and FcγR3A 158F/V polymorphisms exhibited partial responses to treatment with panitumumab, which was not expected to induce ADCC.
Because of the relatively small sample sizes in the RAS, BRAF, and FcγR analyses, there were risks of imbalances in important prognostic factors despite patient randomization into the treatment arms. For this reason, we should consider potential differences in important baseline characteristics. To date, ECOG PS and tumor location are considered powerful prognostic factors for anti-EGFR therapy [26]. In this study, no clear difference in survival was observed between the study arms even though nearly 90% of patients had left-sided tumors, which were expected to have greater responses to anti-EGFR therapy than right-sided tumors. We did not adjust for these prognostic factors because of the small sample sizes, but consideration of these factors would not change the results.
5. Conclusions
In conclusion, our study supports the benefits of panitumumab plus irinotecan versus cetuximab plus irinotecan in the treatment of metastatic CRC in patients with wild-type RAS tumors. On the basis of the lack of an observed response, anti-EGFR therapy is contraindicated for patients with RAS-mutant tumors. The benefits of anti-EGFR therapy for BRAF V600E mutant tumors may be very small or nonexistent, whereas substantial benefits were observed in patients with BRAF non-V600E mutant tumors. However, further data are required to clarify these observations.
Acknowledgments
We thank the patients, investigators, and institutions involved in this study. We also thank the data managers and other support staff of the West Japan Oncology Group (WJOG), especially Dr. Shinichiro Nakamura and Kaori Mori.
Funding
This study was supported by WJOG, a non-profit organization, and funded by WJOG Funds.
References
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359 (2008): 1757-1765.
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26 (2008): 1626-1634.
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369 (2013): 1023-1034.
- Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 33 (2015): 692-700.
- Yamazaki K, Taniguchi H, Yoshino T, et al. Japanese Society of Medical Oncology Clinical Guidelines: Molecular testing for colorectal cancer treatment, Third edition. Cancer Sci 109 (2018): 2074-2079.
- Chen D, Huang JF, Liu K, et al. BRAFV600E mutation and its association with clinicopathological features of colorectal cancer: a systematic review and meta-analysis. PLoS One 9 (2014): e90607.
- Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin CancerRes 20 (2014): 5322-5330.
- Mitani S, Taniguchi H, Sugiyama K, et al. The impact of the Glasgow Prognostic Score on survival in second-line chemotherapy for metastatic colorectal cancer patients with BRAF V600E mutation. Ther Adv Med Oncol 11 (2019): 1758835918820298.
- Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol 27 (2009): 1122-1129.
- Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 25 (2007): 3712-3718.
- Sakai D, Taniguchi H, Sugimoto N, Tamura T, et al. Randomised phase II study of panitumumab plus irinotecan versus cetuximab plus irinotecan in patients with KRAS wild-type metastatic colorectal cancer refractory to fluoropyrimidine, irinotecan and oxaliplatin (WJOG 6510G). Eur J Cancer 135 (2020): 11-21.
- Yoshino T, Muro K, Yamaguchi K, et al. Clinical validation of a multiplex kit for rasmutations in colorectal cancer: Results of the RASKET (RAS KEy Testing) prospective, multicenter study. EBioMedicine 2 (2015): 317-323.
- Osumi H, ShinozakiE, Wakatsuki T, et al. Non-V600E BRAF mutations and EGFR signaling pathway in colorectal cancer. Int J Cancer 145 (2019): 2488-2495.
- Kanda Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48 (2013): 452-458.
- Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 15 (2014): 569-579.
- Price T, Kim TW, Li J, et al. Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur J Cancer 68 (2016): 51-59.
- Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 33 (2015): 692-700.
- Molinari F, Felicioni L, Buscarino M, et al. Increased detection sensitivity for KRAS mutations enhances the prediction of anti-EGFR monoclonal antibody resistance in metastatic colorectal cancer. Clin Cancer Res 17 (2011): 4901-4914.
- Taniguchi H, Okamoto W, Muro K, et al. Clinical validation of newly developed multiplex kit using Luminex xMAP technology for detecting simultaneous RAS and BRAF mutations in colorectal cancer: Results of the RASKET-B study. Neoplasia 20 (2018): 1219-1226.
- Yamazaki K, Taniguchi H, Yoshino T, et al. Japanese Society of Medical Oncology Clinical Guidelines: Molecular testing for colorectal cancer treatment, Third edition. Cancer Sci 109 (2018): 2074-2079.
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 48 (2012): 1466-1475.
- Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol 14 (2013): 749-759.
- Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 548 (2017): 234-238.
- Yaeger R, Kotani D, Mondaca S, et al. Response to anti-EGFR therapy in patients with BRAF non-V600-mutant metastatic colorectal cancer. Clin Cancer Res 25 (2019): 7089-7097.
- Geva R, Vecchione L, Kalogeras KT, et al. FCGR polymorphisms and cetuximab efficacy in chemorefractory metastatic colorectal cancer: an international consortium study. Gut 64 (2015): 921-928.
- Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 28 (2017): 1713-1729.
