Bioavailability of essential amino acids after ingestion of fortified biscuits with a mixture of essential amino acid in healthy subjects
Article Information
Federico Vignati1, Luca Barbieri2, Claudio Macca3, Gianleone Di Sacco1, Paola Galli4, Carmelo Iacobello4, Francesca Maffina4, Monica Marini4, Andrea Vignati1, Michela Del Prete1* and Fabrizio Muratori1
1Division of Endocrinology and Diabetology, Sant’Anna Hospital - ASST Lariana, Como, Italy
2Chef Supervisor, Cucinalinearemetabolica, Italy.
3Division of Dietetics and Clinical Nutrition, Humanitas Gavazzeni Institute, Bergamo, Italy
4Department of Laboratory Diagnostic, Chemical-Clinical Analysis, Spedali Civili Hospital, Brescia, Italy
*Corresponding Author: Michela Del Prete, Division of Endocrinology and Diabetology, Sant’Anna Hospital - ASST Lariana, Como, Italy
Received: 07 October 2023; Accepted: 17 October 2023; Published: 12 January 2024
Citation: Federico Vignati, Luca Barbieri, Claudio Macca, Gianleone Di Sacco, Paola Galli, Carmelo Iacobello, Francesca Maffina, Monica Marini, Andrea Vignati, Michela Del Prete and Fabrizio Muratori. Bioavailability of essential amino acids after ingestion of fortified biscuits with a mixture of essential amino acid in healthy subjects. Journal of Food Science and Nutrition Research. 7 (2024): 07-13.
View / Download Pdf Share at FacebookAbstract
Background and Aim: Protein enriched food or beverages are popular in sport trainers and are used in protein malnourished or sarcopenic subjects. Alternatively, promising results are obtained after administration of free essential amino acids (EAA) mixtures both in health and in disease. In this preliminary study, a biscuit dough fortified containing 8 g of a mixture of nine EAA in 100 g of biscuit was developed.
Methods: To test the bioavailability of EAA of this fortified dough, ten healthy subjects received a fortified biscuit containing 8 g of EAA in 100 g of biscuits. The plasma amino acid profile was measured by HPLC before and every 30 minutes for two hours after the ingestion of these biscuits and compared in the same subjects to the amino acid profile induced by the ingestion of not fortified biscuits. A taste questionnaire using a category scale for rating taste intensity was used to test the taste of both fortified and classic biscuits.
Results: All subjects exhibit a significant increase of EAA levels from baseline (fortified biscuit: p < 0.0026). Compared to the not fortified biscuit, the mean plasma EAA concentrations increase was 48.0 % versus 3.5 % from baseline. A significant statistical difference of the absolute and relative variations of EAA concentration peak was observed after the ingestion of both biscuits (absolute variation p < 0.0001; percent variation p<0.0001). Due to the composition of the EAA mixture, a mean increase up to 66% in branched chain amino acids and lysine was observed after ingestion of the fortified biscuit. All the subjects liked the taste of classic and fortified biscuits.
Conclusions: This preliminary study demonstrates that free EAA contained in this fortified dough are bioavailable and sufficient to induce a clear-cut increase in plasma EAA post ingestion. Such a dough might be used to obtain EAA fortified products, useful in sarcopenia, protein malnutrition and muscle accretion.
Keywords
Bioavailability, Essential amino acids, Fortified biscuits
Article Details
Introduction
A balanced diet is essential for health and should be adapted to the needs derived from concomitant diseases or situations including adequate energy, essential amino acids (EAA), vitamins and trace elements. In particular, muscle anabolism needs adequate protein intake and about 0.8-1.0 g of protein/kg of body weight (BW) are necessary in healthy adults to maintain protein turnover [1]. In elderly subjects, for the presence of anabolic resistance, 1.1-1.2 g of protein/kg BW are recommended and even higher intake of protein in case of acute or chronic disease [2]. Protein source may influence muscle protein synthesis (MPS) being vegetable proteins less effective than animal proteins in muscle accretion [3]. The AA composition of a protein is the main factor influencing nitrogen retention and the presence of adequate amounts of EAA, particularly leucine [2,4], is probably the most important variable in this context. Otherwise, not EAA (NEAA) are dispensable to ameliorate muscle protein synthesis [5,6]. Some vegetable proteins, such those derived from cereals, have a poor protein anabolic action because of the negligible content in lysine, that is considered the limiting AA. To improve the anabolic power of those proteins, lysine fortification [7] or development of mixed dough associating different cereals [8] or milk-derived proteins has been used [9]. More recently a mixed cereals therapeutic food was ameliorated by adding free, crystalline amino acids in little amount [10,11]. EAA fortification is difficult because of the bitter or unpleasant taste of the majority of EAA that can alter the taste of the natural food in which are incorporated [12]. To date, to our knowledge, EAA are administered as medical aids or foods for special medical purposes, but they have never been included in a baked preparation. In order to achieve a high number of possible uses of EAA it would be advisable to be able to administer the EAA by introducing them into easily usable foods (for example a biscuit). The fortification with EAA of a common food like a biscuit is in line with many researches demonstrating that supplementation with well calibrated EAA mixtures might exert equal or even more effective muscle anabolic action than whole protein in elderly subjects [13]. In clinical practice EAA mixtures are used as supplement in sarcopenic patients with chronic disease such as heart failure or chronic obstructive pulmonary disease [14-16]. The aim of our preliminary study was therefore to evaluate the bioavailability of EAAs after cooking through the ingestion of a biscuit containing 8% of EAAs in a series of healthy subjects. A biscuit without the addition of EAA was then administered to the same subjects for comparison.
Material and Methods
Subjects:
In the preliminary study ten healthy subjects (9 males and 1 female) after giving written informed consent were enrolled. The number of subjects enrolled was defined by the voluntary adherence to this study by 10 healthy subjects. The mean age was 44.2±19.2 years (range: 22-65) with a mean BMI of 23.87±2.17 kg/m2 (range: 21.00-28.20). None of the subjects was taking any medication or suffering chronic diseases. Due to its characteristics (food administration) the study did not require submission to an ethics committee.
Study protocol
An international chef (L.B.) has devised a method for cooking a biscuit fortified with EAAs which are able to demonstrate their bioavailability even after cooking (this method was patented in Italy 2017 with patent number 102017000032767). An abstention from physical activity for about 7 days, an overnight fast and after achieving a venous access, all subjects were instructed to eat in five minutes 100 g of biscuits prepared with the EAA-fortified dough (containing 8 g of EAA in 100 g of each biscuit), accompanied by 500 mL of water. Blood samples for AA analysis were collected immediately before and every 30 minutes for 2 hours after the ingestion of biscuits. To assess if classical biscuit dough, that contain natural proteins derived from wheat and egg, might induce significant modifications in amino acid profile, the same procedure was performed (after at least 15 days from the administration of the fortified biscuit) after the ingestion of 100 g of a classic biscuit in the same subjects previously tested. Blood samples have been analyzed using Ion Exchange Chromatography (IEC). EAA were analyzed by using the Biochrom Physiological Amino acids (Cambridge, UK) [17].
EAA-fortified dough
A classic biscuit dough was fortified with 8% of EAA with this proportion: 21,3% Leucine, 17,9% lysine, 10,3% Isoleucine, 10% valine, 9,1% Threonine, 7% phenylalanine, 6,7%, 5,1% methionine, 4,3% tryptophan, 2,9% histidine and two NEAA were in these proportion: 6,7% tyrosine and 5% cysteine. The mixture of EAAs and their percentage was extrapolated from the EAA content present in the egg, a food with a high biological value. The AA composition of ovalbumin that inspired the proportion of amino acid and absolute quantities present in 100 g of fortified biscuits are described in table 1. Because the dough also includes animal protein from chicken yolk, sugar, wheat and fat, the macronutrient composition of both kind of biscuits is described in table 2. A modified taste questionnaire using a category scale for rating taste intensity for salty, sour, bitter, sweet, no flavor and fat flavor and in which the following question was added: do you like the taste of the biscuit? yes – no, was used to assess the taste of classic and fortified biscuits rating from 0 to 12 the palatability [18].
Statistical analysis
Results are expressed as mean ± SE. A two tailed paired student’s t-test was performed to compare differences between basal to peak level for each amino acid and unpaired student’s t test between groups. Data distribution was evaluated with the Kolmogorov-Smirnov test. Data were analyzed using GraphPad Prism software (version 9, GraphPad Software Inc., La Jolla, CA).
Results
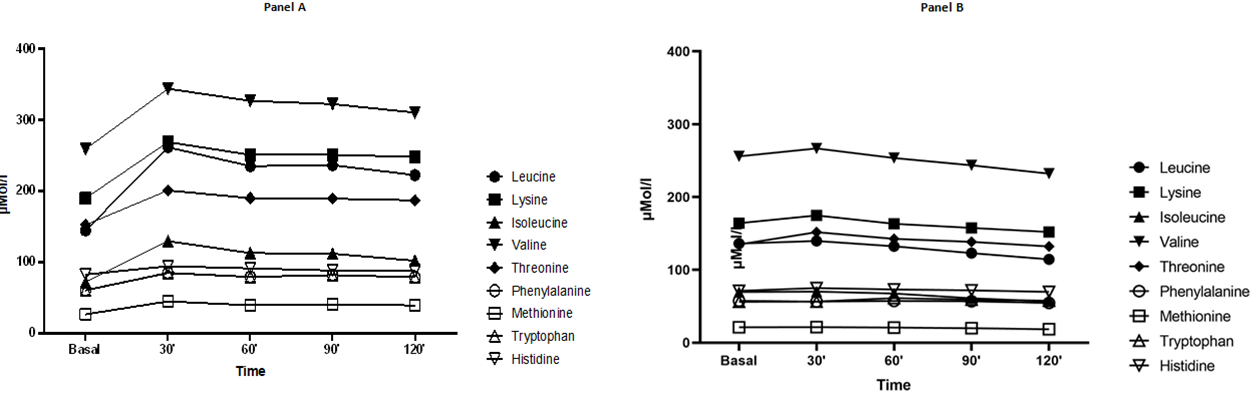
The mean EAA plasma concentration peak, reported as percent increase and observed 30 minutes after fortified biscuit ingestion, was 48.0 ± 23.4% from baseline, while after classic biscuit ingestion was only 3.5±4.3%. Plasma concentrations of all EAA significantly increased after ingestion of fortified biscuits from baseline and was maintained high up to 120 minutes (p < 0.0026; Figure 1). A statistical significance was also observed after the ingestion of the classic biscuit form baseline (p < 0.0036). Figure 1 shows the plasma behavior of each EAA up to 120 minutes from baseline of both fortified and classic biscuits (Figure 1, Panel A and B). Comparing the absolute and the percent variations in EAA concentration peak from baseline after the ingestion of both biscuits there was a statistical significance (absolute variation p < 0.0001; percent variation p<0.0001; Table 3). A preliminary sub-analysis based on the age of participants showed that the mean basal plasma EAA profile did not differ significantly between young and older subjects (p = 0.586) nor the mean peak of plasma EAA after ingestion of fortified biscuits (p = 0.562). After tasted the fortified and the classic biscuits, all subjects responded to the modified taste questionnaire without finding significant differences. All subjects liked the taste of both biscuits (Table 4).
|
Amino acid |
% |
mg/100 g |
|
leucine |
21.37 |
1710 |
|
Isoleucine |
10.34 |
828 |
|
Valine |
10 |
800 |
|
Lysine |
17.93 |
1434 |
|
Methionine |
5.17 |
414 |
|
Threonine |
9.13 |
731 |
|
Histidine |
2.93 |
234 |
|
Phenylalanine |
7.06 |
566 |
|
Tyrosine |
6.72 |
538 |
|
Tryptophan |
4.31 |
345 |
|
Cysteine |
5 |
400 |
Table 1: Amino acidic formula used for enrichment of the dough.
|
Classical biscuit |
8%EAA fortified biscuit |
|
|
Mean value (g) |
100 |
100 |
|
Energy (Kj/Kcal) |
1992/475 |
1966/469 |
|
Fats (g) |
18.7 |
17.2 |
|
CHO (g) |
68 |
62 |
|
Protein (g) |
7.1 |
14.5 |
|
of which free EAA |
- |
8 |
|
Fibers (g) |
2.9 |
2.7 |
|
Salt (g) |
0.95 |
0.87 |
Table 2: Macronutrient’s composition of classical and EAA fortified biscuits.
|
Subjects |
Classic biscuit |
Fortified biscuit |
|||||||
|
Minutes from baseline |
Minutes from baseline |
||||||||
|
30 |
60 |
90 |
120 |
30 |
60 |
90 |
120 |
||
|
Leucine |
µMol/l* |
3.5 |
-3.7 |
-13 |
-21.6 |
81.1 |
117 |
90.6 |
91.8 |
|
%* |
2.6 |
-2.7 |
-9.5 |
-15.8 |
66 |
62.7 |
63.6 |
54 |
|
|
Lysine |
µMol/l |
10.6 |
-0.9 |
-6.5 |
-12.1 |
41.7 |
79 |
61.2 |
61.3 |
|
% |
6.5 |
-0.6 |
-3.9 |
-7.4 |
36.1 |
32.3 |
32.3 |
30.8 |
|
|
Isoleucine |
µMol/l |
0.5 |
-2.2 |
-8.6 |
-12,9 |
80.6 |
57.7 |
40.9 |
40.1 |
|
% |
0.8 |
-3.2 |
-12.4 |
-18.5 |
65.9 |
57.1 |
56 |
42.2 |
|
|
Valine |
µMol/l |
11 |
-2.3 |
-12.2 |
-23.8 |
32.7 |
84.8 |
67.8 |
63.3 |
|
% |
4.3 |
-0.9 |
-4.8 |
-9.3 |
27.3 |
26.2 |
24.4 |
19.7 |
|
|
Threonine |
µMol/l |
16.7 |
7.7 |
3.5 |
-2.9 |
31.9 |
48.5 |
37.7 |
36.9 |
|
% |
12.4 |
5.7 |
2.6 |
-2.1 |
25.7 |
24.8 |
24.3 |
22.8 |
|
|
Phenylalanine |
µMol/l |
-1 |
-0.2 |
-0.7 |
-3 |
40.6 |
24.5 |
18.8 |
21.4 |
|
% |
-1.8 |
-0.3 |
-1.2 |
-5.2 |
25.5 |
31.1 |
35.5 |
30.7 |
|
|
Methionine |
µMol/l |
0.2 |
-0.4 |
-1.3 |
-2.8 |
68.8 |
18.2 |
12.8 |
13.9 |
|
% |
0.8 |
-2.1 |
-5.9 |
-13.1 |
60.9 |
48.8 |
52.6 |
47.2 |
|
|
Tryptophan |
µMol/l |
0.1 |
4.9 |
2.9 |
1.3 |
40.3 |
24.3 |
18.8 |
21.4 |
|
% |
0.2 |
8.7 |
5.2 |
2.3 |
25.5 |
31.1 |
35.5 |
30.7 |
|
|
Histidine |
µMol/l |
3.9 |
2 |
0.8 |
-1.3 |
14.1 |
11.7 |
8.5 |
5.7 |
|
% |
5.4 |
2.9 |
1.1 |
-1.9 |
11.9 |
10.3 |
6.9 |
5.9 |
|
*Absolute and relative variations of mean EAA plasma concentrations from baseline after ingestion of classic and fortified biscuit. T test for unpaired data was p < 0.0001 for absolute variations and p < 0.0001 for relative variations.
Table 3: Absolute and relative mean EAA plasma variations from baseline of classic and fortified biscuits.
|
Classic biscuit |
Fortified Biscuit |
|
|
Mean score |
Mean score |
|
|
Salty |
0 |
0 |
|
Sour |
0 |
0 |
|
Bitter |
0 |
0 |
|
Sweet |
8 |
7 |
|
No flavor |
0 |
0 |
|
Fat flavor |
2 |
3 |
|
Do you like the taste of the biscuit? |
Yes 100% |
Yes 100% |
Table 4: Taste questionnaire scores for classic and fortified biscuits.
Discussion
Our preliminary data demonstrate that the ingestion of this EAA-fortified biscuit can induce a rapid and clear-cut increase in EAA plasma concentrations of about 50% above basal levels in all participants and regardless of age. This observation is of particular interest because, as recently demonstrated, the increase in peripheral EAA concentrations dictates protein synthesis at both the muscle and whole-body level and achieving large EAA gradient is an important determinate of the protein synthetic response to feeding [19]. This result was obtained by enriching the biscuit dough with 8 g of EAA in 100 g of biscuit. This percentage of fortification was chosen because different researchers demonstrated the usefulness of EAA supplementation at doses of 4 grams twice a day [15,16] and those biscuits might be used in portions of 50 grams to obtain a comparable effect. As above mentioned, the palatability of the biscuit was good and without significant differences from the not fortified biscuit. Few data have been published on the post absorptive AAs availability of common foods and [20], in our work, we demonstrate that the ingestion of not fortified biscuit, even if containing high quality protein such as chicken yolk, do not provide sufficient EAA to induce a marked increase in their plasma concentrations, limiting the anabolic action. Protein malnutrition and sarcopenia may be counteracted by adequate protein intake and nutrition guidelines recommend an increase in the daily protein intake above 1,2 g/kg BW in malnourished patients, sarcopenic and hyper catabolic subjects [2]. The nutritional approach is so far based on the concept of adjusting the protein intake by increasing its intake in relation to different situations. Obtaining an optimal MPS or Whole-Body Protein Synthesis stimuli is not always granted by protein ingestion because of the rate of absorption. Only by inducing a rapid increase in the plasma concentration of EAA it would be possible to obtain this result. For this reason, the nutritional approach includes the use of specific dietary supplements based on high biological value proteins such as whey protein alone [21] or with leucine [22]. In our study we observed a significantly greater increase in branched AAs (valine, leucine, and isoleucine) and lysine.
EAAs in free form, unlike proteins, are rapidly absorbed from the intestinal tract without requiring energy expenditure and are rapidly available in the plasma [22]. In this experiment we demonstrated a rapid plasma appearance of EAA which was maintained up to 120 minutes from the baseline. Increasing evidence suggest that EAA supplementation might be used even in sarcopenic subjects to increase a daily amount of EAA [21]. In support of these statements, in an animal model, Dioguardi et al. hypothesized that health and life spam might be promoted by augmenting the ratio of EEA/NEAA ingested [23,24]. A recent work provides the evidence that protein supplements enriched with EAA can achieve rapid and sustained peripheral EAA concentrations enhancing whole-body protein status and supporting MPS during the catabolic stress of underfeeding [25]. Similarly, our study supports the possibility to increase EAA supply and availability by fortifying commonly used aliments. The possibility of providing a biscuit with a pleasant taste that can induce a rapid and significant increase in the levels of EAA could find a wide use in the general population. Currently, protein or EAA supplements are available in soluble or liquid form. Alternatively, due to the growing problem of sarcopenia in elderly subjects, the fortification of a common food like a biscuit with EAA might represent a promising alternative to the current nutritional approaches. A further possible application might be the fortification of common foods in those less developed countries with a shortage of nitrogen products for food, expanding the availability of different formats of products fortified with EAA. In conclusion, our results demonstrates that EAA contained in this fortified dough are bioavailable and sufficient to induce a clear-cut increase in plasma EAA post ingestion phase. Nevertheless, further studies are needed to confirm muscle anabolism, as occur after ingestion of free EAA mixtures, and to predict and monitor the impact of food fortification with EAA in population groups and, where needed, in the general population.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests
FV, LB, FM, and GDS are patent owners of the EAA fortified dough.
Author contributions
All authors contributed to the manuscript editing, read, and approved the final manuscript.
References
- World Health Organisation (WHO) Dietary Reference Intakes for Energy, Carbohydrate, Fibre, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients) World Health Organisation (WHO); Geneva, Switzerland: 2007. (WHO Technical Report Series 935).
- Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 14 (2013): 542-559.
- Gorissen SHM, Crombag JJR, Senden JMG, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50 (2018): 1685-1695.
- Duan Y, Li F, Liu H, et al. Nutritional and regulatory roles of leucine in muscle growth and fat reduction. Front Biosci (Landmark Ed) 20 (2015): 796-813.
- Volpi E, Kobayashi H, Sheffield-Moore M, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78 (2003): 250-258.
- Kato H, Volterman KA, West DWD, et al. Nutritionally non-essential amino acids are dispensable for whole-body protein synthesis after exercise in endurance athletes with an adequate essential amino acid intake. Amino Acids 50 (2018): 1679-1684.
- Pellett PL, Ghosh S. Lysine fortification: past, present, and future. Food Nutr Bull 25 (2004): 107-113.
- Bahwere P, Balaluka B, Wells JC, et al. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk- and peanut paste-based formulation for treating severe acute malnutrition: a noninferiority, individually randomized controlled efficacy clinical trial. Am J Clin Nutr 103 (2016): 1145-1161.
- Briend A, Lacsala R, Prudhon C, et al. Ready-to-use therapeutic food for treatment of marasmus. Lancet. 1999;353(9166):1767-1768.
- Bahwere P, Akomo P, Mwale M, et al. Soya, maize, and sorghum-based ready-to-use therapeutic food with amino acid is as efficacious as the standard milk and peanut paste-based formulation for the treatment of severe acute malnutrition in children: a noninferiority individually randomized controlled efficacy clinical trial in Malawi. Am J Clin Nutr 106 (2017): 1100-1112.
- Sato W, Furuta C, Matsunaga K, et al. Amino-acid-enriched cereals ready-to-use therapeutic foods (RUTF) are as effective as milk-based RUTF in recovering essential amino acid during the treatment of severe acute malnutrition in children: An individually randomized control trial in Malawi. PLoS One 13 (2018): e0201686.
- Delompre T, Guichard E, Briand L, et al. Taste Perception of Nutrients Found in Nutritional Supplements: A Review. Nutrients 11 (2019).
- Paddon-Jones D, Sheffield-Moore M, Katsanos CS, et al. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol 41 (2006): 215-219.
- Kim IY, Park S, Smeets E, et al. Consumption of a Specially-Formulated Mixture of Essential Amino Acids Promotes Gain in Whole-Body Protein to a Greater Extent than a Complete Meal Replacement in Older Women with Heart Failure. Nutrients 11 (2019): 345.
- Lombardi C, Carubelli V, Lazzarini V, et al. Effects of oral amino Acid supplements on functional capacity in patients with chronic heart failure. Clin Med Insights Cardiol 8 (2014): 39-44.
- Aquilani R, D'Antona G, Baiardi P, et al. Essential amino acids and exercise tolerance in elderly muscle-depleted subjects with chronic diseases: a rehabilitation without rehabilitation? Biomed Res Int 20 (2014): 341603.
- Carling RS, McDonald BA, Austin D, et al. Challenging the status quo: A comparison of ion exchange chromatography with liquid chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry methods for the measurement of amino acids in human plasma. Ann Clin Biochem 57 (2020): 277-290.
- Douglas JE, Mansfield CJ, Arayata CJ, et al. Taste Exam: A Brief and Validated Test. J Vis Exp 138 (2018): 8923.
- Church DD, Hirsch KR, Park S, et al. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 12 (2020).
- Rondanelli M, Aquilani R, Verri M, et al. Plasma kinetics of essential amino acids following their ingestion as free formula or as dietary protein components. Aging Clin Exp Res 29 (2017): 801-805.
- Nabuco HCG, Tomeleri CM, Sugihara JP, et al. Effects of Whey Protein Supplementation Pre- or Post-Resistance Training on Muscle Mass, Muscular Strength, and Functional Capacity in Pre-Conditioned Older Women: A Randomized Clinical Trial. Nutrients 10 (2018): 156.
- Deutz NE, Safar A, Schutzler S, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr 30 (2011): 759-68.
- Romano C, Corsetti G, Flati V, et al. Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan. Nutrients 11 (2019).
- Corsetti G, Pasini E, Romano C, et al. Body Weight Loss and Tissue Wasting in Late Middle-Aged Mice on Slightly Imbalanced Essential/Non-essential Amino Acids Diet. Front Med (Lausanne) 5 (2018): 136.
- Gwin JA, Church DD, Hatch-McChesney A, et al. Essential amino acid-enriched whey enhances post-exercise whole-body protein balance during energy deficit more than iso-nitrogenous whey or a mixed-macronutrient meal: a randomized, crossover study. J Int Soc Sports Nutr 18 (2021): 4.