Better Therapeutic Target to Enhance Cisplatin Sensitivity in Cervical Cancer: PARP-1 or β-catenin
Article Information
Minakshi Mann1, Shyam S Chauhan2, Neerja Bhatla3, Sunesh Kumar3, Sameer Bakhshi1, Ritu Gupta4, Sandeep Mathur5, Lalit Kumar1*
1Department of Medical Oncology, Dr. B.R.A. Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
2Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, India
3Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, India
4Laboratory Oncology Unit, Dr. B.R.A. Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
5Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
*Corresponding Author: Dr. Lalit Kumar, Prof. and Head, Department of Medical Oncology, Dr. B.R.A. Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
Received: 10 June 2020; Accepted: 03 July 2020; Published: 20 August 2020
Citation: Minakshi Mann, Shyam S Chauhan, Neerja Bhatla, Sunesh Kumar, Sameer Bakhshi, Ritu Gupta, Sandeep Mathur, Lalit Kumar. Better Therapeutic Target to Enhance Cisplatin Sensitivity in Cervical Cancer: PARP-1 or β-catenin. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 266-282.
View / Download Pdf Share at FacebookAbstract
Development of resistance to cisplatin (CDDP) is a major bottleneck to treat cancer with CDDP, including cervical cancer (CC). We have recently shown that PARP-1 inhibition targets β-catenin signaling and enhance CDDP sensitivity in cervical cancer. This indicates that β-catenin itself could serve as an absolute target to potentiate CDDP cytotoxicity. So, we determined the effect of β-catenin inhibition using JW74 either alone or in combination CDDP using CC cell lines on cell vaibility, apoptosis, cell cycle progression, proliferation, invasion and metastasis, clonogenecity. However, unexpectedly β-catenin inhibitor JW74 failed to significantly enhance CDDP- cytotoxicity in both the cell lines. Since PARP-1 inhibitors inhibited β-catenin also, as determined through our previous work, so, we evaluated effect of JW74 mediated β-catenin inhibition on PARP-1 expression level inside cell. Intriguingly, we found that JW74 treatment enhanced PARP-1 expression as determined through western blotting. Increased PARP-1 serves responsible for CDDP resistance, thereby this seems stands responsible limiting our hypothesis. Further to confirm this, PARP-1 inhibitor PJ34 was added to a combination of JW74+CDDP and this significantly reduced cell survival and proliferation, enhanced cell death and decreased invasion and migration, hence enhanced CDDP sensitivity in CC cells. PJ34 with CDDP served as a better combination to increase caspase-3/7 cleavage, hence apoptosis, than combination of JW74 and CDDP; with PJ34 itself being less toxic to cells. Also PARP-1 expression was determined in human cervical cancer tissue sample at base level and compared to normal tissue sample. PARP-1 expression was significantly enhanced in human cervix cancer tumor samples. In summary, β-catenin inhibition increases PARP-1 expression, thereby, limits ability of β -catenin inhibitors to enhance CDDP cytotoxicity. Enhanced PARP-1 exp
Keywords
CDDP; Drug sensitivity; Wnt signaling; PJ34; JW74
CDDP articles, Drug sensitivity articles, Wnt signaling articles, PJ34 articles, JW74 articles
CDDP articles CDDP Research articles CDDP review articles CDDP PubMed articles CDDP PubMed Central articles CDDP 2023 articles CDDP 2024 articles CDDP Scopus articles CDDP impact factor journals CDDP Scopus journals CDDP PubMed journals CDDP medical journals CDDP free journals CDDP best journals CDDP top journals CDDP free medical journals CDDP famous journals CDDP Google Scholar indexed journals Drug sensitivity articles Drug sensitivity Research articles Drug sensitivity review articles Drug sensitivity PubMed articles Drug sensitivity PubMed Central articles Drug sensitivity 2023 articles Drug sensitivity 2024 articles Drug sensitivity Scopus articles Drug sensitivity impact factor journals Drug sensitivity Scopus journals Drug sensitivity PubMed journals Drug sensitivity medical journals Drug sensitivity free journals Drug sensitivity best journals Drug sensitivity top journals Drug sensitivity free medical journals Drug sensitivity famous journals Drug sensitivity Google Scholar indexed journals Wnt signaling articles Wnt signaling Research articles Wnt signaling review articles Wnt signaling PubMed articles Wnt signaling PubMed Central articles Wnt signaling 2023 articles Wnt signaling 2024 articles Wnt signaling Scopus articles Wnt signaling impact factor journals Wnt signaling Scopus journals Wnt signaling PubMed journals Wnt signaling medical journals Wnt signaling free journals Wnt signaling best journals Wnt signaling top journals Wnt signaling free medical journals Wnt signaling famous journals Wnt signaling Google Scholar indexed journals PJ34 articles PJ34 Research articles PJ34 review articles PJ34 PubMed articles PJ34 PubMed Central articles PJ34 2023 articles PJ34 2024 articles PJ34 Scopus articles PJ34 impact factor journals PJ34 Scopus journals PJ34 PubMed journals PJ34 medical journals PJ34 free journals PJ34 best journals PJ34 top journals PJ34 free medical journals PJ34 famous journals PJ34 Google Scholar indexed journals JW74 articles JW74 Research articles JW74 review articles JW74 PubMed articles JW74 PubMed Central articles JW74 2023 articles JW74 2024 articles JW74 Scopus articles JW74 impact factor journals JW74 Scopus journals JW74 PubMed journals JW74 medical journals JW74 free journals JW74 best journals JW74 top journals JW74 free medical journals JW74 famous journals JW74 Google Scholar indexed journals cervical cancer articles cervical cancer Research articles cervical cancer review articles cervical cancer PubMed articles cervical cancer PubMed Central articles cervical cancer 2023 articles cervical cancer 2024 articles cervical cancer Scopus articles cervical cancer impact factor journals cervical cancer Scopus journals cervical cancer PubMed journals cervical cancer medical journals cervical cancer free journals cervical cancer best journals cervical cancer top journals cervical cancer free medical journals cervical cancer famous journals cervical cancer Google Scholar indexed journals β-catenin articles β-catenin Research articles β-catenin review articles β-catenin PubMed articles β-catenin PubMed Central articles β-catenin 2023 articles β-catenin 2024 articles β-catenin Scopus articles β-catenin impact factor journals β-catenin Scopus journals β-catenin PubMed journals β-catenin medical journals β-catenin free journals β-catenin best journals β-catenin top journals β-catenin free medical journals β-catenin famous journals β-catenin Google Scholar indexed journals cytotoxicity articles cytotoxicity Research articles cytotoxicity review articles cytotoxicity PubMed articles cytotoxicity PubMed Central articles cytotoxicity 2023 articles cytotoxicity 2024 articles cytotoxicity Scopus articles cytotoxicity impact factor journals cytotoxicity Scopus journals cytotoxicity PubMed journals cytotoxicity medical journals cytotoxicity free journals cytotoxicity best journals cytotoxicity top journals cytotoxicity free medical journals cytotoxicity famous journals cytotoxicity Google Scholar indexed journals metastasis articles metastasis Research articles metastasis review articles metastasis PubMed articles metastasis PubMed Central articles metastasis 2023 articles metastasis 2024 articles metastasis Scopus articles metastasis impact factor journals metastasis Scopus journals metastasis PubMed journals metastasis medical journals metastasis free journals metastasis best journals metastasis top journals metastasis free medical journals metastasis famous journals metastasis Google Scholar indexed journals clonogenecity articles clonogenecity Research articles clonogenecity review articles clonogenecity PubMed articles clonogenecity PubMed Central articles clonogenecity 2023 articles clonogenecity 2024 articles clonogenecity Scopus articles clonogenecity impact factor journals clonogenecity Scopus journals clonogenecity PubMed journals clonogenecity medical journals clonogenecity free journals clonogenecity best journals clonogenecity top journals clonogenecity free medical journals clonogenecity famous journals clonogenecity Google Scholar indexed journals
Article Details
1. Introduction
Globally, cervical cancer (CC) ranks as fourth common cancer among females with 5,69,847 new cases and 3,11,365 death reported in 2018 [1]. Approximately 90% of the CC cases occur in countries with low socio-economic status [2]. In India, CC ranks as commonest gynaecological cancer constituting 10% of all cancer-related mortality [1]. Persistent infection by high-risk subtype of human papilloma virus (HPV) and subsequent malignant transformation accounts for 95% cases of CC. HPV 16 and 18 are the main high-risk subtypes causing ≈80% of all CC [3]. CC is majorly treated with concurrent radiation and cisplatin (CDDP)-based chemotherapy. CDDP mediates its cytotoxic effect by interacting with DNA to form DNA–protein and DNA–DNA adducts that activate signaling mechanisms leading to apoptosis [4]. Efficacy of CDDP is often limited due to innate/acquired CDDP resistance among cancer cells [5] leading to lower response to CDDP with an expected overall survival ranging from 10 to 17.5 months [6].
CDDP resistance has been classified into four types: (i) pre-target resistance, prevents CDDP binding to DNA by reducing CDDP accumulation, (ii) on-target resistance, by increased repair of CDDP-DNA adducts formed, (iii) post-target resistance, by errors in signal transduction in response to damaged DNA by CDDP, and (iv) off-target resistance, by interfering signal cross-talk required for CDDP-mediated cell death [7]. Poly (ADP-ribosyl) polymerase (PARP-1) is a nuclear DNA binding protein involved in DNA damage repair by nucleotide excision repair (NER) pathway. PARP-1 has been linked with CDDP resistance in various solid cancers [8] and use of PARP-1 inhibitors in BRCA mutated tumors leads to synthetic lethality and sensitize cancer cell to CDDP [9, 10]. We have recently shown that PARP-1 inhibitors downregulates β-catenin signaling to enhance CDDP sensitivity in CC cells [11]. Since β-catenin is involved in various malignancy and associated with increased cell survival, invasion, advanced stage and poor prognosis [12-17], we hypothesized that inhibition of β-catenin might enhance CDDP cytotoxicity in CC. Pharmacological abrogation was used for β-catenin inhibition through JW74, which is a tankyrase inhibitor. In this study, efficacy of JW74 to enhance CDDP-mediated cytotoxicity was determined and also compared with combination of PJ34 plus CDDP-mediated cytotoxicity.
2. Materials and Methods
2.1 Chemicals and cell culture
Both HeLa and SiHa used in the study were cultured in DMEM high glucose supplemented with 10% FBS and 1% penicillin/streptomycin. KEMOPLAT (1 mg/ml CDDP Injection) was used for CDDP treatment and JW74 (β-catenin inhibitor) was purchased from Sigma-Aldrich. All chemicals and antibodies used in the study were purchased from Sigma-Aldrich and Santa Cruz, respectively.
2.2 Assessment of drug dose response by cell growth
Through MTT-based cytotoxicity assay, concentration as well as time-dependent response to JW4 was analyzed at 48h and 72h for both HeLa and SiHa. IC50 value was determined and used to decide drug concentration for further experiments. For CDDP treatment, 2.5mM and 5mM doses were chosen and for β-catenin inhibition, we chose low doses of JW74 i.e. 5mM and 10mM.
2.3 MTT cytotoxicity assay
5×103 cells/well were plated in 96-well plate and treated with JW74 and CDDP alone or in combination for 48h and 72h. Following treatment, cells were subsequently incubated with 0.5% MTT at 37°C for 4h. The medium was then removed and 100ml DMSO was added to dissolve the formazan. The resulted absorbance was measured at 570 nm using a multi-well spectrophotometer (Bio-Tek, USA).
2.4 Immunoblotting
Whole cell lysate was prepared in RIPA lysis buffer and was resolved on 10% SDS-gel. Protein expression was analyzed using methodology described in our previous study [11]. Briefly, the protein was transferred to PVDF membrane at 40V overnight. After blocking with 3% BSA for 2 h, the target protein was detected by antibodies directed against β-catenin (Cat. No Sc-7963, 1:500) and PARP-1 (Cat. No. Sc-8007, 1:500). Quantification of the target protein levels was conducted with Image J software (NIH, USA).
2.5 Determination of cell cycle progression and cell death
Cell cycle progression and cell death were analyzed through PI staining according to previously described method [11]. Briefly, cells were permeabilized in 70% ethanol. Next day, cells were treated with 200 μg/ml RNase A and stained with 50 μg/ml PI in dark. Cell cycle distribution was determined through FACS Calibur flow cytometer (Becton Dickinson).
2.6 Clonogenic survival assay
Cells were seeded in a very low density (500 for untreated and 1000 for treated) in PD35 and incubated overnight. Following PBS wash, cells were treated with CDDP, JW74 and PJ34 alone or as mentioned combinations for 2 h. Following PBS wash, cells were continuously cultured for next 10 days. Resulted colonies (atleast 30 cells/colony in untreated cells) were fixed with fixing solution (methanol:glacial acetic acid; 3:1) and stained with 0.5% crystal violet and counted under microscope.
2.7 Cell invasion assay
The invasion ability was ascertained using 6-well matrigel invasion plates (8-µm pore size, Corning,) following manufacturers’ protocol. Briefly, 2X105 (HeLa) or 4X105cells (SiHa) cells in basal DMEM-HG, with/without drug were seeded in the upper chamber. Complete DMEM-HG (with 10% FBS) was added in the lower chamber for chemo-attractant. The culture was incubated at 37°C in 5% CO2 atmosphere for 24h. The medium was then removed and cells were washed twice with PBS. Cells were fixed with 4% paraformaldehyde for 30 min and stained with hematoxylin and counterstained with eosin. Non-invaded cells were removed with a cotton bud. The number of cells in 5 different fields of view was counted under microscope.
2.8 Monolayer wound healing assay
The cells were seeded in high density in 6-well plate and resulted monolayer was scratched parallel with a sterile 10μL micropipette tip. Cells were washed with PBS and treated with indicated concentrations of CDDP, JW74 and PJ34 either alone or in indicated combinations. The closure of the wounded area was observed under inverted microscope at 4X at 0 h and indicated . The inhibitory effect of treatment was expressed after normalization with control.
2.9 Detection of apoptosis
Cleaved caspase-3/7 is the active form of caspase-3/7, which are dedicated marker for apoptotic cell death and to assess apoptosis, the Caspase Glo® 3/7 assay (Promega) was used. Caspase-3/7 activity was measured in 96-well flat-bottomed culture plates. 5X103cells were seeded in 100 μl of drug free complete DMEM-HG medium and incubated overnight before drug treatment. Next day, spent media with unadhered cells was discarded, adhered cells were washed with PBS and 150 μl of DMEM-HG containing indicated drug concentration was added and incubated in 5% CO2 incubator at 37°C for respective time points. After treatment, used media was removed and fresh DMEM-HG was added to each well. 100µl of freshly prepared Caspase-Glo® 3/7 reagent was added to each well containing 100µl of blank reaction (vehicle and cell culture medium), negative control (vehicle treated cells) or assay (treated cells in culture medium) and mixed gently and incubated at RT for 1 h. Following incubation, content was transferred carefully to pre labeled white-walled 96-well plate and luminescence of each sample was measured on luminometer (Synergy™ HT Multi-detection, microplate reader, BioTek®). The data obtained were normalized to the control value of each cell line and fold change was plotted as bar graph.
2.10 Immunohistochemistry
Medical record files of CC patients registered during time period 2014-2015 were analyzed. Depending upon the availability of formalin fixed paraffin embedded (FFPE) tissue blocks, 44 cases were selected. Also, 4 non-cancer cervix tissue samples i.e. patients who underwent surgery for non-cancer indications, were used to compare PARP-1 expression in cancer and non-cancerous cervix tissue. For IHC, paraffin sections (4 μm) were deparaffinized in 100% xylene and re-hydrated in series of graded alcohol concentrations and water according to standard protocols. Heat-induced antigen retrieval was performed in 10 mM citrate buffer for 20 min at 100°C. IHC was performed with mouse anti-human PARP-1 antibody (Cat. No. Sc-8007, 1:200). Tissue sections were visualized with diaminobenzidine and counterstained with hematoxylin, using a bright field microscope (vanoxah-fl-2; Olympus Inc., Tokyo, Japan) followed by analysis by pathologist.
2.11 Statistical evaluation
All data are expressed as the mean ± standard deviation from at least three replicates. Comparisons between groups were performed using the two-tailed Student’s t-test with a significance cut-off at < 0.05. Statistical significance in difference in PARP-1 expression in normal cervix tissue vs. cervix tumor tissue was evaluated using student t-test.
3. Results
3.1 β-catenin abrogation by JW74 is dose dependent
JW74 efficiently decreased the expression of β-catenin in dose dependent manner as shown by immunoblots in both the cell lines (Figure 1A and B). 5 μM and 10 μM of JW74 efficiently inhibited β-catenin expression and inhibition was more in SiHa cells than in HeLa cells.
3.2 JW74-mediated β-catenin inhibition is associated with growth inhibition of CC cells
A dose- and time-dependent decrease in cell survival with JW74 and CDDP was observed at both the time points. The IC50 values for JW74 were determined to be 10.5 μM (48 h) and 9.1 μM (72 h) for HeLa cells and 11.0 μM (48 h) and 9.5 μM (72 h) for SiHa cells (Figure 1C and D). Corresponding values for CDDP were 8.25 μM (48 h) and 6.1 μM (72 h) for HeLa cells, and 10.8 μM (48 h) and 7.7 μM (72 h) for SiHa cells [11]. These results indicate that CDDP is more cytotoxic than JW74 and sensitivity towards JW74 is comparable in both HeLa and SiHa cells (Figure 1C and D respectively).
3.3 β-catenin inhibition enhances CDDP sensitivity in CC cells
Combining 5 μM of JW74 with CDDP could not distinctly reduce the IC50 value of CDDP, however, combining 10 μM of JW74 with CDDP significantly decreased the CDDP IC50 to 3.75 fold and 6.75 fold at 48 h and 4.06 fold and 4.27 fold at 72 h, respectively in HeLa and SiHa cells (Figure 1E-I). However, 10 μM JW74 itself was highly toxic and survival reduced to ≈ 50%, whereas survival at 5 μM of JW74 was greater than 75% in both the cell lines (Figure 1C and D); therefore, 5 μM of JW74 was chosen as optimal concentration for further experiments.
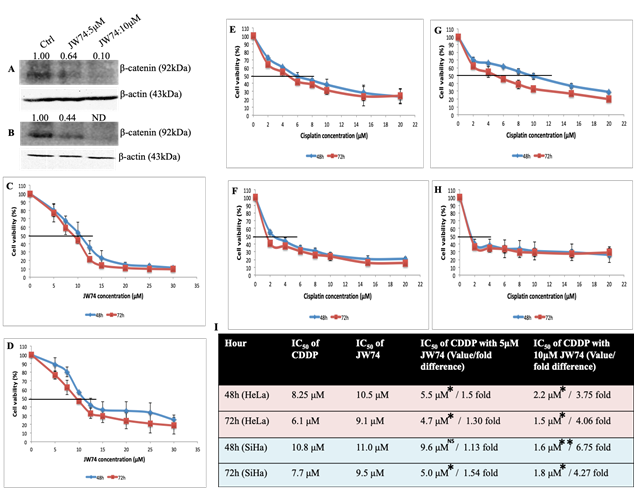
Figure 1: Representative immunoblots con?rming β-catenin inhibition and dose-response effect of JW74 on cell vaibility and combined effect of CDDP and JW74 on IC50 value of CDDP. A and B, immunoblots showing endogenous level of β-catenin in HeLa (A) and SiHa (B) cells after treatment with JW74 (0, 5 and 10µM) for 48h. C and D, HeLa (C) and SiHa (D) cells were treated with indicated doses of JW74 for 48h and 72h, respectively and cell viability was determined by MTT assay. E-I, HeLa cells were treated with different doses of CDDP in combination with 5µM or 10µM of JW74 (E and F). SiHa cells were treated with different doses of CDDP in combination with 5µM or 10µM of JW74 (G and H). Table representing fold change in the IC50 value of CDDP upon combined treatment with JW74 and CDDP vs. CDDP alone in HeLa and SiHa cells at indicated time points (I). All the values are expressed relative to untreated cells (100% control value). β-actin was used as loading control. Error bars represent mean ± SD (n ≥ 3 independent experiments). IC50 values for JW74 at different time points along with their p value is mentioned in the respective graph. ND: Nondetectable; NS: Non-significant; *, p< 0.05; **,p<0.01; ***, p<0.001.
3.4 Effect of β-catenin inhibition on CDDP-induced cell cycle progression and cell death
We further analyzed the combinatorial effect of JW74 and CDDP treatment on CDDP-induced cell cycle progression and cell death. CDDP is not phase specific, however, cells are maximally sensitive in late-G1 phase, just prior to DNA replication [18]. CDDP causes S- or G2/M-phase block at low time point or low doses followed by cell death with increase in either concentration or time point [18, 19]. An increase in G2/M-population was observed in SiHa cells with CDDP treatment at both 48 and 72 h. In HeLa cells, CDDP treatment resulted in an increased S-phase population followed by increase in G2/M population. No difference was observed in the % sub-G1 population as well as cell cycle progression in combinatorial treatment with JW74 and CDDP as compared to CDDP treatment alone in both the cell lines (Figure 2A-D).
3.5 Modulation of the PARP-1 expression through β-catenin inhibition
We determined the effect of JW74 on PARP-1 expression and found increased PARP-1 expression in both the cell lines (Figure 2E and F). Therefore, β-catenin inhibition enhanced PARP-1 expression and this increased PARP-1 could be accountable for insignificant difference in cell death with combinatorial treatment of JW74 and CDDP compared to CDDP alone. Therefore, we next determined the effect of addition of PARP-1 inhibitor PJ34 (10 μM) to combinatorial treatment of JW74 and CDDP and compared to CDDP alone.
3.6 Combined effect of β-catenin and PARP-1 inhibition on CDDP-induced cell death
We determined the % sub-G1 population following PI staining to estimate the cell death. No difference in cell death at 48 h time point was observed in both the cell line following treatment with JW74+PJ34+CDDP as compared to CDDP alone (Figure 2A and 3C). We observed a slight increase in cell death after treatment of HeLa cells with JW74+PJ34+CDDP for 72 h; however, this was not significant as compared to treatment CDDP alone (Figure 2B). In SiHa cells, a marked and significant increase in cell death was observed when JW74 and PJ34 was combined with 5 μM of CDDP as compared to only CDDP or CDDP+JW74 combination (Figure 2D).
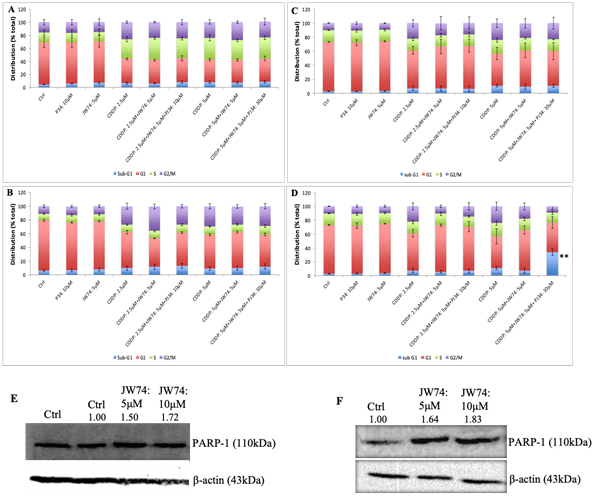
Figure 2: Combined effect of JW74 ± PJ34 & CDDP on cell cycle progression and representative immunoblots con?rming effect of JW74 on PARP-1 expression. A-D, HeLa cells were treated with different doses of CDDP in combination with 5µM of JW74 ± 10µM PJ34 for 48h and 72h (A and B). SiHa cells were treated with different doses of CDDP in combination with 5µM of JW74 ± 10µM PJ34 for 48h and 72h (C and D). E and F, cells were treated with indicated doses of JW74 and resulted effect on PARP-1 expression was determined in HeLa (E) and SiHa cells (F), respectively. β-actin was used as loading control. Error bars represent mean ± SD (n ≥ 3 independent experiments). **, p< 0.01.
3.7 Combined effect of β-catenin and/or PARP-1 inhibition on anti-clonogenecity of CDDP
Colony formation assay was carried out to further examine the anti-proliferation effect of JW74 (and/or PJ34) and CDDP combined treatment vs. CDDP alone. We used 2.5 μM and 5 μM CDDP alone or with 5 μM JW74 and/or 10 μM PJ34. CDDP alone remarkably reduced the colony forming capacity of both the HeLa and SiHa cells (Figure 3A and C). Further, combining CDDP with JW74 reduced the clonogenecity by 1.07 and 1.15 fold in HeLa cells and 1.44 and 2.5 fold in SiHa cells at 2.5 μM and 5μM CDDP, respectively (Figure 3B and D). A combination of JW74+PJ34+CDDP was more effective than combination of JW74 and CDDP in inhibiting the colony forming capacity. Colony forming ability with JW74+PJ34+CDDP significantly decreased to 1.27 fold (HeLa) and 1.95 fold (SiHa) at 2.5 μM CDDP; and 3.87 fold (HeLa) and 5 fold (SiHa) at 5 μM CDDP (Figure 3B and D).
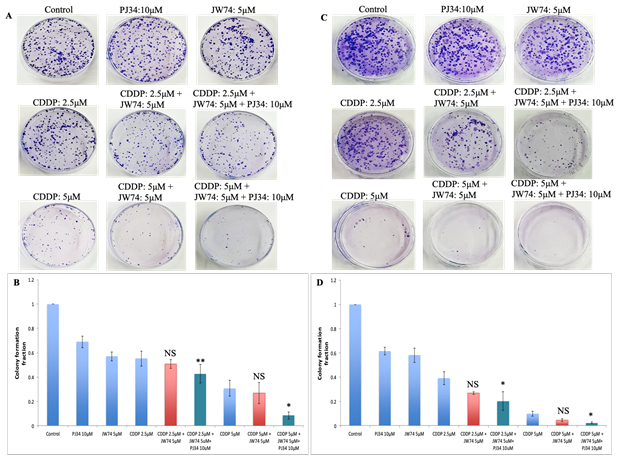
Figure 3: Combined effect of JW74 ± PJ34 & CDDP on colony formation assay. A-D, Representative images for HeLa (A) and SiHa (C) cells treated with 2.5 and 5µM CDDP, 10µM PJ34 and 5µM JW74 alone or combination of CDDP and JW74 ± PJ34 in indicated doses for 2h. Bar graphs showing colony forming ability with respect to control of each group in HeLa (B) and SiHa (D) cells. For each doses, three replicates were performed where the survival of untreated cells (control) was set to one. Error bars represent mean ± SD (n ≥ 3 independent experiments). NS: Non-significant; *, p< 0.05; **, p<0.01.
3.8 Combined treatment with β-catenin and/or PARP-1 inhibitor enhances anti-invasion/ migration effect of CDDP
Wnt signaling regulates migration and invasion of cells, and thereby controls metastasis [20]. The combined effect of JW74 and/or PJ34 and CDDP on the migration was assessed through scratch assay. Combination treatment of JW74 and CDDP decreased cell migration in both the cell lines as compared to CDDP; however, the decrease was not significant (Figure 4A-D). Combination of JW74+PJ34+CDDP was more efficient and significantly reduced the cell migration in both the cell lines used. Migration reduced to 0.65 and 0.55 fold at 2.5 μM and 5μM CDDP, respectively in HeLa cells and to 0.83 and 0.68 fold at 2.5 μM and 5μM CDDP, respectively in SiHa cells (Figure 4B and D). We further determined the combined effect of JW74 and CDDP on cell invasion ability of HeLa and SiHa through transwell invasion assay. CDDP moderately reduced the invasion ability of both the cell lines and combined treatment with JW74 and CDDP further reduced the invasion ability of CC cells (Figure 4E-H). Like migration, cell invasion was significantly reduced to a greater extent upon co-treatment with JW74+PJ34+CDDP as compared to CDDP alone in both the cell lines (Figure 4E-H).
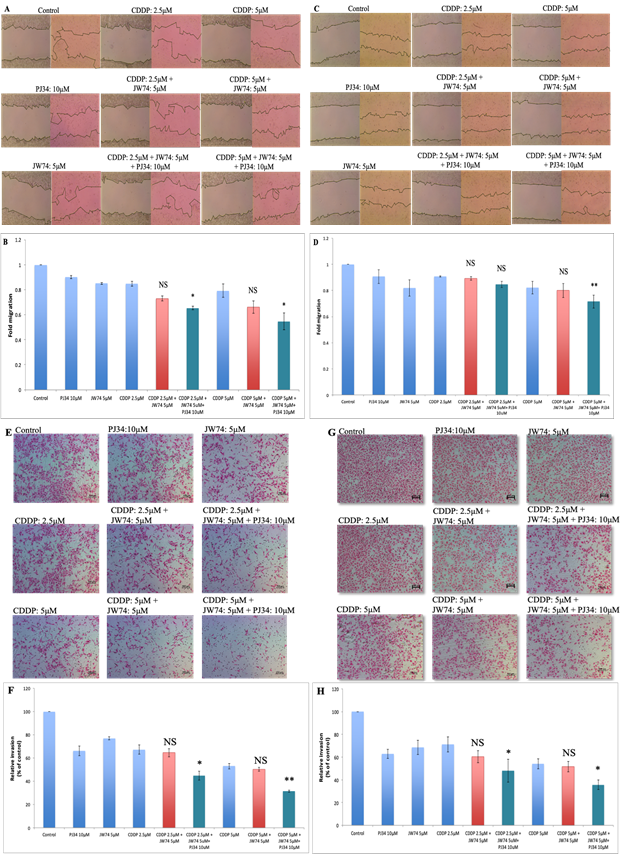
Figure 4: Combined effect of JW74 ± PJ34 & CDDP treatment on the invasion and migration. A-D, representative images (under 4X magnification) of scratch wound healing assay performed after treatment of HeLa (A) and SiHa (C) cells with 2.5 and 5µM CDDP, 10µM PJ34 and 5µM JW74 alone or combination of CDDP and JW74 ± PJ34 in indicated doses. Fold migration (with respect to control) bar graphs of each group in HeLa (B) and SiHa cells (D). E-H, representative images (under 10X magnification) of invaded cells after treatment with 2.5 and 5µM CDDP, 10µM PJ34 and 5µM JW74 alone or combination of CDDP and JW74 ± PJ34 in indicated doses in HeLa (E) and SiHa (G) cells. Fold change in invasion ability (with respect to control) bar graphs of each group in HeLa (F) and SiHa cells (H). Error bars represent mean ± SD (n ≥ 3 independent experiments). NS: Non-significant; *, p< 0.05; **, p<0.01.
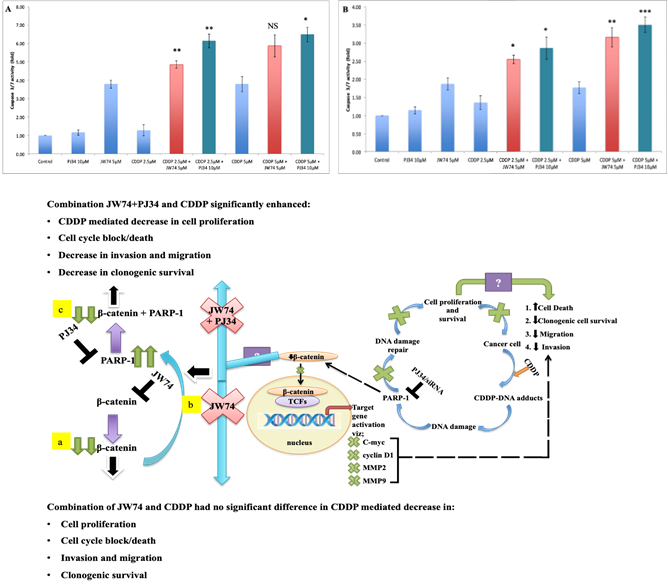
Figure 5: Combined effect of JW74 & CDDP treatment on caspase-3/7 cleavage as compared to combination of PJ34 and CDDP. A-C, HeLa (A) and SiHa (B) cells treated with 2.5 and 5µM CDDP, 10µM PJ34 and 5µM JW74 alone or combination of either CDDP and JW74 or combination of CDDP and PJ34 in indicated doses for 24h. Bar graphs showing caspase-3/7 cleavage with respect to control of each group in HeLa (A) and SiHa (B) cells. C. Model for regulation of β-catenin signaling by PARP-1 inhibition and resulted effect on CDDP sensitivity. Also, showing probable mechanism (enhanced PARP-1 expression) for non-significant effect on augmenting CDDP sensitivity upon β-catenin inhibition through JW74 (a and b) and the resulted effect of PARP-1 inhibition in such condition via PJ34 (c) i.e. PJ34+JW74+CDDDP (C) on CDDP efficacy. For each dose, three replicates were performed where the survival of untreated cells (control) was set to one. Error bars represent mean ± SD (n ≥ 3 independent experiments). NS: Non-significant; *, p< 0.05; **, p<0.01; ***, p<0.001.
3.9 β-catenin inhibition increases CDDP-mediated apoptosis
We determined the combined effect of 5 μM of JW74 and CDDP on CDDP-induced apoptosis through caspase 3/7 cleavage assay. A significant increase in caspase 3/7 cleavage in combination treatment as compared to CDDP alone was observed. However, apoptosis with JW74 alone was ≥ apoptosis caused by 5 μM CDDP; higher concentration of CDDP used in the study (Figure 5A and B).
3.10 PARP-1 inhibition is more efficient than β-catenin inhibition in enhancing CDDP-mediated apoptosis
We next determined the combined effect of PJ34 and CDDP on CDDP-mediated apoptosis and compared with apoptosis due to co-treatment with JW74 and CDDP. Apoptosis caused by PJ34 was lower than apoptosis caused by JW74 as well as CDDP alone (Figure 5A and B). Also, a significant increase in apoptosis was observed in co-treatment with PJ34 and CDDP as compared to CDDP alone. Co-treatment with PJ34 and CDDP caused more apoptosis than co-treatment with JW74 and CDDP.
3.11 Clinicopathological characteristics of cervix cancer patients
Figure 6G summarize clinicopathological characteristics of 44 cervix cancer patients. Majority of these patients were of squamous cell carcinoma histology (86.4 %) and were in locally advanced or advanced stage (stage III and IV: 56.8%).
3.12 Expression of PARP-1 in cervix tumor and non-tumor samples
We correlated the expression of PARP-1 protein in non-tumor cervix tissue and CC samples (Figure 6). Non-tumor cervix samples did not show PARP-1 expression in the epithelial cells (intensity score: 0). Further, low immunoreactivity for PARP-1 was observed in the basal cells but not in the mature cells of the cervix (Figure 6). However, the expression of PARP-1 was significantly higher in CC tissue (p=0.0001, Figure 6) and was independent of tumor stage (Figure 6G-I).
Figure 6: Expression of PARP-1 protein in human normal cervix and cervical cancer samples as determined by IHC. (A-F) Expression of PARP-1 in human normal cervix tissue samples (A and B). Expression of PARP-1 in different stages of cervical cancer (C-F). (G-I) Tables showing demographic details of the study patients (G) and difference in the PARP-1 expression in normal cervix and cervical cancer (H) and also, as per the stage of cervical cancer (I).
4. Discussion
CDDP serves as basis for treatment of solid tumors, including CC [21]. CDDP interacts with N7 on purine base leading to CDDP-DNA adducts [22]. This causes activation of DNA damage repair pathways resulting into either repaired DNA and cell survival or activation of irreversible apoptotic signaling leads to cell death [23]. β-catenin is involved in various human malignancies [24-27], and is actively involved in cell growth, adhesion and stemness [27-29]. Based on our previous findings that PARP-1 inhibition downregulates β-catenin expression and thereby, Wnt signaling [30], here we hypothesized that inhibiting β-catenin could serve as a better approach to enhance CDDP sensitivity in CC. In the present study we tested the efficiency of JW74 to enhance CDDP sensitivity using HeLa and SiHa cells and compared to PARP-1 inhibition. JW74 or 4-[4-(4-Methoxyphenyl)-5-[[[3-(4-methylphenyl)-1,2,4-oxadiazol-5-yl]methyl]thio]-4H-1,2,4-triazol-3-yl] pyridine acts by stabilizing AXIN2, a key component of the β-catenin destruction complex [31, 32]. JW74 efficiently reduced expression of β-catenin in both the cell lines and also, it reduced invasion, migration as well as clonogenic survival and enhanced cell death in CC cells used, however, JW74, hence β-catenin inhibition was less cytotoxic as compared to CDDP. We next determined the combinatorial effect of JW74 and CDDP on CDDP-mediated cytotoxicity either alone or in indicated combinations. JW74 was combined with sub-lethal doses of CDDP i.e. 2.5μM and 5μM. A remarkable decrease in the IC50 value of CDDP was observed in combination with 10μM of JW74. But, being highly cytotoxic at 10μM a lower dose of JW74 (5μM) was used. A decrease in IC50 value of CDDP was observed in both HeLa and SiHa at combinatorial treatment with 5μM JW74 and CDDP, however, decrease was not significant (Figure 1E-I). Other studies have also reported inhibition of β-catenin, insufficient to affect apoptosis in multiple myeloma [26, 33].
Studies show that, β-catenin level increases in S phase, accumulates during G2/M phase and decrease as cell enters G1 phase of cell cycle [34]. Also, β-catenin controls G1/S transition in MDCK cells [35]. Hence, we next determined the effect of JW74 either as single agent or in combination with CDDP on cell cycle progression. JW74 downregulates the expression of CCND1 and CDK6 gene, required for entry to G1 phase [12]; however, JW74 didn’t effect CCNE1 and CDK2 gene, which regulates progression from G1 to S phase [36]. In our study also, JW74 alone didn’t effect the cell cycle progression, whereas CDDP caused G2/M block in SiHa cells and S phase block population in HeLa cells at 48 h and 72 h, however, we could not find any marked increase in cell death or block in combination treatment with JW74 and CDDP (Figure 2A-D).
Similarly, β-catenin inhibition didn’t have marked effect on clonogenic cell proliferation and survival or even any significant differences in invasion and migration in combined treatment with JW74 and CDDP as compared to CDDP alone (Figure 3 and 4). We determined the possible reason for incompetence of JW74 to enhance CDDP cytotoxicity. With referral to our earlier finding; PARP-1 inhibition decreased β-catenin expression [11], we focused on the expression of PARP-1 upon β-catenin inhibition using JW74. Interestingly, we found an increased PARP-1 expression upon JW74 treatment (Figure 2E and F). Also, we found a significant increase in cell death at 72 h in SiHa cell when PJ34 was added to combination of JW74 and CDDP as compared to CDDP alone (Figure 2D). A combination of JW74+PJ34+CDDP significantly reduced the clonogenic survival, cell invasion and migration ability in CC cells as compared to only CDDP (Figure 3 and 4). Indicating, it is enhanced PARP-1 expression upon β-catenin inhibition that limited efficacy of JW74 to enhance CDDP sensitivity in cervical cancer cells.
Additionally, we compared the efficiency of JW74 and CDDP combination vs. combination of PJ34 and CDDP in enhancing CDDP-mediated apoptosis through caspase 3/7 cleavage assay. In combination, PJ34 and CDDP combinatorial treatment was more apoptotic than JW74 and CDDP combination. Also, JW74 itself was more apoptotic, whereas, PJ34 was less toxic than both the JW74 and CDDP doses used in the study. Combining these observations, PARP-1 inhibition was more efficient than β-catenin inhibition in enhancing CDDP-mediated cytotoxicity. Next, we observed constant high expression of PARP-1 protein in CC tissue sample while the expression in normal cervix tissue samples was non-evaluable (Figure 6H). PARP-1 overexpression has been associated with carcinogenesis in malignant melanomas, colorectal, breast, testicular and lymphangioleiomyomatosis [37-41]. Elevated expression of PARP-1 in cervix cancer creates a possibility for the use of PARP-1 inhibitors in CC treatment despite the absence of synthetic lethality in these HPV-associated CC. PARP-1 has been associated with invasiveness and higher tumor grade [42]. However, we didn’t find any correlation between PARP-1 expression and tumor staging, which is line with other reports in gastric, colorectal, ovarian and breast cancer [42-45].
- Conclusion
β-catenin inhibition enhances PARP-1 expression and, hence, serves incompetent to significantly enhance CDDP sensitivity in CC cells. Significant increase in CDDP cytotoxicity uenin inhibitor plus CDDP cocktail further confirms it,. Combination of PARP-1 inhibitor with CDDP is a better approach to increase CDDP sensitivity in CC cells than a combinatorial treatment with β-catenin inhibitor and CDDP. PARP-1 inhibitors can be efficiently used in treating cervix cancer patients both as base level as well as relapse state. However, further preclinical and in vivo studies are warranted to validate these findings and before the clinical utility of such therapeutic approach can be exactly determined.
Funding
“This research was supported by AIIMS-Intramural Grant (A-400), Minakshi Mann thanks AIIMS, New Delhi and ICMR, New Delhi for salary award”.
Acknowledgments
We are thankful to Dr. Parthaprasad Chattopadhyay, Professor, Dept. of Biochemistry, AIIMS, New Delhi for providing HeLa cell line. We are extremely thankful to Dr. Alok C. Bharti, Professor, Dept. of Zoology, University of
Delhi, for providing SiHa cell line as well as for authenticating the cell lines by PCR and mycoplasma testing.
Disclosure
None
Conflicts of Interest
The authors declare no conflict of interest.
Authors’ Contribution
MM performed the experiments and data acquisition. LK and MM designed the study and drafted the manuscript. SC, NB, SK, SB, RG and LK supervised the study. SM was responsible for analysis of IHC slides. All authors read and
approved the final manuscript.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68 (2018): 394-424.
- Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet 393 (2019): 169-182.
- Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, et al. The Lancet Oncology Commission Challenges to effective cancer control in China, India, and Russia. Lancet Oncology 15 (2014).
- Small W, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer 123 (2017): 2404-2412.
- Kelland LR. Preclinical Perspectives on Platinum Resistance. Drugs 59 (2000): 1-8.
- Nakamura H, Taguchi A, Kawana K, et al. Therapeutic significance of targeting survivin in cervical cancer and possibility of combination therapy with TRAIL. Oncotarget 9 (2018): 13451-1361.
- Galluzzi L, Vitale I, Michels J, et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis 5 (2014): e1257.
- Ossovskaya V, Koo IC, Kaldjian EP, et al. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 1 (2010): 812-821.
- Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science 355 (2017): 1152-1158.
- Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376 (2010): 245-251.
- Mann M, Kumar S, Chauhan SS, et al. PARP-1 inhibitor modulate β-catenin signaling to enhance cisplatin sensitivity in cancer cervix. Oncotarget 10 (2019): 4262-4275.
- Wang Z, Li B, Zhou L, et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc Natl Acad Sci U S A 113 (2016): 13150-13155.
- Kypta RM, Waxman J. Wnt/β-catenin signalling in prostate cancer. Nat Rev Urol 9 (2012): 418-428.
- National Academy of Sciences (U.S.) PW, Tjin E, Meijer HP, et al. Proceedings of the National Academy of Sciences of the United States of America.. Vol. 101, Proceedings of the National Academy of Sciences of the United States of America. The Academy (1915): 6122–6127.
- Clevers H, Nusse R. Wnt/β-Catenin Signaling and Disease. Cell 149 (2012): 1192-1205.
- Tsai Y-P, Yang M-H, Huang C-H, et al. Interaction between HSP60 and β-catenin promotes metastasis. Carcinogenesis 30 (2012): 1049-1057.
- Goto M, Mitra RS, Liu M, et al. Rap1 stabilizes beta-catenin and enhances beta-catenin-dependent transcription and invasion in squamous cell carcinoma of the head and neck. Clin Cancer Res 16 (2010): 65-76.
- Sorenson CM, Eastman A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res 48 (1988): 4484-4488.
- Ormerod M, Orr R, Peacock J. The role of apoptosis in cell killing by cisplatin: a flow cytometric study. Br J Cancer 69 (1994): 93-100.
- Valkenburg KC, Steensma MR, Williams BO, et al. Skeletal metastasis: treatments, mouse models, and the Wnt signaling. Chin J Cancer 32 (2013): 380-396.
- Dasari S, Bernard Tchounwou P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol 740 (2014): 364-378.
- Sauvaget C, Ramadas K, Fayette J-M, et al. Socio-economic factors & longevity in a cohort of Kerala State, India. Indian J Med Res 133 (2011): 479-486.
- Giraldi G, Martinoli L, De Luca d’Alessandro E. The human papillomavirus vaccination: a review of the cost-effectiveness studies. Clin Ter 165 (2014): e426-e432.
- Wang Z, Li B, Zhou L, et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc Natl Acad Sci 113 (2016): 13150-13155.
- Kypta RM, Waxman J. Wnt/β-catenin signalling in prostate cancer. Nat Rev Urol 9 (2012): 418-428.
- Derksen PWB, Tjin E, Meijer HP, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci 101 (2004): 6122-6127.
- Clevers H, Nusse R. Wnt/β-Catenin Signaling and Disease. Cell 149 (2012): 1192-1205.
- Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398 (1999): 422-426.
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 434 (2005): 843-850.
- Mann M, Kumar S, Sharma A, et al. PARP-1 inhibitor modulate β-catenin signaling to enhance cisplatin sensitivity in cancer cervix. Oncotarget 10 (2019): 4262-4275.
- Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55 (2002): 244-265.
- Bosch FX, Manos MM, Munoz N, et al. Prevalence of Human Papillomavirus in Cervical Cancer: a Worldwide Perspective. JNCI J Natl Cancer Inst 87 (1995): 796-802.
- Grigson ER, Ozerova M, Pisklakova A, et al. Canonical Wnt Pathway Inhibitor ICG-001 Induces Cytotoxicity of Multiple Myeloma Cells in Wnt-Independent Manner. Williams BO, editor. PLoS One 10 (2015): e0117693.
- Olmeda D, Castel S, Vilaró S, et al. β-Catenin Regulation during the Cell Cycle: Implications in G2/M and Apoptosis. Mol Biol Cell 14 (2003):2844-2860.
- Orford K, Orford CC, Byers SW. Exogenous Expression of β-Catenin Regulates Contact Inhibition, Anchorage-Independent Growth, Anoikis, and Radiation-Induced Cell Cycle Arrest. J Cell Biol 146 (1999): 855-868.
- Lee HC, Lim S, Han JY. Wnt/β-catenin signaling pathway activation is required for proliferation of chicken primordial germ cells in vitro. Sci Rep 6 (2016): 34510.
- Staibano S, Pepe S, Muzio L Lo, et al. Poly(adenosine diphosphate-ribose) polymerase 1 expression in malignant melanomas from photoexposed areas of the head and neck region. Hum Pathol 36 (2005): 724-731.
- Nosho K, Yamamoto H, Mikami M, et al. Overexpression of poly(ADP-ribose) polymerase-1 (PARP-1) in the early stage of colorectal carcinogenesis. Eur J Cancer 42 (2006): 2374-2381.
- Rojo F, García-Parra J, Zazo S, et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann Oncol 23 (2012): 1156-1164.
- Mego M, Cierna Z, Svetlovska D, et al. PARP expression in germ cell tumours. J Clin Pathol 66 (2013): 607-612.
- Sun Y, Gallacchi D, Zhang EY, et al. Rapamycin-Resistant Poly (ADP-Ribose) Polymerase-1
- Overexpression Is a Potential Therapeutic Target in Lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 51 (2014): 738-749.
- Liu Y, Zhang Y, Zhao Y, et al. High PARP-1 expression is associated with tumor invasion and poor prognosis in gastric cancer. Oncol Lett 12 (2016): 3825-3835.
- Dörsam B, Seiwert N, Foersch S, et al. PARP-1 protects against colorectal tumor induction, but promotes inflammation-driven colorectal tumor progression. Proc Natl Acad Sci 115 (2018): E4061-E4070.
- Resta L, Cascarano MA, Cormio G, et al. Preliminary Study on the Significance of BRCA1 and PARP1 Immunohistochemical Expression in Ovarian Cancer. J Clin Exp Pathol 08 (2018): 1-6.
- Green AR, Caracappa D, Benhasouna AA, et al. Biological and clinical significance of PARP1 protein expression in breast cancer. Breast Cancer Res Treat 149 (2015): 353-362.
