Belantamab Mafodotin: Advancing Treatment Paradigms in Relapsed/Refractory Multiple Myeloma
Article Information
Syed Owais Akhtar1, Bibek Gautam2, Kuchi Deekshitha3, Adees Wirtan Sarkees Bedros4, Fiza Arif1, Iqra Saleem5, Laiba Arif1, Nadir Akhtar6*
1MBBS Student, Jinnah Sindh Medical University, Karachi, Pakistan
2General Practitioner, Primary Health Care, AL Khuwaima PHC-Ministry Of Health Oman, Jaalan Bani Buali, Oman.
3MBBS, Internal Medicine, Sri Venkateshwara Medical College, Tirupati, Andhra Pradesh, India
4Graduate, Internal Medicine, School of Medicine, The University of Jordan, Amman, Jordan.
5BDS Doctor, Oral and Maxillofacial Surgery, Fatima Memorial Hospital, Lahore, Pakistan
*Corresponding author: Nadir Akhtar, Department of Zoology, Quaid-i-Azam University, Islamabad, Pakistan
Received: August 18, 2024; Accepted: August 26, 2024; Published: September 04, 2024
Citation: Syed Owais Akhtar, Bibek Gautam, Kuchi Deekshitha, Adees Wirtan Sarkees Bedros, Fiza Arif, Iqra Saleem, Laiba Arif, Nadir Akhtar. Belantamab Mafodotin: Advancing Treatment Paradigms in Relapsed/Refractory Multiple Myeloma. Journal of Cancer Science and Clinical Therapeutics 8 (2024): 271-286.
View / Download Pdf Share at FacebookAbstract
Belantamab Mafodotin (Belamaf) has emerged as a critical therapeutic advancement for patients with relapsed/refractory multiple myeloma (RRMM), addressing the unmet need for novel treatments in this patient population. Multiple myeloma, a malignant proliferation of plasma cells, often progresses to relapsed/refractory stages where standard treatments become ineffective, resulting in poor prognosis and significant morbidity. Belamaf, a B-cell maturation antigen (BCMA)- targeted antibody-drug conjugate, introduces a novel mechanism by delivering a cytotoxic agent specifically to myeloma cells, thereby minimizing off-target effects. The pivotal clinical trials, including DREAMM-2, have demonstrated substantial efficacy, with impressive overall response rates and prolonged progression-free survival, alongside a manageable safety profile dominated by adverse events such as keratopathy and thrombocytopenia. The U.S. Food and Drug Administration (FDA) approved Belamaf for RRMM patients who have received at least four prior therapies, including an anti-CD38 monoclonal antibody, a proteasome inhibitor, and an immunomodulatory agent. Comparative analyses indicate that Belamaf offers distinct advantages over existing treatment modalities, such as immunomodulatory drugs, proteasome inhibitors, and monoclonal antibodies. Nevertheless, challenges persist, including high treatment costs, limited accessibility, and the management of associated toxicities. Future research directions focus on optimizing patient selection, enhancing combination therapies, and streamlining the manufacturing process. The integration of Belamaf into clinical practice underscores the importance of multidisciplinary care and patient education, signifying a hopeful advancement in the treatment landscape of RRMM through continued innovation and collaborative efforts.
Keywords
<p>Belantamab mafodotin, Multiple myeloma, Relapsed/refractory, BCMAtargeted therapy, Novel therapies, Clinical trials, Treatment efficacy, Adverse events, Patient selection.</p>
Belantamab mafodotin articles; Multiple myeloma articles; Relapsed/ refractory articles; BCMAtargeted therapy articles; Novel therapies articles; Clinical trials articles; Treatment efficacy articles; Adverse events articles; Patient selection articles.
Article Details
Introduction
Patients with relapsed/refractory multiple myeloma may have a treatment history that includes initial therapy with a combination of proteasome inhibitors (such as bortezomib), immunomodulatory drugs (such as lenalidomide), and possibly autologous stem cell transplantation. Despite an initial response, they often experience multiple relapses over time, necessitating the use of various salvage therapies such as pomalidomide, carfilzomib, daratumumab, and possibly even clinical trial participation. With each relapse, the duration of response to treatment tends to shorten, and the options for effective therapies become more limited due to acquired resistance and cumulative toxicities from prior treatments. Patients may also develop treatment-related side effects such as peripheral neuropathy, thrombocytopenia, fatigue, and gastrointestinal issues, further impacting their quality of life. The disease burden of relapsed/refractory multiple myeloma, coupled with the physical and emotional toll of multiple treatment regimens, can significantly diminish patients' quality of life. They may experience increased fatigue, pain, emotional distress, financial strain, and limitations in daily activities. The uncertainty of disease progression and the challenges of managing treatment side effects can further exacerbate the physical and emotional burden on patients and their caregivers [1].
Definition
Multiple myeloma (MM) is a hematological malignancy characterized by clonal plasma cell proliferation accompanied by the production of monoclonal protein and signs of endorgan damage [2]. Plasma cells are mature antibody-producing B cells that reside in the bone marrow and are essential for maintaining humoral immunity [3].
Complications
Multiple myeloma (MM) is a hematological cancer associated with significant symptomatic burden. Bone disease, renal insufficiency, cytopenia infection, and peripheral neuropathy, among other disease manifestations and complications, impair patients’ quality of life [4]. Analysis of 161 patients (94 males,67 females) indicated that the average age was found to be 64 years and common complications included anemia (147;91.3%), bone destruction (99;61.5%), and renal dysfunction (52;32.3%).
Hypertension (39.1%) and diabetes (14.9%) were prevalent comorbidities. Infections were found in 78.3% of patients, with 24.6% microbiologically defined and 75.4% clinically defined [5]. The results of one of the systematic reviews demonstrate a significant risk for severe infection, pneumonia, and neutropenia in patients with MM [6]. Bone disease is a cardinal complication of MM. Bone disease in MM results from dysregulation of bone remodeling, with an increase in osteoclast and a decrease in osteoblast activities [4]. Among osteoclast-activating factors, the RANK ligand promotes osteoclast activity by binding RANK, a membrane receptor on osteoclast lineage cells [7]. Bone involvement in MM is associated with skeletal-related events, defined as
pathological/compression fractures, hypercalcemia, or need for radiation therapy or orthopedic/surgical intervention. Patient height may also be reduced due to vertebral fractures/ compressions. Neurologic symptoms, typically weakness or numbness in the extremities, can occur from nerve, root, spinal cord, or cauda equina compression and are not uncommon. Spinal cord compression due to plasmacytoma or bone fragment retropulsion into the spinal canal is a medical emergency. These clinical manifestations cause morbidity and decreased QoL [4]. Hypercalcemia of malignancy (HCM) is a severe metabolic complication, with the highest incidence found in multiple myeloma (MM). Serum levels of β-CTX, tPINP, and osteocalcin (OC) were significantly elevated, while PTH levels were significantly decreased in patients with hypercalcemia. Serum alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) levels were not associated with calcium abnormalities. Additionally, patients with lytic bone lesions had higher levels of tP1NP compared to those without osteolytic disease. In a multivariate analysis considering serum levels of haemoglobin, phosphorus, β-CTX, tPINP, OC, 25-(OH)2D3, PTH, creatinine, and uric acid, the most important factors influencing hypercalcemia were found to be PTH, creatinine, uric acid, and haemoglobin. The presence of hypercalcemia was strongly linked to advanced disease characteristics, including β2 macroglobulin, hemoglobin, creatinine, uric acid, phosphorus, ISS stage, and R-ISS stage. Among the 357 patients, 52 (14.6%) had renal insufficiency, with 31 (59.6%) of these having hypercalcemia. In our study, 91.2% of patients had lytic bone lesions detected through imaging techniques such as MRI, CT, and PET-CT [8]. Depending on the degree of hypercalcemia, patients may be asymptomatic or may present with manifestations that include anorexia, nausea, vomiting, constipation, polyuria, polydipsia, fatigue, weakness, and even confusion or coma. Hypercalcemia may also contribute to the development or exacerbation of renal insufficiency [4]. Renal failure (RF) is one of the most common and most serious complications of MM that can be caused either by excess immunoglobulins that are nephrotoxic or some other causes like hypercalcemia, infection, etc [9].
Epidemiology
The incidence of MM worldwide is increasing with greater than 140 000 people being diagnosed with MM per year. Whereas 5-year survival after a diagnosis of MM has improved from 28% in 1975 to 56% in 2012, the disease remains essentially incurable [10]. Multiple myeloma accounted for 176 404 (14%) of 1 278 362 incidence cases of leukemia, lymphoma, and multiple myeloma in 2020. The age-standardized rate (ASR) of multiple myeloma incidence was 1•78 (95% UI 1•69–1•87) per 100 000 people globally and mortality was 1•14 (95% UI 1•07–1•21) per 100 000 people globally in 2020. Australia and New Zealand (ASR 4•86 [4•66–5•07]), northern America (4•74 [4•69–4•79]), and northern Europe (3•82 [3•71–3•93]) reported the highest incidence. The lowest incidences were observed in western Africa (0•81 [0•39–1•66]), Melanesia (0•87 [0•55–1•37]), and southeastern Asia (0•96 [0•73–1•27]). Overall, more countries had an increase in incidence, especially in men aged 50 years or older. The countries with the highest incidence increase in men older than 50 years were Germany (AAPC 6•71 [95% CI 0•75–13•02] p=0•027), Denmark (3•93 [2•44–
5•45] p=0•00027), and South Korea (3•25 [0•69–5•88] p=0•019). For women aged 50 years or older, Faroe Islands (21•01 [2•15– 43•34] p=0•032), Denmark (4•70 [1•68–7•82],
p=0•0068), and Israel (2•57 [0•74–4•43] p=0•012) reported the greatest increases. The highest mortality was observed in Polynesia (ASR 2•69 [0•74–9•81]), followed by Australia and New Zealand (1•84 [1•73–1•96]) and northern Europe (1•80 [1•73–1•88]). The lowest mortalities were reported in southeastern Asia (ASR 0•82 [0•62–1•09]), eastern Asia (0•76 [0•71–0•81]), and Melanesia (0•73 [0•61–0•87]). Men (1•41 [1•29–1•53]) were found to have mortality higher than women (0•93 [0•85–1•02]). There was an increasing trend of multiple myeloma incidence globally, particularly in men, people aged 50 years or older, and those from high-income countries. The overall decreasing global trend of multiple myeloma mortality was more evident in women [11].
Standard treatment paradigm
The approach to treatment of NDMM is based on two main factors: risk stratification and eligibility for autologous stem cell transplantation (ASCT). Additional diseases (renal failure, extramedullary disease, plasma cell leukemia, neurological, and renal complications) and patient
characteristics (comorbidities, frailty, thrombotic history, etc.) may further modify the approach [12]. Patients eligible for ASCT should get 3–4 cycles of induction therapy followed by stem cell collection. After collection, patients can undergo ASCT (early ASCT) followed by maintenance; or (for select standard-risk patients) they can continue induction therapy for a few more cycles and shift to maintenance, delaying ASCT till the first relapse (delayed ASCT) [12]. Patients ineligible for ASCT get induction therapy followed by maintenance [12].
Induction therapy
Induction therapy is the first treatment for a disease. Induction therapies are also called first-line therapies or primary therapies. The goal of induction chemotherapy is to reduce tumor size to make it easier for radiation therapy to get rid of cancer cells and to reduce the chances of cancer spreading to distant parts of your body [13]. Induction therapy in transplant-eligible patients includes Triplet regimens (VRdRegimen: Bortezomib, lenalidomide, dexamethasone; VCd or CyBorD Regimen: Bortezomib, cyclophosphamide, dexamethasone; VTd Regimen: Bortezomib, thalidomide, dexamethasone; KRd Regimen: Carfilzomib, lenalidomide, dexamethasone; CD Regimen: Ca rfilzomib, cyclophosphamide,dexamethasone), Quadruplet regimens (Dara-VTd Regimen: Daratumumab, bortezomib, thalidomide, dexamethasone;
Dara-VRd Regimen: Daratumumab, bortezomib, lenalidomide, dexamethasone; Dara-KRd
Regimen: Daratumumab, carfilzomib, lenalidomide, dexamethasone; Dara-IRd
Regimen: Daratumumab, ixazomib, lenalidomide, dexamethasone; KRdc
Regimen: Carfilzomib, lenalidomide, cyclophosphamide, dexamethasone; Isa-VRd
Regimen: Isatuximab, bortezomib, lenalidomide, dexamethasone), Multidrug regimens(VDTPACE Regimen: Bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, etoposide; Dara-VCRd Regimen: Daratumumab, bortezomib, cyclophosphamide, lenalidomide, dexamethasone).
Induction therapy in transplant-ineligible patients includes Triplet Regimens (VRd Regimen: Bortezomib, lenalidomide, dexamethasone; KRd Regimen: Carfilzomib, lenalidomide, dexamethasone; IRd Regimen: Ixazomib, lenalidomide, dexamethasone; DRd Regimen: Daratumumab, lenalidomide, dexamethasone; KCd Regimen: Carfilzomib, cyclophosphamide, dexamethasone; KMP Regimen: Carfilzomib, melphalan, prednisone; Dara-Id Regimen: Daratumumab, ixazomib, dexamethasone), Quadruplet Regimens (Dara-VMP Regimen: Daratumumab, bortezomib, melphalan, prednisone;belamaf-VRd
Regimen: Belantamab mafodotin (Belamaf), bortezomib, lenalidomide, dexamethasone) [12].
Figure 3: (Treatment schema for relapsed refractory multiple myeloma and commonly used regimens)
[Figure 3] Treatment schema for newly diagnosed multiple myeloma and. (n.d.). ResearchGate. https://www.researchgate.net/figure/ Treatment-schema-fornewly https://www.researchgate.net/figure/Treatment-schema-for-newly-diagnosedmultiple-myeloma-and-commonly-used-regimens-ASCT_fig1_336442526diagnosedmultiplemyeloma-and-commonly-used-regimens-ASCT_fig1_336442526
Consolidation therapy
Consolidation chemotherapy is administered after initial treatment to target cancer cells that may still be in your body. During consolidation therapy, chemotherapy drugs are administered in higher doses. The combination of chemotherapy drugs administered is often like during induction therapy [13]
Maintenance therapy
Treatment is given to help keep cancer from coming back after it has disappeared following the initial therapy [14]. Lenalidomide maintenance, PI maintenance, PI + IMiD maintenance, and Daratumumab maintenance are most used for maintenance therapy in MM [12].
Relapsed/refractory multiple myeloma
Relapsed multiple myeloma is cancer that returns after a period of remission while Refractory multiple myeloma is myeloma that does not respond to treatment [15]. As MM progresses, patients experience cycles of relapse and remission, with remission periods becoming increasingly shorter as the disease becomes less treatment-sensitive. The treatment of relapsed refractory MM (RRMM) remains a significant clinical challenge. Patients with RRMM are a highly heterogeneous group and choosing the most appropriate treatment requires careful consideration. Furthermore, the number of treatment options for MM is continually growing with no definitive consensus to guide treating clinicians. [16]. When relapse is encountered, management can be complex and challenging owing to the biological heterogeneity of the disease. Over the past two decades, the focus of translational and clinical research has been on overcoming drug resistance. Novel therapeutics are making it from bench to bedside at a rapid rate, resulting in better disease control and longer lives for patients. Available therapeutic options for RRMM includes IMiDs, PIs, monoclonal antibodies, venetoclax (oral BH3 mimetic and BCL2 inhibitor),Selinexor(first-inclass oral selective inhibitor of nuclear export-SINE),CAR-T/ BITE therapy. The modern era of treatment in RRMM is rapidly evolving, with cellular- and immunotherapy being at the forefront of therapeutic innovation. Treatments such as
chimeric antigen receptor T-cell (CAR-T) therapy, bispecific T-cell engagers (BITEs), and Cereblon. E3 Ligase Modulators (CELMoDs) are demonstrating encouraging responses in the most heavily of pre-treated patients. It is well established that next-generation IMIDs such as POM, next-generation PIs such as CAR and IXA, and monoclonals such as DARA, ISA, and ELO will continue to have substantial roles long term in the relapse/refractory setting. Other agents such as VEN and SELI are finding their own niche in very specific situations. Certain pillars of therapy such as ASCT and chemotherapy will continue to exist as options for the right patient in unique scenarios such as renal disease or extramedullary relapse [17].
Belamaf: A novel therapeutic option mechanism of action
GPRC5D is an orphan G-protein coupled receptor on human chromosome 12p13, with an unknown ligand and unclear function in normal tissue and multiple myeloma [18]. GPRC5D protein has been detected in immune cells and epithelial structures of the skin and tongue [19]. It has been reported that approximately 90% of patients with MM exhibit the expression of GPRC5D in MM cells [18] but show only low/limited expression in normal cells such as those in hard keratinized structures including the hair shaft, nail, central region of the tongue and in the motor neurons of the inferior olivary nucleus of the brainstem in the central nervous system by ISH, but without correlative protein detection by IHC [21- 23].
GPRC5D mRNA or protein expression has not been detected in the cerebral cortex, basal ganglia, midbrain, or cerebellum by ISH or IHC [18]. No data is available to describe possible racial differences in GPRC5D expression. GPRC5D is highly expressed in the bone marrow of patients with MM and latent MM versus other hematological cancers [18]. Consequently, effector cell-mediated therapies such as T cell-redirecting bispecific antibodies and CAR-T cells may find it easy to target the selective expression of GPRC5D.
[19] The FDA recently approved Talquetamab, a T cell- redirecting bispecific antibody that targets GPRC5D, for the treatment of relapsed/refractory (RR) multiple myeloma [29]. Several additional therapies that target GPRC5D have also shown promising outcomes in clinical trials [28-30]. Using a patient's T cells that have been genetically modified to produce CARs that include a scFv, a CD3ζ T-cell receptor protein, and a costimulatory domain (often CD28 or 4-1BB), CAR-T treatment targets tumor cells. Usually, a single dose of CAR-T therapy is given. [31] CAR T-cell therapy involves modifying a patient's T lymphocytes to express chimeric antigen receptors (CARs) that target specific cancer cells. These modified T cells are then infused back into the patient, where they can recognize and destroy the cancer cells. Cytokine release is a natural part of the immune response. CAR T-cells can be further engineered to release cytokines in response to specific triggers, such as encountering their target cancer cells. This controlled release of cytokines can enhance the effectiveness of the therapy.
Ectodomain
The CAR's ectodomain structure is like a monoclonal antibody's Fc region, which usually aids in the immune response but can negatively impact CAR-T cells. Interactions between immune cells and the Fc region may cause T cells to age more quickly, reducing their effectiveness. Scientists are looking into changing the Fc region to improve CAR flexibility and prevent unfavorable interactions. To investigate these changes and their potential for cancer treatment, clinical studies are currently being conducted [20]. Freshly formed protein is guided into the endoplasmic reticulum by the signal peptide. The single-chain variable fragment (scFv) serves as the outer domain signal peptide in the setting of a chimeric antigen receptor (CAR). It is made by using a flexible linker to unite the variable segments of the heavy and light immunoglobulin chains. A scFv with a simple outer domain and distinct recognition components makes up the antigen recognition domain, which enables it to identify any antigen with high affinity. A spacer is required for the connection between the transmembrane domain and the antigen-binding domain. The hinge region of IgG1 is the most basic type of spacer, which is usually adequate for most scFv-based constructs [21].
Hinge domain
The hinge domain, modeled after membrane receptor linkers, may be crucial in controlling binding affinity and signal transduction, especially when targeting low-affinity malignant cells or dim antigens. This hinge domain has not received much attention in the past, but preclinical experiments suggest it could be a significant factor [20].
Transmembrane domain
The transmembrane domain (TM) is a protein transmembrane receptor that links the ectodomain and endodomain of a cell membrane. Its activating signal depends on its selection. While clinical studies are needed to demonstrate its benefits, a reformulated TM could be a common approach for CAR-T cell treatments, reducing tonic signals and extending cell durability [14]. The CD28 transmembrane domain is the most stable receptor [24].
Endodomain
The most prevalent part of the endodomain is CD3 ζ, the functional end of the receptor, which contains three ITAMs [22-23]. It is sent to T cells when receptors aggregate and signal upon antigen recognition, requiring co-stimulatory signaling during this phase [21].
Figure 5: Role of hinge domain (HD) and transmembrane domain (TMD) in CAR. (A) Role of HD in the expression modality and membrane transport efficiency as well as CAR signaling threshold. (B) Role of TMD in CAR intracellular dynamics and surface expression stability
[Figure 5] Fujiwara K, Tsunei A, Kusabuka H, et al. Hinge and transmembrane domains of chimeric antigen receptor regulate receptor expression and signaling threshold. Cells 9 (2020): 1182.
BCMA role and belantamab mafodotin mechanism of action
The tumor necrosis factor superfamily includes BCMA, also known as TNFRSF17 or CD269, which is expressed in mature B lymphocytes and plasma cells. Its interactions with its natural ligands, such as B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), govern PC survival and proliferation, leading to the development of multiple sclerosis (MM). Belantamab mandolin, a humanized fucosylated monoclonal IgG1 antibody directed by BCMA, is conjugated to MMAF, which activates caspase- 3-dependent apoptosis. The antibody's fucosylation binds to NK cells carrying Fc, resulting in antibody-dependent cellular cytotoxicity (ADCC). This leads to antibody-dependent cellular phagocytosis (ADCP). The antibody inhibits BCMA's interaction with its natural ligands. (Figure 2) [24].
The drug-BCMA interaction enhances caspase- 3dependent apoptosis upon MMAF intracellular release. The fucosylation of the antibody allows it to interact with Fc- bearing NK cells, resulting in antibody-dependent cellular cytotoxicity (ADCC). Macrophages also cause cell death through antibody-dependent cellular phagocytosis (ADCP), which occurs after apoptotic MM cells release antigens [24]. Belamaf, a single treatment for relapsed or treatment- resistant multiple myeloma, was granted FDA expedited clearance in August 2020. It is a first-in-class biologic for patients who have tried four prior treatments, including an anti-CD38 monoclonal antibody, proteasome inhibitor, and immunomodulatory drug [25]. Belamaf, the first licensed anti-B cell maturation antigen (BCMA), has the potential to improve progression-free survival in patients with multiple myeloma with limited therapy alternatives. BCMA is expressed in all CD138+ myeloma cells. Belamaf's receptor specificity permits it to target exclusively malignant MM plasma cells [25].
Target Antigen-B-Cell Maturations Antigen (BCMA)
The specificity of targeting MM cells is due to the mAb component, which targets the B-cell maturation Antigen (BCMA), a tumor-associated antigen expressed on mature B-cells and plasma cells. BCMA helps plasma cells mature and differentiate and is overexpressed during malignant transformation, making it a suitable target in MM therapy [25]. High-affinity BCMA ligands, B-cell activating factor (BAFF), and APRIL increase the proliferation and viability of MM cells in the bone marrow. BAFF is a BCMA agonist that promotes differentiation, proliferation, and antibody production [25]. The binding of belantamab to BCMA receptors inhibits the pro-survival cytokine-signaling actions of BAFF and APRIL in malignant plasma cells [25]. Belamaf, a first-in-class ADC, combines an afucosylated humanized
IgG1 anti-BCMA mAb with monomethyl auristatin F (MMAF) via a protease-resistant linker [26]. Preclinical investigations have shown belamaf's anti-MM efficacy through various modes of action, including direct apoptosis, antibody dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and immunogenic cell death [26]. Belamaf has the ability to quickly eradicate MM cells while protecting normal bone marrow stromal cells and immune effector cells when treated in co-culture [26].
Adverse effects
Adverse effects of belantamab mafodotin include a significant incidence of keratopathy (≥20%). [27] If ocular toxicity occurs, such as blurred vision, dry eyes, or corneal ulcers, the dosage can be lowered or discontinued and should be stopped if the ocular toxicity is severe [27]. Other less common side effects include thrombocytopenia, infusion- related responses, pyrexia, tiredness, nausea, constipation, diarrhea, arthralgia, back pain, reduced appetite, and upper respiratory infections [27] The most prevalent grade 3 or 4 laboratory abnormalities (≥5%) include reductions in neutrophils, lymphocytes, platelets, and hemoglobin, as well as elevations in gamma-glutamyl transferase and creatinine [27]. Belantamab is a big protein molecule, its excretion in breast milk is thought to be extremely low. However, belantamab is conjugated with mafodotin, a small- molecule poison that can be eliminated in milk. As a result, it is suggested that patients utilize effective contraception and refrain from nursing while on the medicine and for three months following the final dosage [27].
Ocular toxicity
In part one of the DREAMM-1 study, where Belamaf dosages varied from 0.03 mg/kg to 4.60 mg/kg, ocular damage was more common at higher doses than at lower levels [28]. Ocular damage was seen in 63% of part-2 patients who received a belamaf dosage of 3.40 mg/kg. In part 1, 29% (n=11) of patients experienced blurred vision, compared to 46% (n=16) in part 2. In part one of DREAMM-1, 24% (n=9) of patients experienced dry eyes, compared to 34% (n=12) in part two. In DREAMM-2, keratopathy was the most prevalent ocular toxicity (73%, n=141), regardless of its degree (71% in
2.5 mg/kg against 75% in 3.4 mg/kg), and the most common symptoms were similarly blurred vision (22% for 2.5 mg/kg
Figure 7:B-cell maturation and differentiation into PC. These three functionally related receptors are type III transmembrane proteins lacking a signal-peptide and containing cysteine-rich extracellular domains
[Figure 7] Tai Y, and Anderson K C. Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy 7 (2015): 1187-1199. https:// doi.org/10.2217/imt.15.7
versus 30% for 3.4 mg/kg) and dry eyes (14%for 2.5 mg/kg versus 23% for 3.4 mg/kg). [28] Patients with a history of dry eyes were more likely to acquire corneal abnormalities, as seen in DREAMM-2. Belantamab mafodotin is linked with substantial ocular toxicity, particularly in the context of preexisting ocular problems. Serial ocular exams using the KVA scale and artificial tears are the most effective ways to reduce toxicity. Belamaf adverse effects in the eyes should be treated with dosage adjustments, pauses, or discontinuations. To guarantee the safe use of belamaf, patients should be continuously evaluated by ophthalmology and hematology- oncology follow-ups [28].
Overview of current treatment landscape for multiple myeloma
Various combinations of drugs from different classes have been explored for the treatment of newly diagnosed multiple myeloma (NDMM). These include immunomodulatory agents (IMiDs), proteasome inhibitors (PIs), anti-CD38 monoclonal antibodies, alkylating agents like cyclophosphamide and melphalan; corticosteroids such as dexamethasone and prednisone; and other drugs including etoposide, doxorubicin, and cisplatin [29].
IMiDs
Immunomodulatory drugs fight tumors through multiple mechanisms. By binding to cereblon, they increase the ubiquitination and degradation of key transcription factors Ikaros (IKZF1) and Aiolos (IKZF3), which are essential for B cell and plasma cell development. They also boost immune responses by enhancing natural killer and T cell activity and increasing interleukin 2 and interferon-γ levels. IMiDs include thalidomide, lenalidomide, and pomalidomide and iberdomide. Anti CD38 monoclonal antibodies: CD38 is a transmembrane glycoprotein with ecto enzymatic activity, highly and uniformly expressed on multiple myeloma (MM) cells. CD38 antibodies act through pleiotropic mechanisms, including Fc-dependent immune effector functions, direct apoptotic activity, and immunomodulatory effects by eliminating CD38+ immune-suppressor cells. The specificity of this target has spurred interest in new drugs and led to the development of CD38 monoclonal antibodies like Daratumumab (fully human) and Isatuximab (chimeric) [30].
PIs
Proteasome inhibitors (PIs) such as Bortezomib and Carfilzomib cause the accumulation of unfolded and misfolded proteins, leading to apoptosis and cell death through mechanisms like ER stress, reactive oxygen species production, JNK, and p53 activation, the induction of cyclin- dependent kinase inhibitors, and pro-apoptotic proteins [31]. CAR T-cell therapy: Chimeric antigen receptor (CAR) T-cell therapies, which induce T-cell killing of target cancer cells, have significantly improved outcomes for patients with hematologic malignancies. B-cell maturation antigen (BCMA) is the primary target for most CAR T-cell products being investigated for multiple myeloma (MM). Currently, two FDA-approved CAR T-cell therapies for relapsed/
Figure 8: Shows Keratopathy and Visual Acuity Scale (KVA) and recommended treatment modifications of BelantamabMafodotin (BCVA, best corrected visual acuity; belamaf, belantamab mafodotin; G, grade. [28]
[Figure 8] Wahab A, Rafae A, Mushtaq K, et al. Ocular toxicity of Belantamab Mafodotin, An Oncological Perspective of Management in Relapsed and Refractory Multiple Myeloma. Frontiers in Oncology 11 (2021). https://doi.org/10.3389/fonc.2021.678634
Figure 9: IMiDs, including thalidomide, pomalidomide and lenalidomide, possess antimyeloma effects via binding to cereblon, a critical component of the E3 ubiquitin ligase complex
[Figure 9] Cornell R F, and Kassim A A. Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: increased options and increased complexity. Bone Marrow Transplantation 51 (2016): 479-491. https://doi.org/10.1038/bmt.2015.307
refractory multiple myeloma (RRMM) are idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) [32].
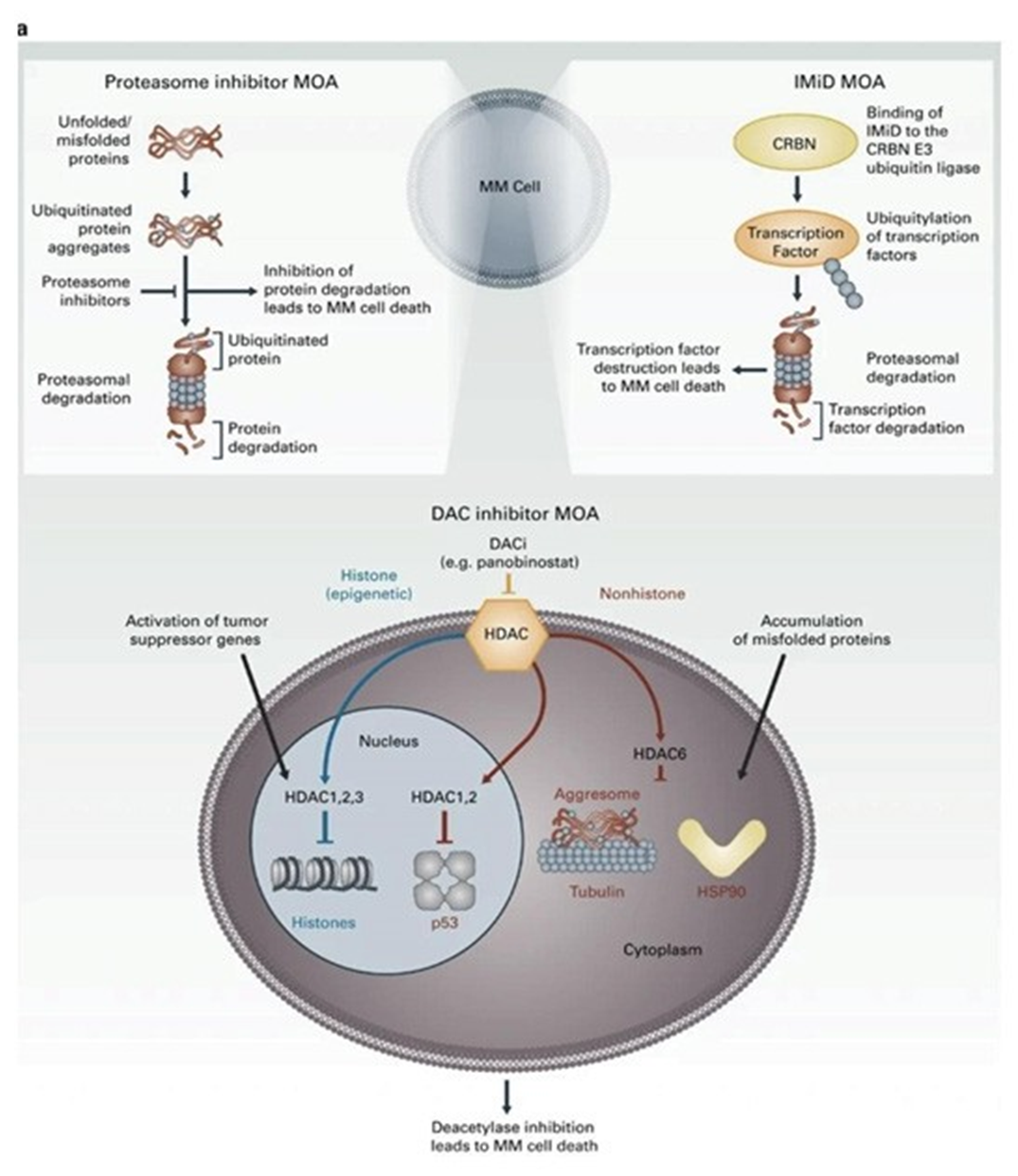
HDAC inhibitors
Histone deacetylase inhibitors (HDAC inhibitors) treat multiple myeloma by blocking the action of histone deacetylases, enzymes that remove acetyl groups from histone proteins. This inhibition leads to increased acetylation of histones, resulting in a more relaxed chromatin structure and altered gene expression. The changes in gene expression can trigger cell cycle arrest, apoptosis, and differentiation in myeloma cells. HDAC inhibitors also enhance the effects of other anti-myeloma agents and can modulate the immune response, contributing to their anti-tumor activity. An example of a histone deacetylase inhibitor used in the treatment of multiple myeloma is panobinostat [33].
Belemaf/blenrep vs. Standard of care
Blenrep is a type of antibody-drug conjugate where a humanized monoclonal antibody targeting B-cell maturation antigen (BCMA) is coupled to the cytotoxic agent auristatin F using a non-cleavable linker [34].
Clinical trial for Belamaf
DREAMM-2 was a phase II, open-label, multicenter trial in RRMM patients who had received ≥3 prior therapies including a PI, an IMiD, and an anti-CD38 mAb. Initially planning to evaluate belantamab mafodotin at 3.4 mg/kg every 3 weeks based on DREAMM1, the trial was amended after FDA discussions on ocular toxicity to include 2.5 mg/ kg. Following safety and efficacy assessments, 2.5 mg/kg every 3 weeks was proposed as the monotherapy dose. The trial spanned North America, Europe, and Australia, with 14% Black or African American participants. The median patient age was 66 years (U.S. MM diagnosis median: 69); 15% were ≥75 years old. In the 2.5 mg/kg group, 27% had high-risk cytogenetics, and 23% had extramedullary disease. All were double refractory to PI/IMiD and had prior anti- CD38 mAb therapy. Stem cell transplantation was prior in 75%, with 2.5 mg/kg patients having a median of 7 prior lines of therapy (range: 3–21). Efficacy in DREAMM-2 was determined by the Independent Review Committee (IRC) assessing ORR using International Myeloma Working Group criteria and duration of response (DOR). Among 97 patients receiving belantamab mafodotin at 2.5 mg/kg every 3 weeks, ORR was 31% (97.5% CI: 21– 43; exact method). Responses included stringent complete response in 2, complete response in 1, very good partial response in 15, and partial response in 12 patients. Median DOR was not reached (95% CI: not reached–not reached); 73% of responders had DOR ≥6 months at median follow-up of 6.3 months. Subgroup analyses generally aligned with primary findings, except for patients with extramedullary disease and those ≥75 years, showing potentially lower ORR compared to the overall cohort [36].
DREAMM-3, a phase III open-label randomized trial comparing belamaf to pomalidomide (Pomalyst) plus low- dose dexamethasone in patients with relapsed/refractory MM, is ongoing. As opposed to the single-arm DREAMM-2 study, DREAMM-3 will provide a direct comparison of belamaf to the standard of care. DREAMM-12 and DREAMM-13 trials will assess safety and efficacy in patients with severe renal and hepatic impairment [36] DRE.AMM- 6 (NCT03544281) ARM A investigated the combination of belantamab mafodotin with lenalidomide and dexamethasone in heavily pre-treated multiple myeloma (MM) patients, who had received 1–11 prior therapies, with 58% having previous lenalidomide treatment. The cohort receiving 1.9 mg/kg every 4 weeks (Q4W) showed the highest overall response rate (ORR) at 75%, likely attributed to a low incidence of grade
≥3 adverse events and subsequent minimal interruptions in
treatment [37]. In DREAMM-6 (NCT03544281) ARM B, 18
patients received belantamab mafodotin at 2.5 mg/kg every 3 weeks alongside bortezomib and dexamethasone. Notably, 89% of patients had prior exposure to bortezomib and half to daratumumab. The overall response rate (ORR) was 78%, particularly high at 75% among those previously exposed to proteasome inhibitors (PIs), with many achieving very good partial response (VGPR) or better outcomes. This was more pronounced in less heavily pre-treated patients.
Keratopathy and thrombocytopenia were the most common adverse events [38]. In phase I/II trial DREAMM-4 (NCT03848845), which evaluated belantamab mafodotin combined with pembrolizumab in patients with relapsed or refractory multiple myeloma who had received ≥3 prior lines of therapy, the overall response rate (ORR) was 47%, with most responses exceedingly very good partial response (VGPR). At a median follow-up of 14.7 months, the median progression-free survival (PFS) was 3.4 months. The most common adverse events (AEs) were keratopathy and blurred vision, occurring in 35% of patients, followed by thrombocytopenia. There were no substantial differences observed in new AEs or efficacy compared to belantamab mafodotin monotherapy [39] .
Future research and challenges
Multiple myeloma remains untreated despite the introduction of targeted biological medicines and immunotherapy in the last decade. Relapse and refractory illness pose significant challenges in pharmacologic therapy. The FDA has authorized the first drug in its class, belantamab mandolin-bumf, to treat relapsed or treatment-resistant multiple myeloma [40]. DREAMM-1, a phase I clinical study, looked at the safety and effectiveness of GSK2857916 (belantamab mafodotin) in two sections. Part I, the dosage- escalation phase, investigated the drug's safety, tolerability, maximum tolerated dose (MTD), and recommended phase II dose (RP2D) in multiple myeloma patients with progressing or refractory illness. Simply said, the book summarizes two clinical studies (DREAMM-1 and DREAMM-2) that investigated the use of a medication known as belamaf in patients with progressive and resistant multiple myeloma. The trials examined various doses of belamaf and assessed its efficacy and safety. In the DREAMM-1 experiment, patients were treated with belamaf, and it was discovered that a dose of 3.4 mg/kg produced promising outcomes, with some patients responding well. Common adverse effects were ocular problems, anemia, and thrombocytopenia. The research indicated that belamaf at the prescribed dose was both efficacious and well-tolerated in individuals with multiple myeloma who had not responded to previous therapies. In the DREAMM-2 study, belamaf's effectiveness and safety were evaluated in two dose groups: 2.5 mg/kg and 3.4 mg/ kg. Both groups had undergone several previous treatments. The findings revealed that belamaf had a response rate at both levels, with the higher dosage group doing somewhat better. Common adverse effects were ocular problems, anemia, and thrombocytopenia. The study revealed that belamaf was helpful in individuals who had not reacted to several prior treatments, and stressed the need for more trials to compare its efficacy to established medicines [40].
Efficacy of Belamaf
In this study, five patients were given belamafat at a dosage of 3.4 mg/kg every three weeks to see how it affected the cornea. To prevent corneal difficulties associated with belamaf, patients were given steroid eye drops before treatment, and any symptoms such as hazy vision or dry eyes were treated with preservative-free lubricant eye drops and additional steroids. All patients reported some corneal difficulties, with three of them having severe grade III adverse events throughout a 32.6-month follow-up period. Because to these concerns, each patient's treatment had to be interrupted, and the dosage was lowered twice throughout the treatment. Neither of the patients had the most severe grade IV or V corneal adverse effects. Importantly, after discontinuing belamaf medication, all patients saw improvement in corneal adverse events within a year. While the optimal strategy to treat belamaf-related eye problems is still being researched, the authors recommend using fewer steroid eye drops, preservative-free lubricants, and changing the dosage. Belamaf performed well in this tiny research, with two patients achieving a strict complete response (sCR), one achieving a full response (CR), and two achieving a very good partial response (VGPR) [40].
Significance of recognition of cart therapy adverse effects
CART cell therapy has demonstrated promising outcomes in clinical studies, but the adverse effects are a significant barrier to its general usage [41]. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are the two most serious side effects, and they can be deadly in some circumstances.CRS is an inflammatory disorder in which T cells become hyperactive and produce many cytokines, resulting in symptoms ranging from moderate to severe. This generally occurs soon after CART cell treatment is administered. Severe instances of CRS can exhibit symptoms like macrophage activation syndrome, including high levels of certain markers in the blood. IFN-γ, an inflammatory cytokine released by immune cells such as CART cells, can increase the severity of CRS. Using IFN-γ inhibitors may diminish the efficacy of CART cells. On the other hand, ICANS is a neurological adverse effect that might develop in certain individuals undergoing CART cell treatment. It can produce symptoms such as disorientation, tiredness, and convulsions. The actual etiology of ICANS is not entirely understood, however, it appears to be associated with CRS in many cases. Additionally, a typical long-term adverse effect of CART cell treatment is B cell depletion, which can lead to decreased immunological function. Although immunoglobulin replacement can assist, close monitoring is required to identify any delayed issues caused by a shortage of B cells [41].
Management of toxicity of cart therapy
Various therapeutic techniques for cytokine release syndrome (CRS), a possible adverse effect of some immunotherapies. Supportive treatment consists of treating the symptoms of low blood pressure and low oxygen levels using intravenous fluids, blood pressure medicines, and oxygen therapy. The benefits include efficiently maintaining organ function and minimizing future damage. However, this strategy fails to address the root cause of CRS.
Tocilizumab: This medicine inhibits the activity of interleukin-6 (IL-6), a major cytokine linked to CRS. It can quickly reverse CRS by targeting cytokines in the CRS cascade. Tocilizumab cannot pass the blood-brain barrier, making it ineffective for treating neurotoxicity caused by CRS. Steroids: These drugs cause nonspecific immunosuppression, which reduces inflammation. They are effective in many cases of CRS that are not responsive to Tocilizumab. However, non-targeted immunosuppression may restrict the efficacy and duration of chimeric antigen receptor T cell (CAR-T) therapy used to treat some malignancies. Researchers have begun investigating defibrotide to determine whether it can help prevent ICANS in patients getting CART cell treatment for a particular form of cancer. Defibrotide is already licensed to treat liver disease in both adults and children. The theory is that defibrotide may protect certain cells in the brain and spinal cord, potentially lowering the risk of ICANS in CART patients. However, there is presently no study on utilizing defibrotide to address the adverse effects of CART therapy before these clinical studies [41]. The future of CART (Chimeric Antigen Receptor T-cell therapy) is to deliver and monitor it in outpatient settings rather than hospitals. This transition necessitates meticulous preparation to maintain patient safety, monitoring, and flexibility to improve patient happiness. There should be clear standards in place to address emergencies quickly, including transporting patients to the hospital if necessary. Monitoring quality measures, such as reaction time to treatments, is critical. Moving CART therapy to outpatient settings allows hospital resources to be used more efficiently, and the expenses associated with this treatment technique may become more advantageous. This modification is likely to increase patient access to CART therapy by allowing them to get treatment at home, possibly expanding the accessibility of this life-saving medicine. Furthermore, the effective implementation of an outpatient cellular therapy program necessitates comprehensive patient and caregiver education, as the patient and caregiver bear a significant portion of the duty for delivering care. All members of the healthcare team should be involved in giving this education at several critical moments during the cellular treatment journey [41].
Inhancing efficacy of belamaf
We can improve therapy effectiveness and outcomes by optimizing patient selection based on biomarkers such as BCMA and GPRC5D, high recurrence risk, toxicity, tumor nutritional load, and immune microenvironment features. A combination therapy, which combines belamaf with mafodotin, selinexor, and melflufen, has demonstrated promising activity and safety in the treatment of relapsed/ refractory MM, overcoming the resistance mechanism and improving the response rate and survival outcomes [42, 43].
Summary of Key Findings
This review has highlighted several critical points regarding the treatment of relapsed/refractory multiple myeloma. Firstly, there is a significant unmet need for novel therapies in this patient population due to the limited efficacy of existing treatment options and the typically poor prognosis. Belamaf (belantamab mafodotin) has emerged as a promising treatment option, primarily due to its novel mechanism of action, which targets B-cell maturation antigen (BCMA) on multiple myeloma cells. Clinical trial data have shown encouraging results, indicating belamaf's potential to improve outcomes for these patients. However, belamaf also has potential advantages and disadvantages compared to other therapies. Its unique mechanism offers a new avenue for treatment, but it also presents challenges such as managing its side effects, particularly ocular toxicity. The ongoing research and clinical trials are crucial in addressing these challenges and further establishing belamaf's role in therapy.
Impact on Clinical Practice
Belamaf is poised to significantly change the treatment paradigm for relapsed/refractory multiple myeloma. It underscores the importance of early referral to specialized centers where patients can be evaluated for eligibility to receive this novel therapy. Moreover, the management of patients receiving belamaf necessitates a multidisciplinary approach, involving hematologists, oncologists, nurses, pharmacists, and other healthcare professionals to provide comprehensive care. Patient education and shared decision- making are also crucial, as patients need to be well-informed about their treatment options, including the potential risks and benefits of belamaf.
Looking ahead, the future of multiple myeloma treatment appears hopeful, with ongoing research and development of novel therapies like belamaf. The contributions of researchers, clinicians, patients, and advocacy groups have been instrumental in advancing the field and improving patient outcomes. As we continue to explore and develop new treatments, the collective efforts of the medical community will be essential in bringing these innovations to fruition and ultimately, in enhancing the lives of patients with multiple myeloma.
References
- Jurczyszyn A, Waszczuk-Gajda A, Castillo J J, et al. Primary refractory multiple myeloma: a real-world experience with 85 Leukemia & Lymphoma/ Leukemia and Lymphoma 61 (2020): 2868-2875.
- Jurczyszyn A, and Suska Multiple myeloma. In Elsevier eBooks (2019).
- Padala S A, Barsouk A, Barsouk A, et al. Epidemiology, staging, and management of Multiple myeloma. Medical Sciences 9 (2021): 3.
- LeBlanc R, Bergstrom D J, Côté J, Kotb R, et Management of Myeloma Manifestations and Complications: The Cornerstone of Supportive Care: Recommendation of the Canadian Myeloma Research Group (formerly Myeloma Canada Research Network) Consensus Guideline Consortium. Clinical Lymphoma Myeloma & Leukemia 22 (2022): 41-56.
- Lin C, Shen H, Zhou S, et al. Assessment of infection in newly diagnosed multiple myeloma patients: risk factors and main characteristics. BMC Infectious Diseases 20 (2020).
- Balmaceda N, Aziz M, Chandrasekar V T, et Infection risks in multiple myeloma: a systematic review and meta- analysis of randomized trials from 2015 to 2019. BMC Cancer 21 (2021).
- Raje N S, Bhatta S, and Terpos Role of the RANK/RANKL pathway in multiple myeloma. Clinical Cancer Research 25 (2019): 12-20.
- Bao L, Wang Y, Lu M, et al. Hypercalcemia caused by humoral effects and bone damage indicate poor outcomes in newly diagnosed multiple myeloma patients. Cancer Medicine 9 (2020): 8962-8969.
- Kundu S, Jha S B, Rivera A P, et Multiple myeloma and renal failure: Mechanisms, diagnosis, and management. Cureus (2022).
- Hemminki K, Försti A, Houlston R, et al. Epidemiology, genetics and treatment of multiple myeloma and precursor International Journal of Cancer 149 (2021): 1980-1996.
- Huang J, Chan S C, Lok V, et al. The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal The Lancet Haematology 9 (2022): 670-677.
- Goel U, Usmani S, and Kumar Current approaches to management of newly diagnosed multiple myeloma. American Journal of Hematology 97 (2022).
- Yetman D. Induction Chemotherapy vs. Consolidation Therapy: What to Healthline (2021).
- Adjuvant therapy: Treatment to keep cancer from Mayo Clinic (2024)
- Ahmed A, and Killeen R (2023, June 8). Relapsed and refractory multiple myelo]\ma. StatPearls - NCBI Bookshelf (2023).
- Ramasamy K, Gay F, Weisel K, et Improving outcomes for patients with relapsed multiple myeloma: Challenges and considerations of current and emerging treatment options. Blood Reviews 49 (2021): 100808.
- Bhatt P, Kloock C, and Comenzo Relapsed/Refractory Multiple Myeloma: A review of available therapies and clinical scenarios encountered in myeloma relapse. Current Oncology 30 (2023): 2322-2347.
- Rodriguez-Otero P, Van De Donk N W C J, Pillarisetti K, et al. GPRC5D as a novel target for the treatment of multiple myeloma: a narrative Blood Cancer Journal 14 (2024b).
- Tomita U, Ishimoto Y, Ri M, et A novel T cell- redirecting anti-GPRC5D × CD3 bispecific antibody with potent antitumor activity in multiple myeloma preclinical models. Scientific Reports 14 (2024).
- Huang R, Li X, He Y, et al. Recent advances in CAR-T cell engineering. Journal of Hematology & Oncology 13 (2020b).
- Shah K, Al-Haidari A, Sun J, et al. T cell receptor (TCR) signaling in health and disease. Signal Transduction and Targeted Therapy 6 (2021).
- Baines A C, Ershler R, Kanapuru B, et FDA Approval Summary: Belantamab Mafodotin for Patients with Relapsed or Refractory Multiple Myeloma. Clinical Cancer Research 28 (2022): 4629-4633.
- Ketchum E B, Clarke A, and Clemmons A B. (2022). Belantamab Mafodotin-BLMF: A novel Antibody- Drug conjugate for treatment of patients with Relapsed/ Refractory Multiple Myeloma. Journal of the Advanced Practitioner in Oncology 13 (2022): 77-85.
- Quach H, Gironella M, Lee C, et (2022). Safety and clinical activity of belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma (RRMM): DREAMM-6 arm-A interim analysis. Journal of Clinical Oncology 40 (2022): 8017.
- Lassiter G, Bergeron C, Guedry R, et al. Belantamab Mafodotin to Treat Multiple Myeloma: A Comprehensive review of disease, drug efficacy and side effects. Current Oncology 28 (2021b): 640-660.
- Becnel M R, and Lee H The role of belantamab mafodotin for patients with relapsed and/or refractory multiple myeloma. Therapeutic Advances in Hematology 11 (2020b).
- Lassiter G, Bergeron C, Guedry R, et al. Belantamab Mafodotin to Treat Multiple Myeloma: A Comprehensive review of disease, drug efficacy and side effects. Current Oncology 28 (2021b): 640-660.
- Wahab A, Rafae A, Mushtaq K, et al. (2021c). Ocular toxicity of Belantamab Mafodotin, An Oncological Perspective of Management in Relapsed and Refractory Multiple Frontiers in Oncology 11 (2021c).
- Goel U, Usmani S, and Kumar Current approaches to management of newly diagnosed multiple myeloma. American Journal of Hematology 97 (2022d).
- Gozzetti A, Ciofini S, Simoncelli M, et al. Anti CD38 monoclonal antibodies for multiple myeloma treatment. Human Vaccines & Immunotherapeutics 18 (2022b).
- Ito S. Proteasome inhibitors for the treatment of multiple Cancers 12 (2020d): 265.
- Rendo M J, Joseph J J, Phan L M, et al. CAR T-Cell Therapy for Patients with Multiple Myeloma: Current Evidence and Blood and Lymphatic Cancer Targets and Therapy 12 (2022b): 119-136.
- Harada T. Hideshima T, and Anderson K C. Histone deacetylase inhibitors in multiple myeloma: from bench to International Journal of Hematology 104 (2016): 300-309.
- Matula Z, Uher F, Vályi-Nagy I, et al. The Effect of Belantamab Mafodotin on Primary Myeloma–Stroma Co- Cultures: Asymmetrical Mitochondrial Transfer between Myeloma Cells and Autologous Bone Marrow Stromal International Journal of Molecular Sciences 24 (2023): 5303.
- Baines A C, Ershler R, Kanapuru B, et al. (2022b). FDA Approval Summary: Belantamab Mafodotin for Patients with Relapsed or Refractory Multiple Myeloma. Clinical Cancer Research 28 (2022b): 4629-4633.
- Ketchum E B, Clarke A, and Clemmons, B. Belantamab Mafodotin-BLMF: A novel Antibody-Drug conjugate for treatment of patients with Relapsed/Refractory Multiple Myeloma. Journal of the Advanced Practitioner in Oncology 13 (2022b): 77-85.
- Quach H, Gironella M, Lee C, et al. Safety and clinical activity of belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma (RRMM): DREAMM-6 arm-A interim Journal of Clinical Oncology 40 (2022b): 8017.
- Popat R, Nooka A, Stockerl-Goldstein K, et DREAMM-6: Safety, Tolerability and Clinical Activity of Belantamab Mafodotin (Belamaf) in Combination with Bortezomib/Dexamethasone (BorDex) in Relapsed/Refractory Multiple Myeloma (RRMM). Blood 136 (2020b): 19-20.
- Trudel S, Nooka A, Fecteau D, et DREAMM 4: A phase I/II single-arm open-label study to explore safety and clinical activity of belantamab mafodotin (GSK2857916) administered in combination with pembrolizumab in patients with relapsed/refractory multiple myeloma (RRMM). Annals of Oncology 30 (2019b): 447.
- Lassiter G, Bergeron C, Guedry R, et al. Belantamab Mafodotin to Treat Multiple Myeloma: A Comprehensive review of disease, drug efficacy and side effects. Current Oncology 28 (2021e): 640-660.
- Zahid A, Siegler E L, and Kenderian S CART Cell Toxicities: New Insight into Mechanisms and Management. Clinical Hematology International 2 (2020b): 149.
- Sheikh S, Lebel E, and Trudel Belantamab mafodotin in the treatment of relapsed or refractory multiple myeloma. Future Oncology 16 (2020): 2783-2798.
- McCurdy A, and Visram The role of belantamab mafodotin, selinexor, and melflufen in multiple myeloma. Current Hematologic Malignancy Reports 17 (2022b): 306-318.