Atypical Appearance of Pneumocystis carinii pneumonia Without Ground-Glass Opacities
Article Information
Aatif Amir Husain1*, Rahul Khamar2, Raees Lunat3, Ruhaid Khurram4
1Intensive Care Department, Imperial College Healthcare NHS Trust, United Kingdom
2Radiology Department, Guy's and St Thomas' NHS Foundation Trust, United Kingdom
3Accident and Emergency Department, Royal Free London NHS Foundation Trust, United Kingdom
4Radiology Department, Royal Free London NHS Foundation Trust, United Kingdom
*Corresponding Author: Dr. Aatif Amir Husain, Intensive Care Department, Imperial College Healthcare NHS Trust, United Kingdom
Received: 24 September 2020; Accepted: 07 October 2020; Published: 10 October 2020
Citation: Aatif Amir Husain, Rahul Khamar, Raees Lunat, Ruhaid Khurram. Atypical Appearance of Pneumocystis carinii pneumonia Without Ground-Glass Opacities. Journal of Radiology and Clinical Imaging 3 (2020): 089-091.
View / Download Pdf Share at FacebookAbstract
Keywords
Pneumocystis; Computed Tomography; Ground-glass; HIV; Atypical Pneumonia
Pneumocystis articles; Computed Tomography articles; Ground-glass articles; HIV articles; Atypical Pneumonia articles
Pneumocystis articles Pneumocystis Research articles Pneumocystis review articles Pneumocystis PubMed articles Pneumocystis PubMed Central articles Pneumocystis 2023 articles Pneumocystis 2024 articles Pneumocystis Scopus articles Pneumocystis impact factor journals Pneumocystis Scopus journals Pneumocystis PubMed journals Pneumocystis medical journals Pneumocystis free journals Pneumocystis best journals Pneumocystis top journals Pneumocystis free medical journals Pneumocystis famous journals Pneumocystis Google Scholar indexed journals Computed Tomography articles Computed Tomography Research articles Computed Tomography review articles Computed Tomography PubMed articles Computed Tomography PubMed Central articles Computed Tomography 2023 articles Computed Tomography 2024 articles Computed Tomography Scopus articles Computed Tomography impact factor journals Computed Tomography Scopus journals Computed Tomography PubMed journals Computed Tomography medical journals Computed Tomography free journals Computed Tomography best journals Computed Tomography top journals Computed Tomography free medical journals Computed Tomography famous journals Computed Tomography Google Scholar indexed journals Ground-glass articles Ground-glass Research articles Ground-glass review articles Ground-glass PubMed articles Ground-glass PubMed Central articles Ground-glass 2023 articles Ground-glass 2024 articles Ground-glass Scopus articles Ground-glass impact factor journals Ground-glass Scopus journals Ground-glass PubMed journals Ground-glass medical journals Ground-glass free journals Ground-glass best journals Ground-glass top journals Ground-glass free medical journals Ground-glass famous journals Ground-glass Google Scholar indexed journals HIV articles HIV Research articles HIV review articles HIV PubMed articles HIV PubMed Central articles HIV 2023 articles HIV 2024 articles HIV Scopus articles HIV impact factor journals HIV Scopus journals HIV PubMed journals HIV medical journals HIV free journals HIV best journals HIV top journals HIV free medical journals HIV famous journals HIV Google Scholar indexed journals Atypical Pneumonia articles Atypical Pneumonia Research articles Atypical Pneumonia review articles Atypical Pneumonia PubMed articles Atypical Pneumonia PubMed Central articles Atypical Pneumonia 2023 articles Atypical Pneumonia 2024 articles Atypical Pneumonia Scopus articles Atypical Pneumonia impact factor journals Atypical Pneumonia Scopus journals Atypical Pneumonia PubMed journals Atypical Pneumonia medical journals Atypical Pneumonia free journals Atypical Pneumonia best journals Atypical Pneumonia top journals Atypical Pneumonia free medical journals Atypical Pneumonia famous journals Atypical Pneumonia Google Scholar indexed journals carinii pneumonia articles carinii pneumonia Research articles carinii pneumonia review articles carinii pneumonia PubMed articles carinii pneumonia PubMed Central articles carinii pneumonia 2023 articles carinii pneumonia 2024 articles carinii pneumonia Scopus articles carinii pneumonia impact factor journals carinii pneumonia Scopus journals carinii pneumonia PubMed journals carinii pneumonia medical journals carinii pneumonia free journals carinii pneumonia best journals carinii pneumonia top journals carinii pneumonia free medical journals carinii pneumonia famous journals carinii pneumonia Google Scholar indexed journals Clinical Image articles Clinical Image Research articles Clinical Image review articles Clinical Image PubMed articles Clinical Image PubMed Central articles Clinical Image 2023 articles Clinical Image 2024 articles Clinical Image Scopus articles Clinical Image impact factor journals Clinical Image Scopus journals Clinical Image PubMed journals Clinical Image medical journals Clinical Image free journals Clinical Image best journals Clinical Image top journals Clinical Image free medical journals Clinical Image famous journals Clinical Image Google Scholar indexed journals Beta D Glucan serology articles Beta D Glucan serology Research articles Beta D Glucan serology review articles Beta D Glucan serology PubMed articles Beta D Glucan serology PubMed Central articles Beta D Glucan serology 2023 articles Beta D Glucan serology 2024 articles Beta D Glucan serology Scopus articles Beta D Glucan serology impact factor journals Beta D Glucan serology Scopus journals Beta D Glucan serology PubMed journals Beta D Glucan serology medical journals Beta D Glucan serology free journals Beta D Glucan serology best journals Beta D Glucan serology top journals Beta D Glucan serology free medical journals Beta D Glucan serology famous journals Beta D Glucan serology Google Scholar indexed journals Lactate Dehydrogenase articles Lactate Dehydrogenase Research articles Lactate Dehydrogenase review articles Lactate Dehydrogenase PubMed articles Lactate Dehydrogenase PubMed Central articles Lactate Dehydrogenase 2023 articles Lactate Dehydrogenase 2024 articles Lactate Dehydrogenase Scopus articles Lactate Dehydrogenase impact factor journals Lactate Dehydrogenase Scopus journals Lactate Dehydrogenase PubMed journals Lactate Dehydrogenase medical journals Lactate Dehydrogenase free journals Lactate Dehydrogenase best journals Lactate Dehydrogenase top journals Lactate Dehydrogenase free medical journals Lactate Dehydrogenase famous journals Lactate Dehydrogenase Google Scholar indexed journals mycobacterium avium complex articles mycobacterium avium complex Research articles mycobacterium avium complex review articles mycobacterium avium complex PubMed articles mycobacterium avium complex PubMed Central articles mycobacterium avium complex 2023 articles mycobacterium avium complex 2024 articles mycobacterium avium complex Scopus articles mycobacterium avium complex impact factor journals mycobacterium avium complex Scopus journals mycobacterium avium complex PubMed journals mycobacterium avium complex medical journals mycobacterium avium complex free journals mycobacterium avium complex best journals mycobacterium avium complex top journals mycobacterium avium complex free medical journals mycobacterium avium complex famous journals mycobacterium avium complex Google Scholar indexed journals
Article Details
Main Point
This article serves to demonstrate Pneumocystis carinii pneumonia may cause severe infection and significant lung changes on computerized tomography scan without containing its hallmark feature - ground-glass opacities.
Clinical Image
A 51-year-old male with a background of advanced HIV and non-compliance with antiretrovirals was referred to the infection team with a 4-month history of cough, dyspnoea, 2-stone weight loss and night sweats. On admission, his viral load was greater than 1 million copies with a CD4+ T cell count of 0.056 ´ 109/L (normal range 0.3 - 1.4 x109/L). Notably, his Beta D Glucan serology and Lactate Dehydrogenase levels were 455.6 pg/mL (<80 pg/mL) and 189 unit/mL (135 – 225 unit/mL), respectively. A contrast-enhanced examination of the chest (Figure 1) revealed widespread nodularity measuring up to 12mm in diameter with areas of cavitation measuring up to 30mm. There was no zonal predominance. An infectious aetiology was strongly suspected however appearances were not in-keeping with key differentials. There was no ground-glass opacification to suggest pneumocystis or cytomegalovirus pneumonia; no significant lymphadenopathy or central cavity calcification to suggest histoplasmosis and no necrotic lymph nodes to implicate TB. Furthermore, the lack of bronchiectatic change and diffuse nature of the nodularity were not in-keeping with mycobacterium avium complex. Following the result of a bronchoalveolar lavage which was PCR positive for pneumocystis carinii, the patient was initiated on a treatment course of co-trimoxazole. Repeat cross-sectional imaging performed 82-days following the initial scan (Figure 2) revealed significant interval resolution of the nodular and cavitating lesions. This case highlights the importance of considering a wide differential when evaluating aetiologies of lung nodularity and cavitation; specifically, the possibility of pneumocystis presenting without its hallmark ground-glass opacification.
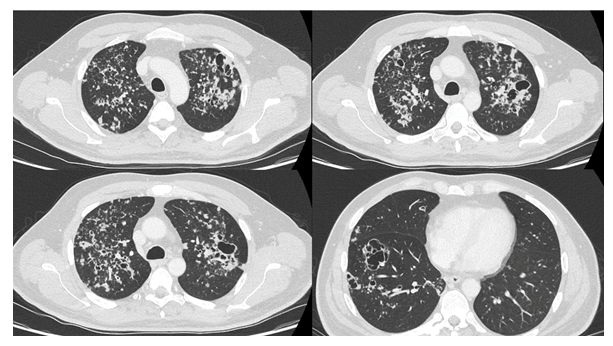
Figure 1: Post-contrast examination of the lungs demonstrating widespread nodularity bilaterally with areas of cavitation in the left upper and right lower lobes. Notably, there is no significant ground-glass opacification.
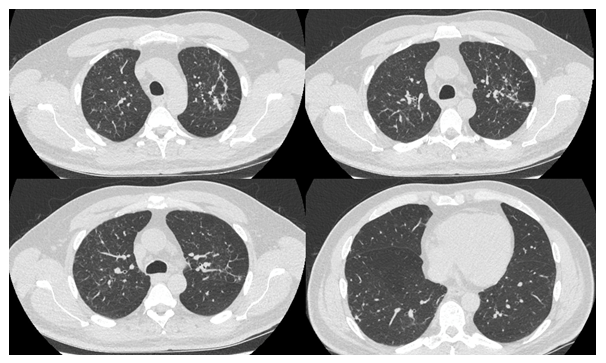
Figure 2: Eighty-two days post Figure 1 following co-trimoxazole treatment. HRCT examination that demonstrates significant interval improvement with resolving nodularity and reduction in size of previously demonstrated cavitations. HRCT = High resolution computed tomography.
