Atezolizumab-Related Sarcoidosis-Like Reaction in Early Breast Cancer Patient: Know It To Diagnose It: A Case Report with Review of the Literature
Article Information
Antonio Tornincasa1, Fabio Canino1, Lucia Trudu1, Monica Barbolini2, Luca Moscetti1,3, Claudia Omarini1, Massimo Dominici2 and Federico Piacentini2,3*
1Oncology Department, University Hospital of Modena, Italy
2Division of Medical Oncology, Department of Medical and Surgical Sciences for Children and Adults, University Hospital of Modena, Italy
3Italian Oncology Group for Clinical Research (GOIRC), Parma, Italy
*Corresponding Author: Federico Piacentini, Division of Medical Oncology, Department of Medical and Surgical Sciences for Children and Adults, University Hospital of Modena, Italy
Received: 28 June 2022; Accepted: 11 July 2022; Published: 29 August 2022
Citation: Antonio Tornincasa, Fabio Canino, Lucia Trudu, Monica Barbolini, Luca Moscetti, Claudia Omarini, Massimo Dominici, Federico Piacentini. Atezolizumab-Related Sarcoidosis-Like Reaction in Early Breast Cancer Patient: Know It To Diagnose It: A Case Report with Review of the Literature. Archives of Clinical and Medical Case Reports 6 (2022): 611-612.
View / Download Pdf Share at FacebookAbstract
Here we report a case of sarcoidosis-like reaction in a patient while on neoadjuvant chemotherapy plus Atezolizumab for an operable HER2- positive breast cancer. The aim of the current article is to raise awareness about Atezolizumab-associated sarcoidosis, even in early breast cancer setting.
Keywords
Atezolizumab; Sarcoidosis;Breast Cancer
Case Report articles
Atezolizumab articles Atezolizumab Research articles Atezolizumab review articles Atezolizumab PubMed articles Atezolizumab PubMed Central articles Atezolizumab 2023 articles Atezolizumab 2024 articles Atezolizumab Scopus articles Atezolizumab impact factor journals Atezolizumab Scopus journals Atezolizumab PubMed journals Atezolizumab medical journals Atezolizumab free journals Atezolizumab best journals Atezolizumab top journals Atezolizumab free medical journals Atezolizumab famous journals Atezolizumab Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Syndrome articles Syndrome Research articles Syndrome review articles Syndrome PubMed articles Syndrome PubMed Central articles Syndrome 2023 articles Syndrome 2024 articles Syndrome Scopus articles Syndrome impact factor journals Syndrome Scopus journals Syndrome PubMed journals Syndrome medical journals Syndrome free journals Syndrome best journals Syndrome top journals Syndrome free medical journals Syndrome famous journals Syndrome Google Scholar indexed journals Sarcoidosis articles Sarcoidosis Research articles Sarcoidosis review articles Sarcoidosis PubMed articles Sarcoidosis PubMed Central articles Sarcoidosis 2023 articles Sarcoidosis 2024 articles Sarcoidosis Scopus articles Sarcoidosis impact factor journals Sarcoidosis Scopus journals Sarcoidosis PubMed journals Sarcoidosis medical journals Sarcoidosis free journals Sarcoidosis best journals Sarcoidosis top journals Sarcoidosis free medical journals Sarcoidosis famous journals Sarcoidosis Google Scholar indexed journals Ophthalmoplegia articles Ophthalmoplegia Research articles Ophthalmoplegia review articles Ophthalmoplegia PubMed articles Ophthalmoplegia PubMed Central articles Ophthalmoplegia 2023 articles Ophthalmoplegia 2024 articles Ophthalmoplegia Scopus articles Ophthalmoplegia impact factor journals Ophthalmoplegia Scopus journals Ophthalmoplegia PubMed journals Ophthalmoplegia medical journals Ophthalmoplegia free journals Ophthalmoplegia best journals Ophthalmoplegia top journals Ophthalmoplegia free medical journals Ophthalmoplegia famous journals Ophthalmoplegia Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Breast Cancer articles Breast Cancer Research articles Breast Cancer review articles Breast Cancer PubMed articles Breast Cancer PubMed Central articles Breast Cancer 2023 articles Breast Cancer 2024 articles Breast Cancer Scopus articles Breast Cancer impact factor journals Breast Cancer Scopus journals Breast Cancer PubMed journals Breast Cancer medical journals Breast Cancer free journals Breast Cancer best journals Breast Cancer top journals Breast Cancer free medical journals Breast Cancer famous journals Breast Cancer Google Scholar indexed journals Retina articles Retina Research articles Retina review articles Retina PubMed articles Retina PubMed Central articles Retina 2023 articles Retina 2024 articles Retina Scopus articles Retina impact factor journals Retina Scopus journals Retina PubMed journals Retina medical journals Retina free journals Retina best journals Retina top journals Retina free medical journals Retina famous journals Retina Google Scholar indexed journals Radiotherapy articles Radiotherapy Research articles Radiotherapy review articles Radiotherapy PubMed articles Radiotherapy PubMed Central articles Radiotherapy 2023 articles Radiotherapy 2024 articles Radiotherapy Scopus articles Radiotherapy impact factor journals Radiotherapy Scopus journals Radiotherapy PubMed journals Radiotherapy medical journals Radiotherapy free journals Radiotherapy best journals Radiotherapy top journals Radiotherapy free medical journals Radiotherapy famous journals Radiotherapy Google Scholar indexed journalsArticle Details
1. Case Presentation
In June 2020, a locally advanced HER2+ breast cancer was diagnosed in a 59-year-old Caucasian woman. No significant comorbidities in her clinical history were known. The core biopsy of the breast nodule of 21x20x23 mm in diameter revealed a grade 3 infiltrating ductal carcinoma, with hormonal receptors negative, MIB1 30% and HER2 positive by ISH. The fine needle aspiration of one axillary lymph node was positive for metastatic cells. Baseline CT and bone scan do not reveal any other suspicious findings.
As part of a clinical trial, the patient started neoadjuvant therapy: Epirubicin plus Cyclophosphamide q21 for three cycles followed by weekly Carboplatin + Paclitaxel and dual blockade with Trastuzumab and Pertuzumab for further three cycles. Atezolizumab 1200 mg was administered concomitantly with the chemotherapy since the beginning, every three weeks for six times. Ultrasonography, mammography and breast MRI at the end of primary therapies revealed no residual disease on breast and axilla, while a chest TC scan showed the appearance of a couple of lung micro-nodules and the enlargement of multiple mediastinal lymph nodes (Figure 1A). No respiratory symptoms were present. A double endoscopic ultrasound-guided fine needle aspiration of the mediastinal lymph nodes was performed. Pathological examination found no evidence of malignant cells, but it revealed non-necrotizing micro-granulomas and mature lymphocytes referable to sarcoidosis-like granulomatous inflammation. ACE and alkaline phosphatase resulted within the normal range. As per pneumological evaluation, systemic therapies were not indicated due to the absence of associated symptoms and no abnormal index of respiratory function. The patient underwent left breast quadrantectomy and sentinel lymph node biopsy, with evidence of complete pathological response in T and N. Radiotherapy on left breast and nodal basin was administered, and adjuvant therapy with Trastuzumab and Pertuzumab was suggested too. Atezolizumab was withhold take into account the pCR and the supposed etio-pathogenetic role in the sarcoidotic reaction. While on anti-HER2 adjuvant treatment, a 6-month-later CT scan of the chest has showed a numerical and dimensional reduction of lung and mediastinal nodes (Figure 1B).
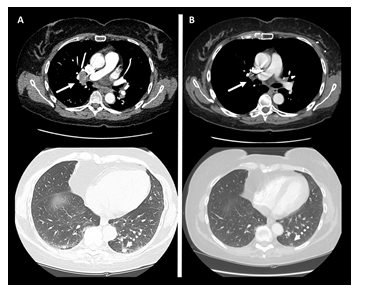
Figure 1: A - Mediastinal (continuous arrow) and lung (handle arrow) nodes while on Atezolizumab. B – Regression of mediastinal (continuous arrow) and lung (handle arrow) nodes after holding Atezolizumab.
2. Discussion
Atezolizumab is a Fc-engineered, humanised IgG1 anti-programmed death-ligand 1 (PD-L1) monoclonal antibody that is approved in combination with nab-paclitaxel for the first-line treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumours have PD-L1 expression ≥ 1%. In this setting, Atezolizumab has shown to improve progression free survival from 5.0 to 7.5 months (hazard ratio, 0.62; 95% CI, 0.49 to 0.78; P<0.001, for PDL1+ cohort) [1], but no overall survival advantage [2]. By removing inhibition of the immune response, Atezolizumab can potentially induce immune-mediated adverse reactions. Sarcoidosis-like reactions are characterized by the presence of non-caseating granulomas especially in intrathoracic lymph nodes, in patients who do not otherwise meet criteria for the diagnosis of systemic sarcoidosis. They can appear during the course of an oncologic disease or as immune checkpoint inhibitors-related adverse event. To date less than 100 cases of patients with sarcoidosis or sarcoid-like reactions are described in literature, in 81% of causes related to melanoma and with a slight predilection for pembrolizumab. To the best of our knowledge, only one case of Atezolizumab-related sarcoidosis-like reactions has been reported in a metastatic PDL1+ triple negative breast cancer [3], and another one in a patient with urothelial cell carcinoma [4]. The present is the first case in early breast cancer setting: it focused on the central role of re-biopsy of suspected nodes that is the only way to exclude a malignancy dissemination, especially in case of discrepancy in tumor evaluation, since there is no imaging technique able to solve the dilemma [5]. It can reasonably be excluded that the understanding malignancy should be at the origin of sarcoiditic reaction, since the achieved pathological complete response on the breast and axilla, while Atezolizumab should be called as the triggering event. Physicians should be aware of Atezolizumab relate sarcoidotic manifestations in order to avoid misinterpretation and to tailor patient treatment properly.
References
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 379 (2018): 2108-2121.
- Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21 (2020): 44-59.
- Lafon M, Blaye C, Kind M, et al. Sarcoidosis-like reaction in metastatic triple negative breast cancer treated by anti-PD-L1. Breast J 25 (2019): 971-973.
- Mitchell MA, Hogan K, Amjadi K. Atezolizumab-induced sarcoid-like granulomatous reaction in a patient with urothelial cell carcinoma. Immunotherapy 10 (2018): 1189-1192.
- Rambhia PH, Reichert B, Scott JF, et al. Immune checkpoint inhibitorinduced sarcoidosis-like granulomas. Int J Clin Oncol 24 (2019): 1171e81.
