Association of Serum 14-3-3ƞ Protein level in Rheumatoid Arthritis Patients in a Tertiary care Hospital in Bangladesh
Article Information
Taskin Jahan1, Shaila Akhtar1, Minhaj Rahim Choudhury2, Ahmed Abu Saleh1, Shaheda Anwar1
1Department of Microbiology and Immunology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
2Department of Rheumatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
*Corresponding author: Taskin Jahan, Department of Microbiology and Immunology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Received: 19 October 2023; Accepted: 29 October 2023; Published: 03 November 2023
Citation: Taskin Jahan, Ahmed Abu Saleh, Shaheda Anwar, Shaila Akhtar, Minhaj Rahim Choudhury. Association of Serum 14-3-3ƞ Protein level in Rheumatoid Arthritis Patients in a Tertiary care Hospital in Bangladesh. Fortune Journal of Health Sciences. 6 (2023): 438-445
View / Download Pdf Share at FacebookAbstract
Aim: The study aimed to demonstrate the association of serum 14-3-3Ƞ protein level with Rheumatoid Arthritis and its disease severity. It also aimed to determine if the disease detection rate increased after the addition of this biomarker to current biomarkers.
Methods: This was a cross-sectional comparative analysis. 40 diagnosed rheumatoid arthritis patients according to the American College of Rheumatology (ACR) criteria 2010, free from other rheumatological diseases and 40 healthy subjects who fulfilled the inclusion criteria were included in this study. Serum 14-3-3Ƞ protein levels were determined by Enzyme Linked Immunosorbent Assay (ELISA).
Result: Mean serum concentration of 14-3-3Ƞ protein was significantly higher (P<0.001) in RA patients in comparison to healthy subjects. Sensitivity of 14-3-3Ƞ was higher (97.5%) than RF (90%) and anti-CCP (92.5%), but specificity was lower (90%), than RF (97.5%) and anti-CCP (100%). Serum 14-3-3Ƞ protein was significantly (P=0.04) associated with RA disease severity evaluated by Disease Activity Score 28 (DAS28). Concentration of this protein was significantly correlated with RF level (p<0.05), DAS 28 score (P<0.05) and HAQ (p<0.01). In addition of 14-3-3η protein to RF and anti-CCP, diagnostic rate increased by 2.5%.
Conclusion: Serum 14-3-3η level was significantly higher in rheumatoid arthritis patients compared to healthy subjects. It was associated with rheumatoid arthritis disease severity. It had a high sensitivity than RF and anti-CCP. Addition of 14-3-3η protein to RF and anti-CCP diagnostic rate of disease increased.
Keywords
Rheumatoid Arthritis, 14-3-3η protein. Rheumatoid factor (RF), Anti-cyclic citrullinated peptide antibodies (Anti-CCP), ELISA
Rheumatoid Arthritis articles, 14-3-3Ƞ protein articles. Rheumatoid factor (RF) articles, Anti-cyclic citrullinated peptide antibodies (Anti-CCP) articles, ELISA articles
Article Details
Abbreviations:
RA- Rheumatoid Arthritis, RF- Rheumatoid Factor, anti-CCP- anti cyclic citrullinated peptide antibodies, HRP- Horse radish peroxidase, TMB- Tetra methyl benzidine, OD- Optical density, PPV- Positive predictive value, NPV - Negative predictive value, LR+ - Positive likelihood ratio, LR_- Negative likelihood ratio.
1. Introduction
Rheumatoid arthritis (RA) is a common systemic inflammatory autoimmune disease that causes synovial inflammation and can result in chronic and irreparable joint degeneration. Numerous factors, such as genetic, environmental, and hormonal, have a role in the development of specific autoimmune processes and the ensuing tissue damage that characterizes the disease [1]. Women are affected 2-3 times more frequently than men [2]. Highest incidence of the disease occurs between the ages of 50-60 years but it can occur at any age [3]. This disorder affects approximately 1.5% of the global population [4]. Among Asian countries prevalence of RA estimated to be 0.42% in China [5], 0.6-1% in Japan [6], 0.7% in India [7], and 0.5% in Pakistan [8]. In Bangladesh, the prevalence of Rheumatoid arthritis is 1.6%, with women having a much greater frequency (2.4%) than men (0.7%) [9]. The most typical feature of the disease is symmetrical pain and swelling of the hands, wrists, feet and knees, although other joints may be affected. Some RA patients may present or later develop disease manifestations in other organs, such as interstitial lung disease, pleural effusion, bronchiectasis, and pericarditis [10]. Untreated RA causes significant joint destruction, impairing physical function and causing workplace incapacity [11]. It is nowadays well known that a patient's prognosis can be greatly enhanced by early RA diagnosis, evaluation of the disease's severity, and application of an efficient treatment plan [12].
The American College of Rheumatology classification criteria 2010, are based on assessments of joint involvement, symptom duration, measurement of acute-phase reactant and serological markers. In case of serological measurements, patients with greater titers of both anti-cyclic citrullinated peptide antibodies (anti-CCP) and rheumatoid factor (RF) are given more importance. Seronegativity is still a significant drawback for these two markers, highlighting the need for new complementary markers that will improve diagnostic sensitivity [13]. More than one-third of RA patients lack the current serological indicators [14]. Seronegative RA patients have a delayed diagnosis and treatment initiation which results in lower chance of remission, extensive joint injury and disability [15]. Current biomarkers (RF and anti-CCP) have only approximately 70% of sensitivity. Clinically precise and timely diagnosis of RA, particularly seronegative RA and early RA, is still challenging [16]. Several biomarkers are under study in hope of increasing diagnostic accuracy of RA. Previous meta-analysis showed, anti-mutated citrullinated vimentin (anti-MCV), had similar sensitivity (64-84%) and specificity (79-96%) as anti-CCP in RA diagnosis [17]. Antibodies to carbamylated protein (anti-car P) another studied novel biomarker for RA did not show increased sensitivity and specificity in comparison to RF and anti-CCP. A previous study showed sensitivity and specificity of anti-car P and anti-CCP were 44%, 89% and 54%, 96% respectively [18]. Serum 14-3-3Ƞ protein, has been studied as novel biomarker for the diagnosis of RA. A previous meta-analysis showed, the sensitivity of this biomarker was higher (73%) compared to RF (70%) and anti-CCP (67%), specificity (88%) was higher than RF (86%) but lower than anti-CCP (96%) [19]. A recent study in Egypt found sensitivity and specificity of 14-3-3Ƞ was higher than RF and anti-CCP in early RA. Sensitivity and specificity of 14-3-3Ƞ, RF, anti-CCP were 86.7%, 61.7%, 68.3% and 96.7%, 88.3%, 95% respectively [20]. In early RA diagnosis, when Serum 14-3-3Ƞ protein added to RF and anti-CCP, diagnostic rate increased from 72% to 78% [21]. So, this study aimed to assess the role of 14-3-3Ƞ in RA diagnosis.
The 14-3-3Ƞ protein may be involved in the pathogenesis of RA by activating pro-inflammatory signaling cascades and inflammatory mediators [22]. The 14-3-3 protein family is a group of highly conserved intracellular chaperone molecules found in all eukaryotes. Hundreds of 14-3-3 binding partners, including protein kinases, phosphatases, receptors, and transcription factors, have been found. 14-3-3 proteins participate in essential physiological functions such as cell-cycle regulation, apoptosis, signal transduction, energy consumption, and protein trafficking via these interactions [23]. 14-3-3 proteins have seven isoforms (β, γ, ε, η, σ, θ, and ζ) [24]. Kilani et al., in 2007, using Western blot analysis demonstrated that, compared to healthy individuals, 14-3-3Ƞ levels were significantly higher in the synovial fluid and serum of arthritis patients and its serum levels strongly correlated with the matrix metalloproteinases (MMP) MMP-1 and MMP-3 [25]. Serum levels of 14-3-3Ƞ were later found to be noticeably greater in patients with RA, than in healthy individuals and patients with different autoimmune and rheumatic disorders in other studies. So, 14-3-3Ƞ may be a RA-specific biomarker and assessment of 14-3-3Ƞ together with RF and/or ACPA may increase their diagnostic sensitivity [21]. The diagnostic utility of 14-3-3Ƞ in conjunction with RF and anti-CCP has been demonstrated in some previous studies. The role of this novel biomarker in rheumatoid arthritis patient diagnosis in our country has not yet been studied. So, the purpose of this study was to determine whether there is any association of this biomarker in rheumatoid arthritis disease and its severity in the Bangladeshi population.
2. Methods
2.1 Study subjects
This study was a cross-sectional comparative analysis involving 40 patients diagnosed with RA according to the 2010 American College of Rheumatology (ACR) criteria [4]. All patients were recruited from the Department of Rheumatology, Bangabandhu Sheikh Mujib Medical University (BSMMU) from January 2023 to March 2023. The exclusion criteria for patients were as follows: patients under 18 years of age and age more than 60 years, patients with other autoimmune diseases, and patients suffering from major illnesses, such as hepatic failure, renal failure, cancer etc. This study also included 40 age and sex matched healthy subjects for comparison. Relevant data were taken from patients, including age, sex, disease duration and number of tender and swollen joints. RA disease severity was assessed by the Disease Activity Score 28 (DAS28) score. Functional status was assessed by the Health Assessment Questionnaire (HAQ). According to the DAS 28 score, 3 patients were in remission, 8 patients were in low disease activity, 18 patients were in moderate disease activity, and 11 patients were in severe disease activity. According to the HAQ, mild disability was present in 26 patients, moderate disability was present in 10 patients, and severe disability was present in 4 patients. Patients laboratory investigations, including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), and anti-cyclic citrullinated peptide antibodies (anti-CCP) were recorded. The study was ethically authorized by the Institutional Review Board (IRB) of BSMMU (No. BSMMU/2022/12791). The study was conducted in compliance with the Helsinki Declaration of 1964 and its subsequent amendments. Informed written consent was taken from all study participants.
2.2 Serum 14-3-3Ƞ, RF and anti-CCP Measurement
Properly stored serum at (- 20 °C), from patients of RA and healthy subjects were used for determining the serum 14-3-3Ƞ, RF and anti-CCP. Serum 14-3-3Ƞ was determined using sandwich ELISA according to the manufacturer’s instruction (ELK biotechnology, cat: ELK8821, Lot:37264305, Wuhan, China). All kit components and samples were brought to room temperature (18-25°C) before use. Wells for Diluted Standard, Blank, and Sample were marked. 7 wells were prepared for Standard, 1 well for Blank, and other wells for samples. 100μL of samples were added into the appropriate wells of micro-plate, covered and incubated for 80 minutes at 37°C. Liquid of each well was poured out and washed with 200μL of diluted wash solution to each well and the remaining liquid from all wells were completely removed by snapping the plate onto absorbent paper. 100μL of Biotinylated Antibody working solution was added to each well, covered with the plate sealer and incubated for 50 minutes at 37°C. Aspiration and wash process were repeated. 100μL of Streptavidin-HRP Working Solution was added to each well, covered the wells with the plate sealer and incubated for 50 minutes at 37°C again after aspiration and wash process, 90μL of TMB Substrate Solution was added to each well, covered with a new Plate sealer. Incubated for 20 minutes at 37°C in the dark. Finally, 50μL of Stop Reagent was added to each well. OD value was measured at 450 nm using an ELISA plate reader. Serum concentration of 14-3-3 eta was determined by comparing with OD value (Figure 1). RF was determined by Nephelometric system using Atellica NEPH 630 System, SIEMENS, USA. RF = 15 IU/ml (International unit/ mililitre) or more was considered positive. Anti-CCP was determined by chemiluminescence Immunoassay system using ADVIA Centaur XPT, SIEMENS, USA. Anti-CCP = 4 U/ml or more interpreted as positive.
2.3 Statistical analysis
The SPSS software package version-27 (Strata Corporation, College Station, Texas) was used to analyze the data. Descriptive analysis of all relevant variables was done by using percentage, mean, standard deviation, and ROC curve. Independent sample t-test was used to analyze mean serum 14-3-3Ƞ protein differences between RA patients and healthy subjects. The area under the curve (AUC) was used to measure the diagnostic usefulness of 14-3-3Ƞ using the receiver operating characteristic (ROC) curve. The association of serum 14-3-3η with RA disease severity was determined by paired t-test. Spearman’s rank correlation coefficient procedure was used to determine the relation between serum 14-3-3η with other serological markers. The incremental benefit is the diagnostic value added by inclusion of 14-3-3Ƞ protein in addition to RF and anti-CCP. It was calculated using the following formula: [(A−B) ÷ B] × 100. P value <0.05 was considered statistically significant.
3. Results
3.1 Clinical and laboratorial characteristics of RA patients
This study was conducted in 40 clinically confirmed cases of rheumatoid arthritis patients according to American College of Rheumatology criteria 2010 and 40 healthy subjects, whose age and sex were matched. Clinical characteristics of the patients with RA are shown in Table 1. RA patients had mean age of [37.22±6.70] years, where age range was [20 – 50] years. Among the patients, 31 were female (77.5%) and 9 were male (22.5%). Male: female was 1:3.4. Mean disease duration was [18.11±7.39] months. Mean value of ESR was 41.17 (5-120) mm in 1st hour, CRP - 14.40 (0.75-71.9) mg/L, RF-163.90 (9.39-615) IU/ml, anti-CCP - 138.75 (0.7-422) U/ml. RF found to be positive in 36 patients (90%) and negative in 4 patients (10%), anti-CCP was positive in 37 patients (92.5%) and negative in 3 patients (7.5%). Mean value of number of tender and swollen joints were 12.17 (0-28) and 3.10 (0-14) respectively. Mean value of DAS28 score and HAQ were [4.29±1.23] and [0.82±0.67] respectively.
Table 1: Clinical and laboratorial characteristics of patients with Rheumatoid Arthritis (n =40)
|
Characteristics |
Cases (n = 40) |
|
Age (years) mean ± SD |
37.22±6.70 |
|
Range |
(20-50) |
|
Gender Male |
9 (22.5%) |
|
Female |
31 (77.5%) |
|
Male: Female |
1? 3:4 |
|
Disease duration (months) |
18.11±7.39 |
|
Number of tender joints [mean(range)] |
12.17 (0-28) |
|
Number of swollen joints |
3.10 (0-14) |
|
ESR (mm in 1st hour) |
41.17 (5-120) |
|
CRP (mg/L) |
14.40 (0.75-71.9) |
|
RF (IU/ml) |
163.90 (9.39-615) |
|
Anti-CCP (U/ml) |
138.75 (0.7-422) |
|
DAS28 [mean ± SD] DAS28, n (%) |
4.29±1.23 |
|
Remission (≤2.6) |
3 (7.5%) |
|
Low (2.6 - ≤3.2) |
8 (20%) |
|
Moderate (3.2- ≤5.1) |
18 (45%) |
|
Severe (>5.1) |
11 (27.5%) |
|
HAQ [mean ± SD] HAQ, n (%) |
0.82±0.67 |
|
Mild disability |
26 (65%) |
|
Moderate disability |
10 (25%) |
|
Severe disability |
4 (10%) |
|
Number of patients with positive RF, n (%) |
36 (90%) |
|
Number of patients with negative RF, n (%) |
4 (10%) |
|
Number of patients with positive anti-CCP, n (%) |
37 (92.5%) |
|
Number of patients with negative anti-CCP, n (%) |
3 (7.5%) |
SD = Standard deviation; ESR = Erythrocyte sedimentation rate; CRP = C-reactive protein; DAS-28 = Disease Activity Score-28; HAQ = Health Assessment Questionnaire; RF = rheumatoid factor; anti-CCP = antibodies to cyclic citrullinated peptide.
3.2 Serum 14-3-3Ƞ protein in RA
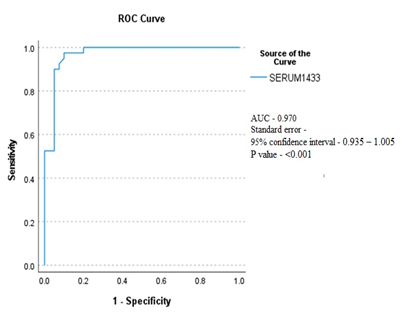
The comparison of serum 14-3-3Ƞ protein levels between RA patients and healthy subjects is shown in Table 2. The mean serum concentration of protein was found significantly higher (P<0.001) in RA patients compared to healthy subjects (1.91±0.83ng/ml vs 0.53±0.31 ng/ml). Serum 14-3-3Ƞ protein comparison between RA patients and healthy subjects with ROC curve analysis determined a significant (P<0.001) AUC of 0.970 (95% CI = 0.935 – 1.005; Figure 2). At a cut-off of ≥0.87 ng/ml, ROC showed a sensitivity of 97.5% and specificity of 90%. Association of serum 14-3-3Ƞ with RA disease severity according to the DAS28 score shown in Table 3. Three RA patients were in remission having DAS 28 score < 2.6. So, they were excluded from assessing disease severity. The association of serum 14-3-3Ƞ with disease severity was assessed by comparing the serum 14-3-3Ƞ level of 26 RA patients who were in low to moderate disease activity [DAS28 score (2.6-5.1)] and 11 RA patients who had severe disease activity [DAS28 score >5.1]. Mean serum 14-3-3Ƞ concentration of RA patients was found to be significantly (p <0.05) associated with RA disease severity.
Table 2: Comparison of serum 14-3-3Ƞ protein concentration in RA patients and healthy subjects (n=80)
|
|
Serum 14-3-3Ƞ (ng/mL) |
P value* |
|
|
Mean±SD |
Median (Range) |
||
|
RA patients (n= 40) |
1.91±0.83 |
1.78 (0.73-3.99) |
<0.001 |
|
Healthy subjects (n=40) |
0.53±0.31 |
0.43 (0.16-1.57) |
|
P value measured by Independent sample t-test
Table 3: Association of serum14-3-3η protein with disease severity according to DAS 28 score in RA patients (n=37)
|
Disease severity by DAS28 score |
serum14-3-3η protein level (ng/mL) |
P- value* |
|
Low to moderate disease activity (n=26) |
1.13±0.40 |
0.049 |
|
Severe disease activity (n=11) |
1.77±0.64 |
|
P value determined by Paired t-test
3.3 Relationship between serum 14-3-3Ƞ protein and clinical and serological parameters
The correlation coefficient shown in Table 4, demonstrated the relationship between serum 14-3-3Ƞ protein concentration with other clinical and serological variables of RA. Serum 14-3-3Ƞ protein concentration was significantly correlated with RF level (p<0.05), DAS 28 score (P<0.05), and HAQ (p<0.01).
Table 4: Correlation coefficients (r) of serum 14-3-3η protein with clinical and serological measures in patients with RA (n=40)
|
Variables |
serum14-3-3η |
DAS28 |
HAQ |
RF |
anti-CCP |
ESR |
CRP |
|
DAS28 |
0.344* |
||||||
|
HAQ |
0.423** |
0.790** |
|||||
|
RF |
0.388* |
0.440** |
0.480** |
||||
|
Anti-CCP |
0.091 |
0.385* |
0.393* |
0.392* |
|||
|
ESR |
0.269 |
0.375* |
0.328* |
0.254 |
0.381* |
||
|
CRP |
0.301 |
0.376* |
0.444** |
0.555** |
0.192 |
0.189 |
Correlation was done using Spearman’s rank correlation coefficient
*P<0.05; **P <0.01;
3.4 Diagnostic performance of serum 14-3-3Ƞ protein, RF, and anti-CCP
The diagnostic performance of biomarkers in RA patients and healthy subjects is presented in Table 5. Serum 14-3-3η was detected above cut-off value in 39 RA patients and 4 healthy subjects. RF detected the above cut-off value in 36 RA patients and one healthy subject. Anti-CCP was detected above cut-off value in 37 RA patients and none of the healthy subjects. The sensitivity of RF, anti-CCP, 14-3-3η was 90%, 92.5%, 97.5% respectively and the specificity of RF, anti-CCP, 14-3-3η was 97.5%, 100%, 90% respectively. PPV and NPV of RF, anti-CCP, 14-3-3η were 97.3%, 100%, 90.6% and 90.7%, 93%, 97.2% respectively. NPV and Sensitivity of biomarker 14-3-3η was higher than both RF and anti-CCP, but specificity was lower than RF and anti-CCP.
Table 5: Diagnostic performance of RF, anti-CCP, serum 14-3-3η in RA patients (n=40)
|
Biomarkers |
Sensitivity |
Specificity |
PPV |
NPV |
LR+ |
LR_ |
|
RF |
90% |
97.50% |
97.30% |
90.70% |
36 |
0.1 |
|
Anti-CCP |
92.50% |
100% |
100% |
93% |
0.93 |
0.07 |
|
14-3-3η |
97.50% |
90% |
90.60% |
97.20% |
9.75 |
0.02 |
3.5 Incremental benefit of adding serum 14-3-3η protein to RF and anti-CCP
Among 40 RA patients RF, anti-CCP, and 14-3-3η were positive in 36 (90%), 37 (92.5%), and 39 (97.5%) patients respectively. The addition of anti-CCP to RF increased diagnostic detection from 90% to 97.5%. The addition of 14-3-3η to RF increased the diagnostic rate from 90% to 100% and the addition of 14-3-3η to anti-CCP increased the diagnostic rate from 92.5% to 100%. RF and / or anti-CCP detected in 97.5% of patients. When all three markers combined together diagnostic rate increased to 100%. So, when 14-3-3η was added to RF and anti-CCP, diagnostic rate increased by 2.5% (Table 6).
Table 6: Complementarity between diagnostic markers in RA patients (n=40)
|
Diagnostic markers |
RA patients, n =40 |
Incremental benefit |
|
RF+ |
36 (90%) |
- |
|
Anti-CCP+ |
37 (92.5%) |
- |
|
14-3-3η+ |
39 (97.5%) |
- |
|
RF and / anti-CCP+ |
39 (97.5%) |
3 |
|
RF and / 14-3-3η+ |
40 (100%) |
4 (10%) |
|
Anti-CCP and / 14-3-3η+ |
40 (100%) |
3 (7.5%) |
|
RF and / anti-CCP and/14-3-3η+ |
40 (100%) |
1 (2.5%) |
Note: Incremental benefit of serum14-3-3Ƞ protein means additional value added by inclusion of 14-3-3Ƞ protein in addition to RF and anti-CCP. An incremental benefit was calculated as follows- [(A−B) ÷ B] × 100 [21].
Here,
A= Total patients with positive RF and/anti-CCP and/ 14-3-3Ƞ =40
B= Total patients with positive RF and/anti-CCP = 39
Incremental benefit of adding 14-3-3Ƞ protein to RF and anti-CCP = ×100 = 2.5%
4. Discussion
Early detection of rheumatoid arthritis, evaluation of disease severity at diagnosis, and initiation of an appropriate treatment strategy can improve a patient's prognosis and prevent joint destruction. More than one-third of patients lack current serological indicators, so new biomarkers are needed to increase diagnostic sensitivity [14]. Serum 14-3-3η is an authenticated biomarker for RA [22]. This study showed serum levels of 14-3-3Ƞ were significantly (P <0.001) higher in RA patients [1.78 (0.73 – 3.99) ng/ml] than in healthy subjects [0.43 (0.16-1.57) ng/ml]. This finding was in line with the study of Maksymowych et al [21] where serum level was higher in RA patients [1.12 (0.22–6.87) ng/ml] than in healthy controls. Other studies also showed that 14-3-3Ƞ serum levels were significantly higher (P value<0.0001) in RA patients than in healthy individuals [26, 27]. In this study, analysis of the ROC curve of serum 14-3-3Ƞ protein showed that RA patients compared to healthy subjects had a significant (P<0.001) AUC of 0.970 (95% CI = 0.935 – 1.005), at a cut-off value ≥0.87 ng/ml, which is in line with a study in China, where AUC was 0.979, P < 0.0001, when the cutoff value was 0.879 ng/ml [28]. Here, ROC curve analysis of 14-3-3Ƞ, at a cut-off value ≥0.87 ng/ml, yield sensitivity of 97.5%, specificity 90%, PPV= 90.6%, NPV=97.2%, positive LR = 9.75. In this study, the sensitivity, specificity, and positive LR of 14-3-3Ƞ were higher than in a previous meta-analysis of 14-3-3Ƞ in China where sensitivity, specificity, and positive likelihood ratio were 73%, 88%, and 5.98 respectively [19].
When assessing the role of 14-3-3Ƞ with RA disease severity, this study found a significant association (P= 0.04) of 14-3-3Ƞ protein with disease severity assessed by DAS28 score. This was in line with a study that also found 14-3-3Ƞ significantly associated with RA disease severity [21]. There was also a significant correlation of 14-3-3Ƞ with DAS 28, HAQ and RF in this study. A previous study in Iraq showed, 14-3-3Ƞ was correlated with DAS 28, RF, ESR, and number of swollen joints [29]. Another study found a correlation of 14-3-3Ƞ with RF and anti-CCP but they did not find any correlation with DAS28 and HAQ in established RA [21]. This study showed that the sensitivity of 14-3-3Ƞ was 97.5%, much higher than the sensitivity of RF and anti-CCP which were 90% and 92.5% respectively. However, the specificity of 14-3-3Ƞ was 90%, lower than RF and anti-CCP which were 97.5% and 100% respectively. In a previous meta-analysis of 14-3-3Ƞ, sensitivity was found to be 73%, higher than the sensitivity of RF (70%) and anti-CCP (67%) and specificity was 88%, which was higher than RF (86%) but lower than anti-CCP (96%) [19]. Another study found that sensitivity of 14-3-3Ƞ (77%) was lower than sensitivity of RF (84%) and anti-CCP (79%). The Specificity of 14-3-3Ƞ (92.6%) was higher than RF (85%) but lower than anti-CCP (99%) [21].
Variation of these findings with our study may be due to differences in cut-off value. In this study cut-off value was considered ≥0.87 ng/ml, whereas some studies considered cut-off deal as >0.19 ng/ml [21, 27], >0.39ng/ml [26]. Disease duration may be another cause of result variation. In some studies, patients were divided into early and established RA cases according to disease duration [21, 27, 28]. but in this study, there was no such difference in patients according to disease duration. Differences in ethnicity and sample size may act as other influencing factors for such variations in results. The addition of serum 14-3-3Ƞ to RF and anti-CCP increased the detection rate by 10% and 7.5% respectively. These findings nearly matched a study by where addition of serum 14-3-3Ƞ to RF and anti-CCP increased detection rate by 6% and 9% respectively. RF and/or anti-CCP were positive for 97.5% of patients; addition of 14-3-3Ƞ increased the diagnostic rate by 2.5% in this study, which was in line with a survey of where addition of serum 14-3-3Ƞ to RF and /anti-CCP increased diagnostic rate by 2% [27].
5. Conclusion
According to the study's findings, the serum14-3-3Ƞ protein level is significantly higher in rheumatoid arthritis patients than in healthy individuals, and it is also associated with the disease severity as measured by the DAS28 score. Comparing this protein to RF and anti-CCP, it has a better sensitivity and NPV. Adding 14-3-3Ƞ protein to RF and anti-CCP improves the diagnostic rate of RA.
Acknowledgment
We thank all patients and healthy subjects who participated in this study and all the individuals who helped us to complete the study.
Funding
No specific funding sources.
Conflict of Interest
There is no conflict of interest.
References
- Mcinnes IB & Schett G. The Pathogenesis of Rheumatoid Arthritis. The NEW ENGLAND JOURNAL of MEDICINE 365 (2011): 2205–2219.
- Aletaha D & Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA - Journal of the American Medical Association 320 (2018): 1360–1372.
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nature Reviews Disease Primers 4 (2018): 18001.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and Rheumatism 62 (2010): 2569–2581.
- Zeng, Zhu, Tan, et al. Disease burden and quality of life of Rheumatoid arthritis in China: A systemic review. Chinese Journal of Evidence-Based Medicine 13 (2013): 300–307.
- Yamanaka H, Sugiyama N, Inoue E, et al. Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Modern Rheumatology 7595 (2013): 33–40.
- Malaviya AN, Kapoor SK, Singh RR, et al. Prevalence of rheumatoid arthritis in the adult Indian population. Rheumatology International 13 (1993): 131–134.
- Akhter E, Bilal S, & Haque U. Prevalence of arthritis in India and Pakistan: A review. Rheumatology International 31 (2011): 849–855.
- Zahid-Al-Quadir A, Zaman MM, Ahmed S, Bhuiyan MR, et al. Prevalence of musculoskeletal conditions and related disabilities in Bangladeshi adults: a cross-sectional national survey. BMC Rheumatology 4 (2020): 1–14.
- Sparks JA. In the Clinic® rheumatoid arthritis. Annals of Internal Medicine 170 (2019): 1–15.
- Verstappen SMM, Bijlsma JWJ, Verkleij H, et al. Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Care and Research 51 (2004): 488–497.
- Vermeer M, Kuper HH, Hoekstra M, et al. Implementation of a treat-to-target strategy in very early rheumatoid arthritis: Results of the Dutch Rheumatoid Arthritis Monitoring remission induction cohort study. Arthritis and Rheumatism 63 (2011): 2865–2872.
- Burr ML, Viatte S, Bukhari M, et al. Long-term stability of anti-cyclic citrullinated peptide antibody status in patients with early inflammatory polyarthritis. Arthritis Research and Therapy 14 (2012).
- Mjaavatten MD, Van Der Heijde DM, Uhlig T, et al. Should anti-citrullinated protein antibody and rheumatoid factor status be reassessed during the first year of followup in recent-onset arthritis? A longitudinal study. Journal of Rheumatology 38 (2011): 2336–2341.
- Coffey CM, Crowson CS, Myasoedova E, et al. Evidence of Diagnostic and Treatment Delay in Seronegative Rheumatoid Arthritis: Missing the Window of Opportunity. Mayo Clinic Proceedings 94 (2019): 2241–2248.
- Wei JCC, Leong PY, & Liu GY. Chaperone/scaffolding/adaptor protein 14-3-3η (eta): A diagnostic marker of rheumatoid arthritis. International Journal of Rheumatic Diseases 23 (2020): 1439–1442.
- Luime JJ, Colin EM, Hazes JMW, et al. Does anti-mutated citrullinated vimentin have additional value as a serological marker in the diagnostic and prognostic investigation of patients with rheumatoid arthritis? A systematic review. Annals of the Rheumatic Diseases 69 (2010): 337–344.
- Shi J, van Steenbergen HW, van Nies JAB, et al. The specificity of anti-carbamylated protein antibodies for rheumatoid arthritis in a setting of early arthritis. Arthritis Research and Therapy 17 (2015): 1–6.
- Wang D, Cui Y, Lei H, et al. Diagnostic accuracy of 14-3-3 η protein in rheumatoid arthritis?: A meta-analysis. International Journal of Rheumatic Diseases 23 (2020): 1443-1451.
- Alashkar DS, Elkhouly RM, Abd Elnaby AY, et al. Will 14-3-3η Be a New Diagnostic and Prognostic Biomarker in Rheumatoid Arthritis? A Prospective Study of Its Utility in Early Diagnosis and Response to Treatment. Autoimmune Diseases 2022 (2022): 1-8.
- Maksymowych WP, Naides SJ, Bykerk V, et al. Serum 14-3-3η is a novel marker that complements current serological measurements to enhance detection of patients with rheumatoid arthritis. Journal of Rheumatology 41 (2014): 2104–2113.
- Maksymowych WP, van der Heijde D, Allaart CF, et al. 14-3-3Η Is a Novel Mediator Associated with the Pathogenesis of Rheumatoid Arthritis and Joint Damage. Arthritis Research and Therapy 16 (2014): 1–11.
- Obsilova V, & Obsil T. Structural insights into the functional roles of 14-3-3 proteins. Frontiers in Molecular Biosciences 9 (2022): 1–15.
- Cau Y, Valensin D, Mori M, et al. Structure, Function, Involvement Diseases and Targeting of 14-3-3 Proteins: An Update. Current Medicinal Chemistry 25 (2017): 5–21.
- Kilani RT, Maksymowych WP, Aitken A, et al. Detection of high levels of 2 specific isoforms of 14-3-3 proteins in synovial fluid from patients with joint inflammation. Journal of Rheumatology 34 (2007): 1650–1657.
- Mohamed AH, Abdellatif S, & El-noshokaty EH. Serum level of 14-3-3η (eta) protein as a diagnostic marker for rheumatoid arthritis and potential correlation with disease activity. MOJ Orthopedics & Rheumatology 7 (2018): 3–7.
- Shovman O, Gilburd B, Watad A, et al. The diagnostic value of 14-3-3η protein levels in patients with rheumatoid arthritis. Best Practice & Research Clinical Rheumatology 32 (2019): 610-617.
- Gong X, Xu S, Wu Y, et al. Elevated serum 14-3-3η protein may be helpful for diagnosis of early rheumatoid arthritis associated with secondary osteoporosis in Chinese population. Clinical Rheumatology 36 (2017): 2581-2587.
- Ali, Jinan & Saleh, Basil & Isho Gorial, Faiq. Validity of 14-3-3η Protein in Diagnosis of Rheumatoid Arthritis among Iraqi Patients. International Journal of Science and Research (IJSR) 6 (2017): 2194–2197.