Association of Mean Pressure Gradient, Pressure Half Time, Regurgitant Area, and Heart Rate with Mitral Valve Orifice Area Prior and Post Edge-To-Edge Mitral Valve Repair
Article Information
Iryna Dykun, Peter Lüdike, Sultan Poyraz, Alexander Y. Lind, Tienush Rassaf, Amir A. Mahabadi*
1Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center, University of Duisburg-Essen, Essen, Germany
*Corresponding Author: Amir A. Mahabadi, MD, Department of Cardiology and Vascular Medicine, West German Heart and Vascular Center, University of Duisburg-Essen, Essen, Germany
Received: 13 November 2020; Accepted: 20 November 2020; Published: 15 February 2021
Citation: Iryna Dykun, Peter Lüdike, Sultan Poyraz, Alexander Y. Lind, Tienush Rassaf, Amir A. Mahabadi. Association of Mean Pressure Gradient, Pressure half time, Regurgitant area, and Heart rate with Mitral valve orifice area prior and post edge-to-edge Mitral valve repair. Cardiology and Cardiovascular Medicine 5 (2021): 150-161.
View / Download Pdf Share at FacebookAbstract
Background: Mitral valve orifice area (MVOA) is a key parameter in edge-to-edge mitral valve repair. 3-dimensional transesophageal echocardiography (TOE) assessment of MVOA is the gold standard. In clinical routine, mitral valve stenosis is frequently estimated by mean pressure gradient (MPG) and pressure half time (PHT), which are influenced by the degree of regurgitation and heart rate (HR).
Objectives: We aimed to determine the association of MPG, PHT, regurgitant area, and HR with MVOA with mitral valve regurgitation in patients undergoing interventional mitral valve repair. Methods: TOE images from patients undergoing edge-to-edge mitral valve repair were evaluated. MVOA was measured from 3-dimensional volume datasets using multiplanar reformations. Linear regression analysis was used to determine the association of MPG and PHT with MVOA.
Results: By implantation of 1.24 devices in 51 patients (aged 80±13years, 49%male), MVOA was reduced from 5.77±2.17cm² to 2.96±1.03cm². MVOA was modestly inversely correlated with MPG (r=-0.45, p<0.0001) and PHT (r=-0.56, p<0.0001). MPG (unadjusted Beta-estimate (95%CI):-0.99(-1.38--0.60), p<0.0001, r-square=0.20) and PHT (-1.23(-1.59--0.87), p<0.0001, r-square=0.31) were significantly associated with MVOA. Prediction of MVOA improved when combining the information of MPG, PHT, HR and regurgitant area (r-square=0.516).
Conclusion: MPG and PHT modestly predict 3-dimensional MVOA. However, only combining the information of MPG and PHT with patient’s HR and regurgitation allows reliable prediction of MVOA.
Keywords
Edge-to-edge mitral valve repair; MitraClip; Mitral Valve Orifice Area; Mean Pressure Gradient; Mitral Regurgitation; Interventional Therapy
Edge-to-edge mitral valve repair articles; MitraClip articles; Mitral Valve Orifice Area articles; Mean Pressure Gradient articles; Mitral Regurgitation articles; Interventional Therapy articles
Article Details
1. Introduction
Interventional therapy using the edge-to-edge technology is increasingly performed in severe mitral valve regurgitation in patients with increased surgical risk, improving outcome and rehospitalization rates [1-3]. Accordingly, current guidelines suggest interventional mitral valve repair as a treatment option in patients with secondary MR and high surgical risk [4]. In recent years, the most widely catheter-based strategy used to treat MR is the MitraClipTM device (Abbott Vascular, Menlo Park, California, USA). Edge-to-edge repair of the mitral valve attaches the anterior and posterior mitral valve leaflets, creating a double orifice and reduction of the mitral valve orifice area (MVOA). These changes in anatomy frequently make post-interventional mitral valve stenosis a frequent concern [5,6]. MVOA, as assessed from 3-dimensional transesophageal echocardiography (TOE) is the gold standard for the assessment of potential stenosis of the mitral valve prior and post interventional mitral valve reconstruction therapy [7-11]. However, its assessment requires TOE and state-of-the art equipment regarding echocardiographic systems and probes. But especially in routine follow-up, transthoracic echocardiography (TTE) without ability for quantification of 3-dimensional MVOA is performed in the majority of cases. In TTE, evaluation of a potential mitral valve stenosis is performed by assessment of mean pressure gradient (MPG) and pressure half time (PHT). While these variables can be relevantly affected by heart rate or degree of regurgitation, little is known how well their quantification can actually predict 3-dimensional MVOA prior and post edge-to-edge interventional mitral valve repair. Therefore, we assessed the ability of a combination of easily assessable measures from TTE to predict 3-dimensional MVOA in patients prior and post edge-to-edge interventional mitral valve repair.
2. Materials and Methods
Study subjects
We retrospectively included consecutive patients undergoing interventional edge-to-edge mitral valve repair (MitraClip-NT devices, Abbott, Chicago, Illinois, U.S) at our center between May 2018 and April 2019. Patients with moderate-severe or severe MR, symptomatic despite guideline-directed optimal medical therapy were included and evaluated by a multidisciplinary Heart Team. Otherwise, no specific in- or exclusion criteria were applied. Both patients with functional and degenerative mitral valve regurgitation were eligible. All patients with TOE assessment prior and during interventional therapy were included. Patients with insufficient image quality or incomplete image acquisition, not allowing for complete assessment of all intended variables were excluded. The study was approved by the local ethics committee (19-8721-BO).
Echocardiographic measurements
Echocardiographic assessment was performed on an Epiq 7C system equipped with a X8-2 probe (Philips Healthcare, Amsterdam, the Netherlands) providing a range frequency of 2.0–8.0-MHz and both 2D and 3D matrix arrays (Philips Medical Systems). Functional MR was defined as impaired coaptation of the mitral leaflets due to the left ventricular and annular dilatation or restricted leaflet motion. Degenerative MR was defined as disruption in any part of the mitral apparatus [12]. MR severity was graded based on the combination of the jet area, vena contracta and the size of flow convergence zone.
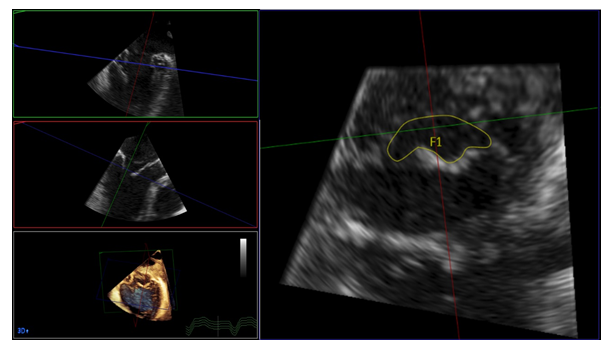
All echocardiographic measurements were performed according to the ESE/EACVI recommendations [13-17]. In brief, the mitral valve inflow was assessed from continuous wave Doppler images and utilized for quantification of mean MPG and PHT prior interventional therapy. In case of atrial fibrillation, at least 3 measurements were performed and mean values were calculated. After one or more clips were placed, the beam of CW Doppler was located in the center of the largest orifice and the postprocedural MPG and PHT were assessed. Heart rate was assessed at the timepoint of continuous wave Doppler assessment. 3-dimensional MVOA was assessed using the QLAB software Version 2.3 (Philips Healthcare, Amsterdam, the Netherlands). 3-dimensional datasets, acquired over 4 heartbeats, were used and MVOA was manually traced at the maximal opening of the valve during early LV filling after adjusting cut planes (Figure 1) [14,18]. Likewise, the EORA was quantified from 3-dimensional datasets, acquired over 4 heartbeats.
LV ejection fraction was measured using biplane method at LV end-systole and LV end-diastole in the apical 2- and 4-chamber views in TTE examination prior and after interventional therapy [19]. Likewise, RV/RA gradient, left atrial volume index left ventricular end-systolic volume, left ventricular end-diastolic, were quantified from TTE prior the implantation and from follow-up examination. As MV inflow parameters were not routinely assessed as part of the follow-up examinations as per institutional standards, only data from prior MV repair is provided. The left atrial volume was measured from apical four chamber view at the end systole, shortly before the mitral valve opens in four- and two-chamber view and indexed to BSA. With standard thransthoracic pulsed wave-Doppler echocardiography using the apical four chamber view, the peak early-diastolic (E), atrial systolic (A) transmitral flow velocities, the deceleration time of the E wave (DT) were measured and the velocity of mitral annual motion at the lateral ring (lateral E`) using PW tissue Doppler was mesuered according to the ESE/EACVI recommendations for the evaluation of left ventriclular diastolic function [13].
Figure 1: 3-dimensional analysis of mitral valve orifice area (MVOA). Datasets, acquired over 4 heartbeats were analyzed offline. Using multiplanar reformations, an image of the mitral valve orifice was created at maximal opening of the valve during early diastole. The MVOA was then traced manually.
Interventional mitral valve repair
The implantation procedures were performed as recommended by the respective manufacturer (Abbott, Chicago, Illinois, U.S.). In brief, venous access was generated via a femoral vein. After transseptal atrial puncture, the guiding catheter was advanced into the left atrium, allowing to proceed and position the device above the mitral annulus plane. Finally, the clip was implanted by grasping the anterior and posterior mitral valve leaflets and adaption of the leaflets is achieved by closure of the device. If reduction of mitral valve regurgitation by implantation of a single clip was not sufficient, at least one further device was implanted.
Statistical analysis
The baseline characteristics are presented as mean ± standard deviation for continuous variables and as frequency and percentages for categorical variables. All echocardiographic variables were normally distributed. Pearson correlation coefficient was used to determine the correlation of MVOA with other echocardiographic measures. Univariate and multivariate linear regression analysis was used to determine the association of MPG, PHT, EORA, and heart rate with MVOA. For multivariable analysis, two models were used contained MPG, PHT, EORA, and heart rate and model 2 additionally adjusted for age, gender, BMI, left ventricular ejection fraction at baseline, and hypertension. For regression analysis, all included variables were checked for co-linearity where no concern was observed. Effect sizes are depicted per each standard deviation chance of echo characteristic. All analyses were performed using the SAS software (version 9.4, SAS Institute Inc.). A p-value of <0.05 indicated statistical significance.
3. Results
Overall, echocardiographic measurement of 51 patients (mean age 80.0 ± 13.3 years, 49% male) both prior and post mitral valve repair were included (102 datasets). The majority of patients had functional mitral regurgitation. Detail patient characteristics of the cohort are depicted in table 1. For interventional mitral valve repair, a mean of 1.24 devices were implanted (1 device in 40 cases, 2 devices in 10 cases, and 3 devices in a single case), leading to a reduction in MVOA from 5.8 to 3.0 cm². Details of the mitral valve characteristics prior and post interventional valve repair are depicted in Table 2.
Echocardiographic measures of mitral valve stenosis
MPG and PHT were modestly inversely correlated with MVOA (Pearson correlation coefficient for MPG: -0.45, p<0.0001, for PHT: -0.56, p<0.0001). In contrast, MVOA was positively correlated with EORA (0.65, p<0.0001). With HF, we observed no relevant correlation (0.06, p=0.5). In regression analysis, both MPG and PHT were significantly associated with MVOA (table 3). This association was independent of adjustment for each other, heart rate, EORA remained stable when additionally controlling for age, gender, BMI, left ventricular ejection fraction at baseline, and hypertension. In univariate analysis, r-square was 0.202 for MPG and 0.312 for PHT. Combining the information from both parameters, r-square improved to 0.372. Further adding EORA and heart rate improved the r-square to 0.516, ultimately further improving the prediction of MVOA. When adding age, gender, BMI, left-ventricular ejection fraction at baseline, and hypertension to the model, r-square was only modestly improved. Stratifying by measures prior and post interventional mitral valve repair, associations of MPG, PHT, and HR with MVOA were not significantly different, while for EORA, associations were significantly weakened after mitral valve repair (Table 4).
|
n=51 |
|
|
Age, years |
80.0 ± 13.3 |
|
Gender, male |
25 (49) |
|
Body mass index, kg/m² |
25.7 ± 4.6 |
|
Hypertension, n (%) |
50 (98) |
|
Diabetes melitus, n (%) |
12 (23.5) |
|
Hyperlipidemia, n (%) |
41 (80.4) |
|
Coronary artery disease, n (%) |
28 (45.1) |
|
LV-ejection fraction, % |
42.9 ± 14.8 |
|
SysPAP, mmHg |
47.8 ± 13.8 |
|
Functional MR |
35 (68.6) |
|
ACE inhibitor/angiotensin II blocker/Sacubitril |
46 (92) |
|
Beta-blocker |
47 (94) |
|
Diuretics |
48 (96) |
|
Aldosterone antagonist |
26 (52) |
|
NT-proBNP at baseline, pg/ml |
4,821 ± 4,726 |
|
NT-proBNP at follow-up, pg/ml |
2,945 ± 3,139 |
LV: Left ventricular; SysPAP: systolic pulmonary artery pressure; MR: mitral regurgitation
Table 1: Patient characteristics
|
Variable |
Prior MV repair (n=51) |
Post MV repair (n=51) |
|
Mitral Valve |
||
|
Mean pressure gradient, mmHg |
1.86 ± 1.11 |
3.18 ± 1.47 |
|
Pressure half time, ms |
53.3 ± 24.0 |
89.4 ± 30.2 |
|
MVOA, cm² |
5.77 ± 2.17 |
2.96 ± 1.03 |
|
EORA, cm² |
0.70 ± 0.49 |
0.16 ± 0.17 |
|
Heart rate, bpm |
69.4 ± 11.8 |
72.9 ± 20.1 |
|
LV and LA |
||
|
LV-ejection fraction, % |
43.4 ± 14.7 |
40.7 ± 13 |
|
LVESV, ml |
81.5 ± 58.7 |
79.6 ±65.2 |
|
LVEDV, ml |
133.4 ± 72.1 |
134.8 ± 70.1 |
|
LAVI, ml/m² |
68.0 ± 34.8 |
63.3 ± 36 |
|
Right ventricle |
||
|
RV/RA gradient, mm Hg |
47.7 ± 14.6 |
42.7 ± 10.2 |
|
Mitral inflow parameter |
||
|
Peak E-wave velocity, cm/s |
95.4 ±27.9 |
--* |
|
Peak A-wave velocity, cm/s |
75.7 ± 45.0 |
--* |
|
E Deceleration time, ms |
154.7 ± 55.8 |
--* |
|
Peak E`-wave velocity, cm/s |
10.6 ± 4.8 |
--* |
|
E/E` Ratio |
10.8 ± 5.4 |
--* |
|
E/A Ratio |
1.5 ± 0.9 |
--* |
MV: Mitral valve, MVOA: Mitral valve orifice area, EORA: Effective orifice regurgitant area, LV: left ventricle, LA: Left atrium, LVESD: left ventricular end-systolic volume, LVEDD: left ventricular end-diastolic volume, LAVI: left atrial volume indexed to Body Surface Area, RV: right ventricle, RA: right atrium
*Mitral inflow parameter are available only at the baseline
Table 2: Echocardiographic Characteristics prior and post interventional mitral valve repair
|
Unadjusted |
Echocardiographic measures adjusted* |
MV adjusted** |
||||
|
Beta-estimate (95%CI) |
p-value |
Beta-estimate (95%CI) |
p-value |
Beta-estimate (95%CI) |
p-value |
|
|
Mean pressure gradient |
-0.99 |
<0.0001 |
-0.42 |
0.03 |
-0.40 |
0.05 |
|
Pressure half time |
-1.23 |
<0.0001 |
-0.55 |
0.007 |
-0.53 |
0.01 |
|
EORA |
1.42 |
<0.0001 |
1.00 |
<0.0001 |
0.99 |
<0.0001 |
|
Heart rate |
-0.14 |
0.30 |
0.06 |
0.73 |
0.09 |
0.62 |
MVOA: Mitral valve orifice area, EORA: Effective orifice regurgitant area, CI: Confidence interval. *Model contains mean pressure gradient, pressure half time, EORA, heart rate. ** Model ancillary includes age, gender, BMI, left ventricular ejection fraction at baseline, and hypertension.
Table 3: Univariable and multivariable linear regression analysis for the association of MVOA with other echocardiographic measures
|
Prior mitral valve repair |
Post mitral valve repair |
|||
|
Beta-estimate (95%CI) |
p-value |
Beta-estimate (95%CI) |
p-value |
|
|
Mean pressure gradient |
-0.51 |
0.11 |
-0.34 |
0.02 |
|
Pressure half time |
-0.71 |
0.02 |
-0.50 |
0.0004 |
|
EORA |
1.01 |
0.0007 |
0.29 |
0.046 |
|
Heart rate |
0.05 |
0.87 |
0.05 |
0.75 |
Table 4: Univariable linear regression analysis for the association of MVOA with other echocardiographic measures stratified by prior and post mitral valve repair.
4. Discussion
In the present study, we evaluated whether routinely assessed measures of mitral valve stenosis can reliably estimate 3-dimensional MVOA prior and post interventional mitral valve repair. We found that both MPG and PHT were significantly associated with MVOA. However, the prediction of MVOA can be improved when combining these measures together with heart rate and information on the degree of regurgitation. Our results suggest that prior and post mitral valve repair, both MPG and PHT together with the patient’s heart rate and assessment of the degree of mitral valve regurgitation might improve the prediction of true MVOA.
Interventional mitral valve repair via adaptation of the anterior and posterior mitral valve leaflets leads to changes in mitral valve geometry. Predominantly, this procedure reduces the anterior-posterior valve diameter, while also decreeing the medial-lateral diameter as well as overall annulus size [5,20]. Reduction of annulus dimensions is associated with improved mitral regurgitation, especially in functional genesis [9]. Together with the coaptation of the anterior and posterior mitral valve leaflet, this leads to a reduction in the MVOA. In our cohort, the relative reduction in MVOA following the procedure, however, was lower than in other studies, potentially due to the high proportion of patients treated with only a single device [5]. In terms of degree of mitral regurgitation prior to interventional therapy, our cohort was comparable with the COAPT cohort. While in our study only 2 of 51 patients (3,9%) had a baseline EORA of <0.3cm², this was also the case in the minority of patients in the COAPT trial (14%) [21]. The fact that the association of EORA on MVOA was weaker after mitral valve repair suggests that the degree of mitral valve regurgitation only relevantly impacts assessment of mitral valve stenosis if relevant mitral valve regurgitation is present. In terms of predictors of mitral valve stenosis after interventional mitral valve repair, preprocedural MVOA was the best single predictor in patients treated with one device. In contrast, for patients receiving 2 or more devices, other anatomical measures outperformed 3-dimensional orifice area [8]. These results are in line with our findings and others, indicating that multiple parameters should be accounted for assessment of potential mitral valve stenosis and optimal patient selection [22-24]. In terms of changes in cardiac parameters due to interventional mitral valve repair, we not only observed a reduction in EROA and decreased MVOA but also a relevant reduction in NT-proBNP as marker of cardiac stress. Likewise, left atrial volume index and the gradient between right ventricle and right atrium during systole decreased. In contrast, we observed a slight decrease in LV ejection fraction, most likely caused by the increased afterload due to the reduction in regurgitation.
In clinical routine using transthoracic echocardiography, MPG is frequently used for assessment of potential mitral valve stenosis. However, the present findings direct towards the recommendation of a multi-parameter approach for assessment of MVOA in patients prior and post interventional mitral valve repair. In this cohort of patients with severe mitral valve regurgitation prior to therapy, the regurgitant volume leads to a relative increased pressure gradient despite normal MVOA. Likewise, MPG is highly dependent on the duration of the LV-filling period and hence the HF. Therefore, on transthoracic echocardiography, the information of MPG, PHT, HF and EORA should be combined to provide best assessment of the anatomical mitral valve opening area for evaluation of patients prior and after interventional mitral valve repair.
5. Limitations
There are several limitations of this study. First, our results are based on a retrospective cohort. Given its cross-sectional design, we cannot establish causality. In addition, the present data represent a single center experience. Techniques in device implantation may be different compared to other centers, leading to uncertainties of generalization of our results. For example, sufficient reduction of mitral regurgitation was archived by a single device in the majority of patients in our center, which may not be the case in other hospitals. However, given the high volume of interventional atrio-ventricular valve repair performed in our center by a dedicated team with highly standardized protocols and overall high success rate (also reflected by reduction in EORA in this cohort), overall experience of our center is not of concern.
6. Conclusions
MPG and PHT modestly predict 3-dimensional MVOA prior and post edge-to-edge mitral valve repair, independent of heart rate and regurgitant area. However, combining the information of MPG and PHT with patient’s heart rate and regurgitation allows reliable prediction of MVOA. Therefore, all 4 variables should be taken into account when estimating MVOA in interventional mitral valve repair, whenever quantification of 3-dimensional true opening area is not available.
Author Contributions:
Conceptualization, Iryna Dykun, Tienush Rassaf and Amir Abbas Mahabadi; Data curation, Iryna Dykun, Peter Luedike, Sultan Poyraz , Alexander Y. Lind , Matthias Riebisch and Amir Abbas Mahabadi; Formal analysis, Iryna Dykun, Peter Luedike, Sultan Poyraz and Amir Abbas Mahabadi; Funding acquisition, Iryna Dykun; Investigation, Iryna Dykun, Peter Luedike, Sultan Poyraz , Alexander Y. Lind and Matthias Riebisch; Methodology, Iryna Dykun, Peter Luedike and Amir Abbas Mahabadi; Project administration, Iryna Dykun, Peter Luedike, Tienush Rassaf and Amir Abbas Mahabadi; Supervision, Tienush Rassaf and Amir Abbas Mahabadi; Validation, Iryna Dykun, Peter Luedike and Amir Abbas Mahabadi; Writing – original draft, Iryna Dykun and Sultan Poyraz; Writing – review & editing, Iryna Dykun, Peter Luedike, Alexander Y. Lind , Matthias Riebisch, Tienush Rassaf and Amir Abbas Mahabadi.
All authors have read and agreed to the published version of the manuscript.
Funding:
This research received no external funding.
Acknowledgments:
No funding received.
Conflicts of Interest:
Iryna Dykun, Peter Luedike, Sultan Poyraz, Alexander Y. Lind, Mathias Riebisch, Tienush Rassaf and Amir A. Mahabadi declare that they have no conflict of interest.
References
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. New England Journal of Medicine 379 (2018): 2307-2318.
- Sannino A, Smith RL, Schiattarella GG, et al. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a systematic review and meta-analysis. JAMA Cardiology 2 (2017): 1130-1139.
- Iung B, Armoiry X, Vahanian A, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. European Journal of Heart Failure 21 (2019): 1619-1627.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. European Heart Journal 38 (2017): 2739-2791.
- Noack T, Kiefer P, Mallon L, et al. Changes in dynamic mitral valve geometry during Percutaneous edge–edge mitral valve repair with the MitraClip system. Journal of Echocardiography 17 2019): 84-94.
- Levack MM, Jassar AS, Shang EK, et al. Three-dimensional echocardiographic analysis of mitral annular dynamics: implication for annuloplasty selection. Circulation 126 (2012): S183-S188.
- Bleakley C, Eskandari M, Aldalati O, et al. Impact of 3D echocardiography on grading of mitral stenosis and prediction of clinical events. Echo Research and Practice 5 (2018): 105-111.
- Itabashi Y, Utsunomiya H, Kubo S, et al. Different indicators for postprocedural mitral stenosis caused by single-or multiple-clip implantation after percutaneous mitral valve repair. Journal of Cardiology 71 (2018): 336-345.
- Schueler R, Momcilovic D, Weber M, et al. Acute changes of mitral valve geometry during interventional edge-to-edge repair with the MitraClip system are associated with midterm outcomes in patients with functional valve disease: preliminary results from a prospective single-center study. Circulation: Cardiovascular Interventions 7 (2014): 390-399.
- Jassar AS, Brinster CJ, Vergnat M, et al. Quantitative mitral valve modeling using real-time three-dimensional echocardiography: technique and repeatability. The Annals of Thoracic Surgery 91 (2011): 165-171.
- Noack T, Mukherjee C, Kiefer P, et al. Four-dimensional modelling of the mitral valve by real-time 3D transoesophageal echocardiography: proof of concept. Interactive Cardiovascular and Thoracic Surgery 20 (2015): 200-208.
- Braun D, Lesevic H, Orban M, et al. Percutaneous edge-to-edge repair of the mitral valve in patients with degenerative versus functional mitral regurgitation. Catheterization and Cardiovascular Interventions 84 (2014): 137-146.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update From the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography 29 (2016): 277-314.
- Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. European Heart Journal–Cardiovascular Imaging 13 (2012): 1-46.
- Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). European Journal of Echocardiography 11 (2010): 307-332.
- Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. European Heart Journal–Cardiovascular Imaging 14 (2013): 611-644.
- Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. European Heart Journal 32 (2011): 2189-2214.
- Biaggi P, Felix C, Gruner C, et al. Assessment of mitral valve area during percutaneous mitral valve repair using the MitraClip system: comparison of different echocardiographic methods. Circulation: Cardiovascular Imaging 6 (2013): 1032-1040.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal-Cardiovascular Imaging 16 (2015): 233-271.
- Schmidt FP, von Bardeleben RS, Nikolai P, et al. Immediate effect of the MitraClip® procedure on mitral ring geometry in primary and secondary mitral regurgitation. European Heart Journal–Cardiovascular Imaging 14 (2013): 851-857.
- Asch FM, Grayburn PA, Siegel RJ, et al. Echocardiographic outcomes after transcatheter leaflet approximation in patients with secondary mitral regurgitation: the COAPT trial. Journal of the American College of Cardiology 74 (2019): 2969-2979.
- Lubos E, Schlüter M, Vettorazzi E, et al. MitraClip therapy in surgical high-risk patients: identification of echocardiographic variables affecting acute procedural outcome. JACC: Cardiovascular Interventions 7 (2014): 394-402.
- Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC: Cardiovascular Imaging 12 (2019): 353-362.
- Hagendorff A, Doenst T, Falk V. Echocardiographic assessment of functional mitral regurgitation: opening Pandora's box?. ESC Heart Failure 6 (2019): 678-685.