Association of Interleukin-10 with Severity of Axial Spondyloarthritis in Patients Attending in A Tertiary Care Hospital in Dhaka City
Article Information
Raisa Enayet Badhan1, Ahmed Abu Saleh2, Shaheda Anwar2*
1Department of Microbiology, Sheikh Hasina National Institute of Burn and Plastic Surgery, Dhaka
2Department of Microbiology and Immunology, Bangabandhu Sheikh Mujib Medical University, Shahbag, Dhaka
*Corresponding Author: Shaheda Anwar, Microbiology and Immunology Department, Bangabandhu Sheikh Mujib Medical University, Shahbag, Dhaka.
Received: 25 September 2022; Accepted: 03 October 2023; Published: 09 October 2023.
Citation: Raisa Enayet Badhan, Ahmed Abu Saleh, Shaheda Anwar. Association of Interleukin-10 with Severity of Axial Spondyloarthritis in Patients Attending in A Tertiary Care Hospital in Dhaka City. Fortune Journal of Rheumatology 5 (2023): 17-21.
View / Download Pdf Share at FacebookAbstract
Objective: The aim of the study was to determine the association of interleukin-10 (IL-10) level with severity of axial spondyloarthritis (axSpA).
Materials and methods: According to Assessment of Spondyloarthritis International Society (ASAS) criteria, 38 patients with axSpA (clinically diagnosed by Rheumatologist, attending outpatient department of Rheumatology, Bangabandhu Sheikh Mujib Medical University, BSMMU) and 38 healthy controls (resident doctors, laboratory staffs of BSMMU and general people) were enrolled. Blood samples were collected after taking informed written consent and data in a predesigned data collection sheet. Serum IL-10 levels were measured by Enzyme Linked Immunosorbent Assay (ELISA) at Microbiology and Immunology Department, BSMMU.
Results: Among 38 patients, 63.2% were male. According to Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), 14 patients were in mild to moderate group and 24 in severe group. Serum level of IL-10 were significantly higher in patients compared to controls (3.69 pg/ml vs 0.88 pg/ml, P<0.001). Mean IL-10 levels in inactive disease group were comparatively higher than active disease group (P<0.001).
Conclusion: Serum level of IL-10 is significantly elevated in axSpA patients and is negatively correlated with disease activity.
Keywords
IL-10, ELISA, BASDAI
IL-10 articles, ELISA articles, BASDAI articles.
IL-10 articles IL-10 Research articles IL-10 review articles IL-10 PubMed articles IL-10 PubMed Central articles IL-10 2023 articles IL-10 2024 articles IL-10 Scopus articles IL-10 impact factor journals IL-10 Scopus journals IL-10 PubMed journals IL-10 medical journals IL-10 free journals IL-10 best journals IL-10 top journals IL-10 free medical journals IL-10 famous journals IL-10 Google Scholar indexed journals ELISA articles ELISA Research articles ELISA review articles ELISA PubMed articles ELISA PubMed Central articles ELISA 2023 articles ELISA 2024 articles ELISA Scopus articles ELISA impact factor journals ELISA Scopus journals ELISA PubMed journals ELISA medical journals ELISA free journals ELISA best journals ELISA top journals ELISA free medical journals ELISA famous journals ELISA Google Scholar indexed journals BASDAI articles BASDAI Research articles BASDAI review articles BASDAI PubMed articles BASDAI PubMed Central articles BASDAI 2023 articles BASDAI 2024 articles BASDAI Scopus articles BASDAI impact factor journals BASDAI Scopus journals BASDAI PubMed journals BASDAI medical journals BASDAI free journals BASDAI best journals BASDAI top journals BASDAI free medical journals BASDAI famous journals BASDAI Google Scholar indexed journals Spondyloarthritis articles Spondyloarthritis Research articles Spondyloarthritis review articles Spondyloarthritis PubMed articles Spondyloarthritis PubMed Central articles Spondyloarthritis 2023 articles Spondyloarthritis 2024 articles Spondyloarthritis Scopus articles Spondyloarthritis impact factor journals Spondyloarthritis Scopus journals Spondyloarthritis PubMed journals Spondyloarthritis medical journals Spondyloarthritis free journals Spondyloarthritis best journals Spondyloarthritis top journals Spondyloarthritis free medical journals Spondyloarthritis famous journals Spondyloarthritis Google Scholar indexed journals sacroiliac joint involvement articles sacroiliac joint involvement Research articles sacroiliac joint involvement review articles sacroiliac joint involvement PubMed articles sacroiliac joint involvement PubMed Central articles sacroiliac joint involvement 2023 articles sacroiliac joint involvement 2024 articles sacroiliac joint involvement Scopus articles sacroiliac joint involvement impact factor journals sacroiliac joint involvement Scopus journals sacroiliac joint involvement PubMed journals sacroiliac joint involvement medical journals sacroiliac joint involvement free journals sacroiliac joint involvement best journals sacroiliac joint involvement top journals sacroiliac joint involvement free medical journals sacroiliac joint involvement famous journals sacroiliac joint involvement Google Scholar indexed journals spine articles spine Research articles spine review articles spine PubMed articles spine PubMed Central articles spine 2023 articles spine 2024 articles spine Scopus articles spine impact factor journals spine Scopus journals spine PubMed journals spine medical journals spine free journals spine best journals spine top journals spine free medical journals spine famous journals spine Google Scholar indexed journals ankylosing spondylitis articles ankylosing spondylitis Research articles ankylosing spondylitis review articles ankylosing spondylitis PubMed articles ankylosing spondylitis PubMed Central articles ankylosing spondylitis 2023 articles ankylosing spondylitis 2024 articles ankylosing spondylitis Scopus articles ankylosing spondylitis impact factor journals ankylosing spondylitis Scopus journals ankylosing spondylitis PubMed journals ankylosing spondylitis medical journals ankylosing spondylitis free journals ankylosing spondylitis best journals ankylosing spondylitis top journals ankylosing spondylitis free medical journals ankylosing spondylitis famous journals ankylosing spondylitis Google Scholar indexed journals cytokines articles cytokines Research articles cytokines review articles cytokines PubMed articles cytokines PubMed Central articles cytokines 2023 articles cytokines 2024 articles cytokines Scopus articles cytokines impact factor journals cytokines Scopus journals cytokines PubMed journals cytokines medical journals cytokines free journals cytokines best journals cytokines top journals cytokines free medical journals cytokines famous journals cytokines Google Scholar indexed journals Joint pain articles Joint pain Research articles Joint pain review articles Joint pain PubMed articles Joint pain PubMed Central articles Joint pain 2023 articles Joint pain 2024 articles Joint pain Scopus articles Joint pain impact factor journals Joint pain Scopus journals Joint pain PubMed journals Joint pain medical journals Joint pain free journals Joint pain best journals Joint pain top journals Joint pain free medical journals Joint pain famous journals Joint pain Google Scholar indexed journals neck pain articles neck pain Research articles neck pain review articles neck pain PubMed articles neck pain PubMed Central articles neck pain 2023 articles neck pain 2024 articles neck pain Scopus articles neck pain impact factor journals neck pain Scopus journals neck pain PubMed journals neck pain medical journals neck pain free journals neck pain best journals neck pain top journals neck pain free medical journals neck pain famous journals neck pain Google Scholar indexed journals
Article Details
1. Introduction
Spondyloarthritis (SpA) can be divided into peripheral and axial forms. The latter is distinguished by a predominance of spine or sacroiliac joint involvement. The radiographic form of axial SpA, ankylosing spondylitis (AS), and nonradiographic axial SpA (nr-axSpA) are both referred to as axial SpA (axSpA). Both are two distinct stages of the same illness [1]. Adults between 20 to 60 years of age mostly affected, which places a significant socioeconomic burden on their families [2]. In the world's population, AS is thought to affect 0.7% to 3.2% of people [3]. Prevalence is 1.2% (0.7-1.8) in Bangladesh [4]. Only 1%-5% HLAB27 positive people develop AS, despite the fact that HLA-B27 is the dominant gene linked [5]. In addition, there are notable variations in the distribution of HLA-B27 and its subtypes throughout the world [6]. Research has looked into how genetic factors including non-MHC genes are linked. AS has been linked to at least 36 genetic variations in non- major histocompatibility complex (non- MHC) areas [7]. It has Th2 type cytokine secretion pattern [8]. Proinflammatory cytokines are inhibited by IL-10, and Th1 cytokines are typically decreased as a result [9]. Interleukin-10 production may be noticeably higher in AS patients than in healthy controls, according to earlier investigations. Numerous cytokines and immune regulators' expression levels are also genetically influenced. Therefore, it is quite plausible that genetic variations would affect the pattern of cytokine release in AS5. Monocytes and lymphocytes are the main producers of IL-10, which has been demonstrated to have anti-inflammatory effects and to play a role in inhibiting autoimmune and inflammatory issues. It controls the balance of T helper1 (Th1) vs. T helper2 (Th2) cytokines, which is a key factor in controlling the balance between immunity and autoimmune disease, by down-regulating the expression of Th1 cytokines [10]. The BASDAI score, which incorporates patient self-reported sensations assessed from questionnaire, is frequently used to assess disease activity in AS patients. Joint pain, localized soreness, fatigue, and morning stiffness are included in BASDAI, which measures disease activity. The range of possible BASDAI scores is 0 to 10. Score less than 4 denotes inactive disease, score 4 to 6 denotes the need for a combination of test indicators to identify the disease status. Scores 6 to 10 are indicative of an active illness [11]. According to a Taiwanese study, IL-10 levels were higher in mild to moderate illness and lower in severe. Thus, they demonstrated a link between IL-10 levels and the severity of the axSpA condition [12]. M2 macrophages can help us better understand the role of IL-10 in the etiology, these findings are contentious because IL-10 is an anti-inflammatory cytokine. As the innate immune system's effector cells, macrophages play a crucial role in initiation and resolution of inflammation. Macrophages are divided into two groups: M1 (classically activated) and M2 (alternatively activated), which are primarily involved in tissue remodeling and the production of inflammatory cytokines. The phenotype of macrophages can change with IL-10, going from M1 to M2 [13]. Many studies regarding pro inflammatory cytokines are conducted but studies regarding anti inflammatory cytokines are few. In order to establish its potential as a prognostic marker, this study examined the serum levels of IL-10 in both patients and healthy controls, as well as their relationship to disease activity.
2. Materials & Methods
This cross sectional study was conducted from September 2021 to August 2022 at the Department of Microbiology and Immunology in Bangabandhu Sheikh Mujib Medical University (BSMMU), Shahbagh, Dhaka.
2.1 Study population
- Patient group: A total number of 38 axSpA patients attending at out patient department (OPD) of Rheumatology, BSMMU were selected as cases.
- Control group: Total 38 healthy controls were selected from resident doctors and laboratory staffs of Department of Microbiology and Immunology, BSMMU and general people were selected as controls.
2.2 Selection of study population
2.2.1 Inclusion criteria: Patients were selected according to the Assessment of spondyloarthritis international
society (ASAS) criteria for diagnosis of axial spondyloarthritis (axSpA) after taking informed written consent. Persons having no diagnosed autoimmune and/ or rheumatological diseases, related to patient group and belonging to the same ethnic group as the patients were selected as controls.
2.2.2 Exclusion criteria: Pregnancy and breast feeding, alcohol abuse, acute infection and uncontrolled diabetes
were the exclusion criterias for cases. Person having family history of axSpA and other rheumatological disorder, pregnancy and acute infection were the exclusion criteria for controls.
2.3 Cytokine measurement
Three ml blood from each subjects were centrifuged at 4000 rpm for 5 min and stored at -20°C upto the study period. Properly stored serum were used for determining the level of IL-10 using a sandwich ELISA according to manufacturer’s instruction (Elabscience, USA; catalog no: E-EL-H6154).
2.3.1 Statistical analysis:
All the data were rechecked, coded, entered in a data base, and analyzed using Statistical Package for the Social Sciences (SPSS) version 22 (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). The categorical variables were expressed as numbers (n) and percentages (%), while continuous variables were expressed as Mean ± Standard Deviation. To observe the association of IL-10 level in case and control, Mann–Whitney U test was performed. Statistically significant P value was considered as less than 0.05 for all statistical analysis.
3. Results
This cross sectional study was conducted on 38 clinically confirmed axial spondyloarthritis patients and 38 healthy controls for determining the association of IL-10 with disease severity in a tertiary care hospital in Dhaka city. Table 1 shows the Clinico-demographic characteristics of patients. The comparison of IL-10 levels between cases and controls are shown in Table 2. The mean serum levels of IL-10 were found significantly higher in ax-SpA patients compared to controls (Mean± SD 4.12± 1.76 pg/ ml vs 0.90± 0.15 pg/ ml, P<0.001). Figure 1 showing, the comparison of IL-10 between cases and controls. Here IL-10 level is significantly higher in the cases than that of controls. The disease activity are categorized in Table 3 into two groups based on BASDAI scoring system. The scoring include inactive disease group (score <4) and active disease group (score >4). Among 38 patients 14 (36.8%) patients were in inactive disease group and 24 (63.2%) were in active disease group. Mean IL-10 levels in active group were significantly lower than inactive group (P<0.001) according to BASDAI score. Figure 2 shows that the serum level of IL-10 in patients group were negatively correlated with disease activity according to BASDAI score (P<0.001).
Table 1: Clinico-demographic characteristics of the patients with axial SpA (n=38)
|
Parameters |
Patients with axial SpA |
|
Age (years) Mean±SD |
34.66±8.08 |
|
Range |
(21-49) |
|
Gender Male |
24 (63.2%) |
|
Female |
14 (36.8%) |
|
Male : Female ratio |
1.7:1 |
|
Axial spondyloarthritis duration (years) |
3.18±1.93 |
|
Age at onset (years) |
31.5±7.92 |
|
Peripheral arthritis |
27 (71.05%) |
|
Enthesitis |
26 (68.42%) |
|
Dactylitis |
25 (65.79%) |
|
Uveitis |
6 (15.79%) |
|
Psoriasis |
8 (21.05%) |
|
Inflammatory bowel disease |
4 (10.53%) |
|
Positive family history |
25 (65.8%) |
|
C-Reactive protein (mg/L) |
26.9±17.5 |
|
HLA-B27 positive |
34 (89.5%) |
|
Sacroiliitis on X-ray |
38 (100.0%) |
Note: SD= Standard deviation
HLA-B27= Human leukocyte antigen B27
Table 2: Comparison of serum IL-10 level in ax-SpA patients and controls (n=76)
|
Interleukin-10 (pg/ml) |
Case |
Control |
P-value |
|
(n=38) |
(n=38) |
||
|
Mean±SD |
4.12±1. 76 |
0. 90±0.15 |
<0. 001* |
|
Median |
3.69 |
0.88 |
|
|
Range (min-max) |
1. 37 -9.40 |
0. 54 -1.24 |
P-value measured by Mann-Whitney U test,
*indicates significant.
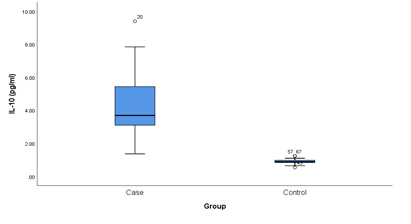
Figure1: Box plot showing the comparison of Interleukin-10 level between cases and controls. Mean Whitney test was performed. Median is represented by the horizontal line. The box represent the interquartile range and the whiskers represent the overall range.
*indicate significant
Table 3: Association of disease severity with serum IL-10 level according to BASDAI score (n=38)
|
Cytokine level |
Disease severity by BASDAI score |
P-value |
|
|
Inactive |
Active |
||
|
(n=14) |
(n=24) |
||
|
Interleukin-10 (pg/ml) |
|||
|
Mean±SD |
5.68±1.66 |
3. 21±1.05 |
<0.001* |
|
Median |
5.7 |
3.31 |
|
P-value reached from Mann-Whitney U test,
*indicate significant.
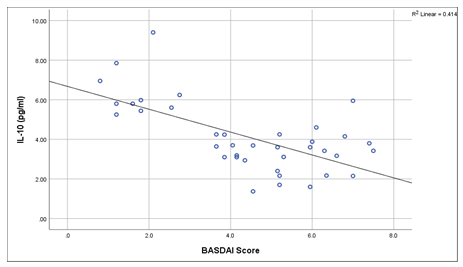
Figure 2: Correlation of BASDAI score with IL-10 levels of the axSpA cases. Spearman's rho correlation were done to see the association.
*indicate significant.
4. Discussion
Ankylosing spondylitis (AS), a common subtype of SpA, is associated with chronic inflammation of the sacroiliac and peripheral joints and the enthesis and it has been confirmed that genetic and environmental factors play important role in its pathogenesis.. There are increasing evidences to suggest the roles of non-major histocompatibility complex (non-MHC) genes in AS. Cytokine encoding genes associated with Ankylosing spondylitis, can interfere with the production of these cytokines and may be a contributory factor of developing Ankylosing spondylitis [14]. With respect to the Th2 cytokine secretion pattern in AS, there has been some interest in the role of IL-10 in the pathogenesis [5]. The study demonstrated the elevated levels of serum IL-10 in axSpA patients compared to healthy controls similar to a Brazilian study [13]. This finding is also in consistence with Madej et al (2015) [1], Lv et al (2011) [5] and Baeten et al (2001) [8]. Another Brazilian study published in 2018 found that, people with AS have high levels of the anti-inflammatory cytokine IL-10. This increased IL-10 expression may be a suppressive reaction to inflammation. 2011 saw reports of elevated IL-10 serum levels in AS patients from China. The elevated levels of IL-10 most likely indicated a suppressive feedback pathway [15]. Similar to the Chinese study, a recent study of a mixed ethnic group found that AS patients had greater serum levels of IL-10 than controls did [16]. Increased levels of IL-10 and IL-10 positive T cells were detected in synovial fluid of AS patients in other studies [17]. Although these results seem controversial as IL-10 is an anti-inflammatory cytokine, a way to better understand the role of IL-10 in the pathogenesis of disease can be through the expression of M2 macrophages. IL-10 can alter the phenotype of macrophages from M1 to M2 [13]. In this study, among the 38 axSpA patients 14 patients were in inactive group and 24 were in active group according to BASDAI score. A higher concentration of IL-10 was found in inactive disease group (5.68±1.66 pg/ml) compared to active disease group (3.21±1.05 pg/ml), estimated P value is <0.001. According to BASDAI scoring, this study revealed a negative correlation of disease activity with IL-10 levels. Chou et al (2006) also found a negative correlation of IL-10 level with the activity of axSpA based on BASDAI score [12]. Another Chinese study by Wen et al (2017) also showed a negative correlation of IL-10 with the activity of axSpA [18]. So, this study was conducted to demonstrate the serum level of IL-10 and to correlate it with disease severity. From the findings, it is evident that higher level of IL-10 and the association with disease severity might be useful for clinicians to predict the prognosis of axSpA. Limitation of the study is, taking drug history from all patients was not possible. That may influence the result. Further studies could be done to find out the association of other anti inflammatory cytokines with severity of Axial spondyloarthritis.
Conclusion
Serum levels of IL-10 is significantly elevated in axSpA patients .Serum IL-10 level is negatively correlated with disease activity assessed by BASDAI score.
Acknowledgments
We would like to thank all the staff of the Department of Microbiology and Immunology, Bangabandhu Sheikh Mujib Medical University, Dhaka. We are grateful to all the patients and healthy controls who have participated in the study. Funding is partially contributed by University grant.
Declarations Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Madej M, Nowak B, Swierkot J, et al. Cytokine profiles in axial spondyloarthritis. Reumatologia/Rheumatology 53 (2015): 9-13.
- Bakland G, Gran JT, Becker-Merok A, et al. Work disability in patients with ankylosing spondylitis in Norway. The Journal of rheumatology 38 (2011): 479-484.
- Ranganathan V, Gracey E, Brown MA, et al. Pathogenesis of ankylosing spondylitis—recent advances and future directions. Nature Reviews Rheumatology 13 (2017): 359-367.
- Hasan ATMT and Alim MA. The Lag Time of Diagnosis of Axial Spondyloarthritis: A Bangladesh Perspective. American Journal of Internal Medicine 8 (2020): 254-257.
- Lv C, Wang Y, Wang J, et al. Association of Interleukin-10 gene polymorphisms with ankylosing spondylitis. Clinical and Investigative Medicine 34 (2011): 370-376.
- Wu X, Wang G, Zhang L, et al. Genetics of Ankylosing Spondylitis—Focusing on the Ethnic Difference Between East Asia and Europe. Frontiers in genetics 12 (2021): 863.
- Brown MA, Kenna T, Wordsworth BP. Genetics of ankylosing spondylitis—insights into pathogenesis. Nature Reviews Rheumatology 12 (2016): 81-91.
- Baeten D, Van Damme N, Van den Bosch F, et al. Impaired Th1 cytokine production in spondyloarthropathy is restored by anti-TNFα. Annals of the Rheumatic Diseases 60 (2001): 750-755.
- Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy-review of a new approach. Pharmacological reviews 55 (2003): 241-269.
- Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology 19 (2001): 683.
- Fallahi S, Jamshidi AR, Gharibdoost F, et al. Urolithiasis in ankylosing spondylitis: Correlation with Bath ankylosing spondylitis disease activity index (BASDAI), Bath ankylosing spondylitis functional index (BASFI) and Bath ankylosing spondylitis metrology index (BASMI) 3 (2012): 508-513.
- Chou CT, Huo AP, Chang HN, et al. Cytokine production from peripheral blood mononuclear cells in patients with ankylosing spondylitis and their first-degree relatives. Archives of medical research 38 (2007): 190-195.
- Deng B, Wehling-Henricks M, Villalta SA, et al. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. The Journal of Immunology 189 (2012): 3669-3680.
- Du J, Sun J, Wen Z, et al. Serum IL-6 and TNF-α Levels Are Correlated with Disease Severity in Patients with Ankylosing Spondylitis. Laboratory Medicine 53 (2022): 149-155.
- Rabelo CF, Baptista TS, Petersen LE, et al. Serum IL-6 correlates with axial mobility index (Bath Ankylosing Spondylitis Metrology Index) in Brazilian patients with ankylosing spondylitis. Open Access Rheumatology: Research and Reviews 10 (2018): 21.
- Braga M, Lara-Armi FF, Neves JS, et al. Influence of IL10 (rs1800896) polymorphism and TNF-α, IL-10, IL-17A, and IL-17F serum levels in ankylosing spondylitis. Frontiers in immunology 12 (2021): 2502.
- Keller C, Webb A, Davis J. Cytokines in the seronegative spondyloarthropathies and their modification by TNF blockade: a brief report and literature review. Annals of the rheumatic diseases 62 (2003): 1128-1132.
- Wen JT, Zhang DH, Fang PF, et al. Role of Th1/Th2 cytokines in the diagnosis and prognostic evaluation of ankylosing spondylitis. Genet Mol Res 16 (2017): 16019322.
