Association between COVID-19 Vaccine and New-Onset Atrial Fibrillation
Article Information
Kunal M. Ajmera
Department of Hospital Medicine, Calvert Health Medical Center, Prince Frederick, MD, USA
*Corresponding Author Kunal M. Ajmera, Department of Hospital Medicine, Calvert Health Medical Center, Prince Frederick, MD 20678, USA
Received: 24 December 2021; Accepted: 05 January 2022; Published: 10 January 2022
Citation: Kunal M. Ajmera. Association between COVID-19 Vaccine and New-Onset Atrial Fibrillation. Cardiology and Cardiovascular Medicine 6 (2022): 1-6.
View / Download Pdf Share at FacebookAbstract
Since the availability of the vaccine against severe acute respiratory syndrome, coronavirus 2 (SARS-CoV-2), the etiologic agent for COVID-19, at least 67% of the US population has been vaccinated with at minimum one dose, and 58.2% of the U.S. population has received two doses. Here, we present the first report of a patient who developed new-onset atrial fibrillation (AF) after receiving a booster dose of the Moderna COVID-19 vaccine. The benefit of the COVID-19 vaccine certainly outweighs the risks of adverse cardiac effects; however, recipients with known cardiac risk factors should be appropriately advised. The mechanism by which the COVID-19 vaccine causes adverse cardiac effects is presently unknown and is likely complex and multifactorial. Prospective studies are needed urgently to understand the mechanism and pathophysiology of such serious adverse events.
Keywords
COVID-19; Cardiovascular disease; COVID-19 vaccine; Atrial fibrillation
Article Details
1. Introduction
Since the beginning of the coronavirus disease (COVID-19) pandemic, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), almost 50 million people in the U.S. [1] and almost 275 million people worldwide [2] have been infected. Much of the control over this rapidly spreading, global pandemic has been achieved through widespread vaccination and the use of masks. As of December 24, 2021, at least 72.7% of the US population has been vaccinated with at least one dose, and 61.7% have been fully vaccinated with two doses. Additionally, almost 32% of the population has received a booster dose [3]. Here, the first case of new-onset atrial fibrillation (AF) after a booster dose of the Moderna COVID-19 vaccine in an elderly female patient is reported. Although we have a better understanding of the COVID-19 disease, its pathophysiology, and its effects on various organs such as the lung, brain, pancreas, heart, and kidneys two years into the pandemic, more data on the adverse events related to the vaccine have been emerging almost daily. These adverse events need to be better understood.
2. Methods
A systematic review of medical literature with the keywords "COVID-19," "Atrial Fibrillation," "cardiovascular complications," "COVID-19 vaccine" was performed on PubMed, Google Scholar, and ScienceDirect databases. Articles that included definition, pathophysiology, mechanism of action, and etiology were identified. All articles, including reviews (narrative and systematic), meta-analysis, literature review, randomized controlled trials (RCTs), case-control cohorts, case series, and case reports, were screened for relevant content. Out of the many articles, full texts of the relevant retrieved articles were accessed and used in this paper, in addition to data obtained from the CDC website. Pertinent information was summarized and organized for ease of understanding.
3. Case Presentation
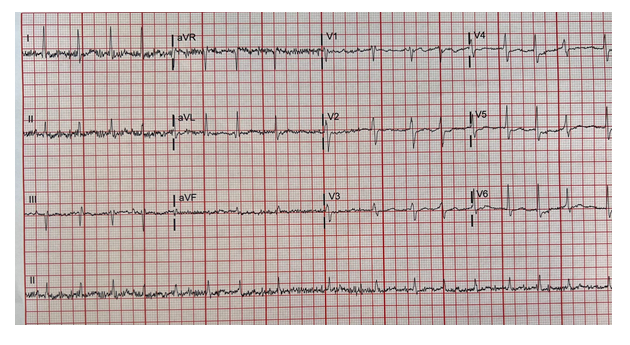
The patient was an 85-year-old, African American woman with a medical history notable for long-standing hypertension, hyperlipidemia, gout, chronic kidney disease stage 3, hypothyroidism, a cerebrovascular accident, and coronary artery disease s/p percutaneous coronary intervention (PCI). She presented to the emergency room (ER) secondary to chest pain and shortness of breath. The patient received the Moderna COVID-19 vaccine booster dose the day before presenting to the ER. She tolerated the first two doses well without any significant adverse effects. She described her chest pain as left-sided and non-radiating. The patient was unable to provide any other information regarding her chest pain and shortness of breath. However, her symptoms resolved on arrival to the ER. The patient's home medication list included allopurinol, amlodipine, hydralazine, metoprolol, pravastatin, mirtazapine, omeprazole, salmeterol/fluticasone inhaler, furosemide, losartan, and triamterene/hydrochlorothiazide. Her family history was positive for a cardiac disorder, cancer, and hypertension. The patient had never smoked cigarettes, drank alcohol, or abused drugs. Her vital signs on arrival to the ER showed a temperature of 99.6 oF, a pulse rate (HR) of 120 beats per minute (bpm), a respiratory rate of 24 breaths/minute, and a blood pressure of 152/51 mmHg. Pertinent positives on physical examination included an irregular heart rhythm with tachycardia. On admission, an electrocardiogram (ECG) showed AF with a HR of 116 bpm, a QRS of 79, and no ST-segment elevation or depression (Figure 1). Blood work in the ER on day 0 showed that the patient was in acute renal failure with a serum creatinine at 2.6, almost double her baseline. Troponin levels were minimally elevated at 0.16 ng/mL, followed by 0.23 ng/mL, 0.41ng/mL, and 0.36 ng/mL on repeat tests, likely related to tachycardia. The remaining blood work results are described in table 1.
|
Reference Range |
||
|
WBC |
10.6 |
4.0-11.0 10x3/ul |
|
Hb |
11.5 |
13.0-18.0 g/dL |
|
MCV |
94.5 |
80-95 fl |
|
Platelet |
245 |
150-450 10x3/uL |
|
Na |
143 |
136-145 mmol/L |
|
K |
3.9 |
3.5-5.1 mmol/L |
|
Glucose |
134 |
75-100 mg/dL |
|
AG |
12 |
8-16 mEQ/L |
|
S. Creat |
2.6 |
0.6-1.3 mg/dL |
|
GFR |
19 |
(>60 mL/min) |
|
Ca |
9.3 |
8.5-10.1 mg/dL |
|
Mg |
2.1 |
1.8-2.4 mg/dL |
|
T. Bili |
0.3 |
0.2-1.0 mg/dL |
|
AST |
23 |
15-37 U/L |
|
ALT |
15 |
12-78 U/L |
|
Alk Phos |
73 |
45-117 U/L |
|
Albumin |
3.4 |
3.4-5.0 g/dL |
|
TSH |
1.56 |
0.35-3.74 uIU/mL |
|
pro-BNP |
142 |
5-450 pg/mL |
Table 1: Blood work on day 0: WBC: White Blood Cell, Hb: Hemoglobin, MCV: Mean Corpuscular Volume, Na: Sodium, K: Potassium, AG: Anion Gap, S. creat: Serum Creatinine, GFR: Glomerular Filtration Rate, Ca: calcium, Mg: magnesium, T. Bili: Total Bilirubin, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, Alk Phos: Alkaline Phosphatase, TSH: Thyroid Stimulating Hormone, BNP-Beta Natriuretic Peptide.
Her chest X-ray showed no acute cardiopulmonary disease, and her ventilation-perfusion (V/Q) scan showed no evidence of pulmonary embolism. The patient was treated with two doses of intravenous Diltiazem, with an improvement in her heart rate. On day 1 of admission, a cardiology consult was requested to evaluate the patient. The cardiologist initiated therapy with oral-maintenance Diltiazem and a renally adjusted dose of apixaban based on a high CHADS2VASC score. An echocardiogram showed mild, concentric, left ventricle hypertrophy with normal systolic function and grade I diastolic dysfunction. The left ventricle ejection fraction was around 55-60%. No hemodynamically-significant valvular stenosis or regurgitation was noted. On day 2 of admission, the patient’s condition continued to improve. Her HR remained well-controlled at around 79-84 bpm. Her kidney function improved, with serum creatinine levels reduced to 1.8 mg/dL. The patient was discharged in stable condition.
4. Discussion
Cardiac complications related to COVID-19 infections are well-known. The exact mechanism leading to these complications is not very clear; however, a proposed mechanism includes a high affinity of the viral-surface spike protein to the human angiotensin-converting enzyme receptor type 2 (ACE2) [4]. This enzyme is present in an abundant quantity in the heart muscle and lungs [5], enabling the virus to enter the heart muscle, to then cause direct damage to myocytes, and ultimately, arrhythmias. In addition, ACE2 indirectly activates the renin-angiotensin system by counteracting angiotensin II, leading to high blood pressure, congestive heart failure, and atherosclerosis [6]. In a cohort study, Bhatla et al. also proposed that it is the severity of the COVID-19 infection- rather than a direct viral infection in the heart- that is associated with cardiac arrhythmias, including AF [7]. Mechanisms such as cytokine storms and hypoxia have also been suggested as potential etiologic factors leading to cardiac arrhythmias in COVID-19 patients [8,9]. AF is the most commonly reported arrhythmia in COVID-19 patients, it is often newly-onset, and it is associated with the worst outcomes [10]. Widely available vaccines in the US are mainly, two mRNA vaccines, Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273), and the Janssen vaccine made of modified DNA of adenovirus (Johnson & Johnson) [11]. Per the Vaccine Adverse Event Reporting System (VAERS), there have been a total of 2406 documented cases of AF in vaccine recipients as of 12/24/2021. Of those cases, 149 cases were associated with the Janssen vaccine, 944 with the Moderna vaccine, and 1313 with the Pfizer-BioNTech vaccine [12]. Out of which 157 vaccine recipients have died post-vaccination. Fifteen vaccine recipients had pre-existing cardiovascular diseases, such as CAD, CHF, or CVA. No data is available on the personal history of medications usage amongst these vaccine recipients that can potentially cause cardiac adverse events. These data are given in detail in table 2.
Additionally, 7857 total recipients have experienced some form of an adverse cardiac event, such as pericarditis (1961 recipients), myocarditis (2492 recipients), cardiac arrest (973 recipients), ventricular fibrillation (213 recipients), or ventricular tachycardia (257 recipients) after receiving one of the three primary vaccines (Janssen, Moderna, PfizerBioNTech) [12] as of 12/24/2021. Some recipients experienced more than one cardiac side effect. Out of these vaccine recipients, 776 have died to date [12]. Both male and female recipients, equally, have experienced these adverse events.
|
Vaccine Type |
Sex |
Events Reported |
|
Female |
68 |
|
|
Male |
76 |
|
|
JANSSEN |
Unknown |
5 |
|
Total |
149 |
|
|
Female |
482 |
|
|
Male |
450 |
|
|
MODERNA |
Unknown |
12 |
|
Total |
944 |
|
|
Female |
677 |
|
|
Male |
614 |
|
|
PFIZER-BioNTech |
Unknown |
22 |
|
Total |
1,313 |
|
|
Total |
2,406 |
|
Table 2: Incidence of AF post-covid-19 vaccination
Even though the VAERS database is the only way to learn about vaccine adverse events, it is essential to keep in mind that this data set has limitations, such as passive data collection and underreporting. Therefore, real-world data might reveal higher incidences of adverse cardiac events. At this time, it is not clear why our patient developed AF after receiving a booster dose of the Moderna COVID-19 vaccine, nor why so many other recipients also experience cardiac side effects post-vaccine. Even though causal association cannot be established between the COVID-19 vaccine and cardiac adverse events from VAERS data, the onset of atrial fibrillation immediately post-COVID-19 vaccine in our patient indeed points towards the temporal association. From VAERS data it is also evident that the recipients with pre-existing cardiovascular disease had higher incidents of developing post-vaccination AF than recipients without. The role of autoimmunity has been proposed as a risk factor for developing rhabdomyolysis post-COVID-19 vaccine [11] and new-onset diabetes post-COVID-19 infection [13,14] and needs to be explored. It is possible that a dysregulated immune system in autoimmune conditions starts to make autoantibodies against cardiac myocytes or cardiac ion channels when stimulated by the vaccine. Such autoantibodies can then trigger damage to cardiomyocytes causing myocarditis or ion channels causing arrhythmias. It is also not yet determined whether this patient’s vaccine-related AF is temporary or long-term. A larger-scale, prospective clinical trial is needed urgently to understand the mechanism and pathophysiology by which the COVID-19 vaccine may cause new-onset AF and other cardiac manifestations.
5. Conclusion
The benefit of the COVID-19 vaccine certainly outweighs the risks of adverse effects. The mechanism by which the COVID-19 vaccine may cause cardiac adverse effects is likely complex and multifactorial and needs to be investigated further. Vaccine recipients with pre-existing cardiovascular disease and those on medications that can cause cardiac arrhythmias should be made aware of the risk of adverse cardiac effects from the vaccine. High-risk recipients should be monitored closely and treated appropriately.
References
- COVID Data Tracker Weekly Review, Centers for Disease Control and Prevention (2020).
- World Health Organization (WHO), Coronavirus (COVID-190 Dashborad (2021).
- COVID-19 Vaccinations in the United States (2021)
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181 (2020): 271-280.
- Zhao Y, Zhao Z, Wang Y, et al. Single-Cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med 2020 (5): 756-759.
- Tikellis C, Thomas MC. Angiotensin-Converting Enzyme 2 (ACE2) Is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 12 (2012): 256-294.
- Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 17 (2020): 1439-1444.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395 (2020): 1054-1062.
- Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol 17 (2020): 259-260.
- Spinoni EG, Mennuni M, Rognoni A, et al. Contribution of atrial fibrillation to in-hospital mortality in patients with COVID-19. Circ Arrhythm Electrophysiol 14 (2021): e009375.
- Ajmera KM. Fatal case of rhabdomyolysis post-COVID-19 vaccine. Infect Drug Resist 14 (2021): 3929-3935.
- United States Department of Health and Human Services (DHHS), Public Health Service (PHS), Centers for Disease Control (CDC) / Food and Drug Administration (FDA), Vaccine Adverse Event Reporting System (VAERS) 1990 - 12/17/2021, CDC WONDER On-line Database (2021).
- Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol 57 (2020): 1265-1266.
- Ajmera KM. COVID-19 and its association with new-onset diabetes. Archives of Clinical and Medical Case Reports 5 (2021): 855-861.