Assessment of Nutritional Status and Its Association with Inflammation among Patients on Continuous Ambulatory Peritoneal Dialysis
Article Information
Marjoa Humaira Mekhola1*, Md. Ayub Ali Chowdhury2, Kazi Shahnoor Alam3, Sourav Saha4, Md. Shahadat Hossain5, Samira Mahjabeen6, Ayesha Samdani7, Nazma Azim8, A. K. M Shahidur Rahman1
1Medical Officer, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
2Professor and Head of the Department (Former), Department of Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
3Professor and Head of the Department, Department of Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
4Associat Professor and Head of the Department, Department of Nephrology, Mainamoti Medical College and Hospital, Cumilla, Bangladesh
5Assistant Registrar, Department of Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
6Medical Officer, Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
7Senior Medical Officer, Outpatient Department (OPD), BIRDEM General Hospital, Dhaka, Bangladesh
8Programmar, Management Information Systems (MIS), National Institute of Cancer Research & Hospital (NICRH), Dhaka, Bangladesh
*Corresponding Author: Dr. Marjoa Humaira Mekhola, Medical Officer, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Received: 17 April 2023; Accepted: 24 April 2023; Published: 09 May 2023
Citation: Mekhola MH, Chowdhury MAA, Alam KS, Saha S, Hossain MS, Mahjabeen S, Samdani A, Azim N, Rahman AKMS. Assessment of Nutritional Status and Its Association with Inflammation among Patients on Continuous Ambulatory Peritoneal Dialysis. Archives of Nephrology and Urology. 6 (2023): 44-50.
View / Download Pdf Share at FacebookAbstract
Continuous ambulatory peritoneal dialysis (CAPD) is one of the therapeutic options in end stage renal disease (ESRD). Malnutrition is prevalent among patients on CAPD. This study was aimed to assess the nutritional status and its association with inflammation among patients on CAPD. This study was conducted at the Department of Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh from July 2018 to June 2019. A total of sixty-nine (69) ESRD patients on CAPD were enrolled. Demographic profile, clinical history and nutritional status of each study patient were recorded. All relevant investigations were done accordingly. Their 24 hours urine and 24 hours effluent were collected for urea and creatinine levels to detect- adequacy of dialysis, residual renal function and normalized protein catabolic rate (nPCR). Malnutrition inflammation score (MIS) was performed following standard procedure. In this study, malnutrition inflammation score (MIS) is higher among CAPD patients. It was observed that, 78.3% patients were malnourished. Serum albumin, serum pre-albumin, total iron binding capacity (TIBC) and body mass index (BMI) showed significant negative correlation with MIS; however high sensitive C-reactive protein (hsCRP) showed positive correlation with MIS. This study concluded that, malnutrition inflammation score (MIS) is related to inflammation in continuous ambulatory peritoneal dialysis (CAPD) patients.
Keywords
Continuous Ambulatory Peritoneal Dialysis (CAPD); End Stage Renal Disease (ESRD); Inflammation; Malnutrition; Malnutrition Inflammation Score (MIS)
Continuous Ambulatory Peritoneal Dialysis (CAPD) articles; End Stage Renal Disease (ESRD) articles; Inflammation articles; Malnutrition articles; Malnutrition Inflammation Score (MIS) articles.
Continuous Ambulatory Peritoneal Dialysis articles Continuous Ambulatory Peritoneal Dialysis Research articles Continuous Ambulatory Peritoneal Dialysis review articles Continuous Ambulatory Peritoneal Dialysis PubMed articles Continuous Ambulatory Peritoneal Dialysis PubMed Central articles Continuous Ambulatory Peritoneal Dialysis 2023 articles Continuous Ambulatory Peritoneal Dialysis 2024 articles Continuous Ambulatory Peritoneal Dialysis Scopus articles Continuous Ambulatory Peritoneal Dialysis impact factor journals Continuous Ambulatory Peritoneal Dialysis Scopus journals Continuous Ambulatory Peritoneal Dialysis PubMed journals Continuous Ambulatory Peritoneal Dialysis medical journals Continuous Ambulatory Peritoneal Dialysis free journals Continuous Ambulatory Peritoneal Dialysis best journals Continuous Ambulatory Peritoneal Dialysis top journals Continuous Ambulatory Peritoneal Dialysis free medical journals Continuous Ambulatory Peritoneal Dialysis famous journals Continuous Ambulatory Peritoneal Dialysis Google Scholar indexed journals End Stage Renal Disease articles End Stage Renal Disease Research articles End Stage Renal Disease review articles End Stage Renal Disease PubMed articles End Stage Renal Disease PubMed Central articles End Stage Renal Disease 2023 articles End Stage Renal Disease 2024 articles End Stage Renal Disease Scopus articles End Stage Renal Disease impact factor journals End Stage Renal Disease Scopus journals End Stage Renal Disease PubMed journals End Stage Renal Disease medical journals End Stage Renal Disease free journals End Stage Renal Disease best journals End Stage Renal Disease top journals End Stage Renal Disease free medical journals End Stage Renal Disease famous journals End Stage Renal Disease Google Scholar indexed journals Inflammation articles Inflammation Research articles Inflammation review articles Inflammation PubMed articles Inflammation PubMed Central articles Inflammation 2023 articles Inflammation 2024 articles Inflammation Scopus articles Inflammation impact factor journals Inflammation Scopus journals Inflammation PubMed journals Inflammation medical journals Inflammation free journals Inflammation best journals Inflammation top journals Inflammation free medical journals Inflammation famous journals Inflammation Google Scholar indexed journals Malnutrition articles Malnutrition Research articles Malnutrition review articles Malnutrition PubMed articles Malnutrition PubMed Central articles Malnutrition 2023 articles Malnutrition 2024 articles Malnutrition Scopus articles Malnutrition impact factor journals Malnutrition Scopus journals Malnutrition PubMed journals Malnutrition medical journals Malnutrition free journals Malnutrition best journals Malnutrition top journals Malnutrition free medical journals Malnutrition famous journals Malnutrition Google Scholar indexed journals Malnutrition Inflammation Score articles Malnutrition Inflammation Score Research articles Malnutrition Inflammation Score review articles Malnutrition Inflammation Score PubMed articles Malnutrition Inflammation Score PubMed Central articles Malnutrition Inflammation Score 2023 articles Malnutrition Inflammation Score 2024 articles Malnutrition Inflammation Score Scopus articles Malnutrition Inflammation Score impact factor journals Malnutrition Inflammation Score Scopus journals Malnutrition Inflammation Score PubMed journals Malnutrition Inflammation Score medical journals Malnutrition Inflammation Score free journals Malnutrition Inflammation Score best journals Malnutrition Inflammation Score top journals Malnutrition Inflammation Score free medical journals Malnutrition Inflammation Score famous journals Malnutrition Inflammation Score Google Scholar indexed journals total iron binding capacity articles total iron binding capacity Research articles total iron binding capacity review articles total iron binding capacity PubMed articles total iron binding capacity PubMed Central articles total iron binding capacity 2023 articles total iron binding capacity 2024 articles total iron binding capacity Scopus articles total iron binding capacity impact factor journals total iron binding capacity Scopus journals total iron binding capacity PubMed journals total iron binding capacity medical journals total iron binding capacity free journals total iron binding capacity best journals total iron binding capacity top journals total iron binding capacity free medical journals total iron binding capacity famous journals total iron binding capacity Google Scholar indexed journals Kidney Disease Outcomes Quality Initiatives articles Kidney Disease Outcomes Quality Initiatives Research articles Kidney Disease Outcomes Quality Initiatives review articles Kidney Disease Outcomes Quality Initiatives PubMed articles Kidney Disease Outcomes Quality Initiatives PubMed Central articles Kidney Disease Outcomes Quality Initiatives 2023 articles Kidney Disease Outcomes Quality Initiatives 2024 articles Kidney Disease Outcomes Quality Initiatives Scopus articles Kidney Disease Outcomes Quality Initiatives impact factor journals Kidney Disease Outcomes Quality Initiatives Scopus journals Kidney Disease Outcomes Quality Initiatives PubMed journals Kidney Disease Outcomes Quality Initiatives medical journals Kidney Disease Outcomes Quality Initiatives free journals Kidney Disease Outcomes Quality Initiatives best journals Kidney Disease Outcomes Quality Initiatives top journals Kidney Disease Outcomes Quality Initiatives free medical journals Kidney Disease Outcomes Quality Initiatives famous journals Kidney Disease Outcomes Quality Initiatives Google Scholar indexed journals body mass index† articles body mass index† Research articles body mass index† review articles body mass index† PubMed articles body mass index† PubMed Central articles body mass index† 2023 articles body mass index† 2024 articles body mass index† Scopus articles body mass index† impact factor journals body mass index† Scopus journals body mass index† PubMed journals body mass index† medical journals body mass index† free journals body mass index† best journals body mass index† top journals body mass index† free medical journals body mass index† famous journals body mass index† Google Scholar indexed journals protein equivalent of nitrogen appearance articles protein equivalent of nitrogen appearance Research articles protein equivalent of nitrogen appearance review articles protein equivalent of nitrogen appearance PubMed articles protein equivalent of nitrogen appearance PubMed Central articles protein equivalent of nitrogen appearance 2023 articles protein equivalent of nitrogen appearance 2024 articles protein equivalent of nitrogen appearance Scopus articles protein equivalent of nitrogen appearance impact factor journals protein equivalent of nitrogen appearance Scopus journals protein equivalent of nitrogen appearance PubMed journals protein equivalent of nitrogen appearance medical journals protein equivalent of nitrogen appearance free journals protein equivalent of nitrogen appearance best journals protein equivalent of nitrogen appearance top journals protein equivalent of nitrogen appearance free medical journals protein equivalent of nitrogen appearance famous journals protein equivalent of nitrogen appearance Google Scholar indexed journals Total iron binding capacity articles Total iron binding capacity Research articles Total iron binding capacity review articles Total iron binding capacity PubMed articles Total iron binding capacity PubMed Central articles Total iron binding capacity 2023 articles Total iron binding capacity 2024 articles Total iron binding capacity Scopus articles Total iron binding capacity impact factor journals Total iron binding capacity Scopus journals Total iron binding capacity PubMed journals Total iron binding capacity medical journals Total iron binding capacity free journals Total iron binding capacity best journals Total iron binding capacity top journals Total iron binding capacity free medical journals Total iron binding capacity famous journals Total iron binding capacity Google Scholar indexed journalsArticle Details
1. Introduction
Continuous ambulatory peritoneal dialysis (CAPD) is one of the therapeutic options of renal replacement therapy (RRT) in end stage renal disease (ESRD). CAPD is a treatment option used by about 11% of the world's dialysis population [1]. Because of its relative simplicity, patient freedom and better preservation of residual renal function (RRF), CAPD utilization is gradually increasing in the developing countries [1, 2]. Despite its potential benefits, CAPD is underutilized in low- and middle-income countries [2, 3].
Patients treated with haemodialysis/CAPD are often malnourished, as evident by low visceral protein levels, decreased anthropometric measurements and low scores of subjective global assessments (SGA) [4, 5]. Malnutrition is a common finding in patients on CAPD because of nutrient loss during dialysis, anorexia, protein catabolism and co-morbidities etc [3]. On the other hand, recent reports have shown that these patients tend to gain body weight and body fat in the first 2 years of CAPD and some of them may develop obesity [6]. The simultaneous existence of overweight and obesity as well as of protein-energy malnutrition (sarcopenia) is a particular nutritional pattern seen in CAPD [7]. To identify patients at high risk for negative outcomes, the Nutrition Kidney Disease Outcomes Quality Initiatives (K/DOQI) proposed a panel of the nutritional index that should be used in these patients [8]. The nutritional parameters recommended include- body weight (with empty peritoneal cavity), height, body mass index (BMI), waist circumference, skin-fold thicknesses, and mid-arm muscle circumference [9, 10]. Other nutritional measurements that should be assessed including serum albumin, pre-albumin, dietary interviews and diaries and the protein equivalent of nitrogen appearance (PNA) [8, 11].
Inflammation can be defined as a localised protective response elicited by injury or destruction of tissues that serves to destroy, dilute or sequester both the injurious agent and injured tissue [12]. Hence, it is a physiological response and in the form of an acute response to infections, trauma, or toxic injury, that helps the body to defend against any pathophysiological insults [13]. There are several factors that contribute to inflammatory condition in a patient on CAPD like: loss of residual renal function (RRF), peritoneal fluid and catheter, peritonitis, peritoneal membrane dysfunction and endotoxemia [11, 14]. However, if inflammation becomes prolonged and persistent in the form of the so called chronic acute-phase reaction, it may lead to adverse consequences, such as decline in appetite, increased rate of protein depletion in skeletal muscle, hyper-catabolism, endothelial damage, and atherosclerosis [12]. The inflammatory status seems to have a pivotal role in increasing the protein catabolism, as increased concentration of tumour necrosis factor-α (TNF-α) and interleukin 6 (IL-6) can lead to anorexia, insulin resistance, activation of the ubiquitin-proteasome pathway and increased resting energy expenditure [15]. All these conditions are associated with decreased skeletal protein stores in CAPD patients [15]. Other factors associated with wasting in CAPD patients include aging, poor physical activity, genetic predisposition, diabetes mellitus and other co-morbidities [15]. There are several markers that can be measured to gauge the level of inflammatory burden, such as C-reactive protein (CRP), serum total iron binding capacity (TIBC) and serum albumin, serum pre-albumin [16].
Nutritional status of a patient with chronic disease could be measured by several tools [17-19]. But clinical condition of patients on CAPD is affected by both malnutrition and inflammatory condition and therefore, should be assessed in a combined way. Malnutrition inflammation score (MIS) significantly correlates with clinical, nutritional, inflammatory, anthropometric parameters and anaemia indices in CAPD patients [17-19]. MIS is a 4-point scale, quantitative nutrition screening tool, consists of four main parts, including - patient’s related medical history, physical examination, BMI and laboratory parameters [17-19]. Regular assessment of nutritional status and inflammatory condition of patients on CAPD may reduce mortality and morbidity and bring long-term better outcomes. There is scarce evidence that assesses nutritional status and inflammatory condition among CAPD patients. This cross-sectional study was undertaken to assess the nutritional status and inflammatory markers of CAPD patients by using malnutrition inflammation score (MIS).
2. Methodology
This cross-sectional study was conducted at the Department of Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh from July 2018 to June 2019. The study was approved by the institutional review board (IRB), NIKDU, Dhaka, Bangladesh. A total of sixty-nine (69) ESRD patients on continuous ambulatory peritoneal dialysis (CAPD) were enrolled by purposive sampling technique following selection criteria. Adult (age >18 years) ESRD patients on CAPD for more than 1 month were included. Patients having any episodes of peritonitis within last 1 month, patients with cognitive impairment and critically ill patients were excluded from the study. Demographic profiles and clinical history along with previous medical data of each study patient were recorded. All relevant investigations like- serum creatinine, blood urea, high sensitive C-reactive protein (hsCRP), fasting blood sugar (FBS), serum triglyceride (TG), serum cholesterol, serum high density lipoprotein cholesterol (S. HDL- C), serum albumin level, serum pre-albumin, serum calcium (S. Ca), serum phosphate (S. PO4), serum uric acid and total iron-binding capacity (TIBC) were measured. Their 24-hour urine and 24-hour effluent were collected for urea and creatinine levels to detect- adequacy of dialysis (Kt/v urea), residual renal function (RRF) and normalized protein catabolic rate (nPCR). Malnutrition inflammation score (MIS) was performed following standard procedure.
2.1 Study procedures
After selection of the patients, the purpose and procedure of the study was explained. Informed written consent was taken from each study patient for enrollment in this study. The study subjects were advised to arrive at the place of study in the morning of the scheduled date with 12 hours fasting state. They were also asked to bring 24 hours’ urine sample and 24 hours’ effluent sample from each bag of last 24 hours. For anthropometric measurements of the patient’s height and post-dialysis weight were taken. Then body mass index (BMI) was calculated using weight (in kilograms)/height (in meters)2 formula. Mid-arm circumference (MAC) was measured by using a flexible, non-stretchable, measuring tape. The biceps skin-fold (BSF) thickness and triceps skin-fold (TSF) thickness were measured by trained nutritionist using Harpenden skin-fold caliper on the right arm of study patients. An overnight fasting blood sample (6 ml venous blood) from each study patient was collected for biochemical analysis. All biochemical parameters were measured by routine methods at the Department of Laboratory Medicine, NIKDU, Dhaka, Bangladesh. The demographic information, clinical examination findings and laboratory investigations reports were recorded in a predesigned data collection sheet.
2.2 Malnutrition–Inflammation Score (MIS) [18]
The Malnutrition–Inflammation Score (MIS) is a scoring system used to evaluate malnutrition and inflammation of a patient with chronic disease. The MIS was created using the seven components of the conventional subjective global assessment (SGA) and combining them with three new elements: body mass index (BMI), serum albumin, and total iron-binding capacity (TIBC). The MIS is made up of ten components that are derived from a patient's medical history, physical examination, BMI, and laboratory parameters. Each component of the score is graded on a scale of 0 (normal) to 3 (severely abnormal). The total of the MIS's ten components ranges from 0 (normal) to 30 (severe malnutrition and inflammation).
2.3 MIS scoring
The total score was calculated. The cut-off score derived from the highest sensitivity – (1-specificity) point and that was 5. This cut-off score was used to classify patients with nutritional risk where patients with score <5 were classified as normal nutrition status whilst patients with score ≥5 were classified as malnourished [19].
2.4 Statistical analysis
Statistical analysis of the results was done by using computer-based statistical software (SPSS) version- 22. Quantitative data were presented as mean with standard deviation (SD) and qualitative data were presented by frequencies with percentages. The Pearson correlation test was performed to detect the correlation and unpaired ‘t’ test was done to analyze quantitative variables. A p value less than 0.05 was considered statistically significant.
3. Results and Observations
This cross-sectional study was conducted to evaluate the nutritional status and inflammatory condition among CAPD patients. A total of 69 patients were included in this study according to selection criteria. Out of 69 study patients; 39(56.5%) were male and rest 30(43.5%) were female (Figure- 1). Of them, 49.3% had diabetes mellitus (DM), 30.4% had glomerulonephritis (GN), 15.2% had hypertension (HTN), 2.9% had obstructive uropathy (OU) and 1.4% had polycystic kidney disease (PKD) (Figure- 2). Most of the study patients (78.3%) were found malnourished (Figure- 3).
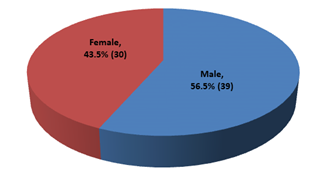
Figure- 1: Distribution of the study patients according to gender (N= 69)
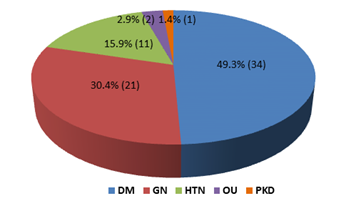
Figure- 2: Distribution of the study patients according to primary disease (N= 69)
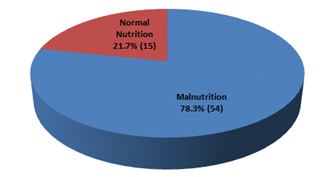
Figure- 3: Nutritional status of the study patients (N= 69)
It was observed that, mean age of the study patients was 58.3 ± 13.7 years, mean body mass index (BMI) was 26.1±4.0 kg/m2, mean systolic blood pressure (SBP) was 140 ± 21 mm of Hg and mean diastolic blood pressure (DBP) was 83 ± 11 mmHg. Mean mid arm circumference (MAC) was 25.94 ± 3.99 cm, mean waist size was 91.99 ± 8.92 cm, mean hip size was 99.06 ± 8.04 cm, mean waist-hip ratio (WH ratio) was 0.93 ± 0.08, mean biceps skin-fold thickness was 3.83 ± 2.62 cm and mean triceps skin-fold thickness was 9.74 ± 5.14 cm. Mean duration of dialysis was 9.75 ± 14.7 months (Table- 1).
|
Variables |
Mean ± SD |
Minimum – maximum |
|
Age (year) |
58.3 ± 13.7 |
19 – 89 |
|
BMI (kg/m2) |
26.1 ± 4.0 |
14.4 – 35.0 |
|
Systolic BP (mmHg) |
140 ± 21 |
100 – 180 |
|
Diastolic BP (mmHg) |
83 ± 11 |
40 – 110 |
|
MAC (cm) |
25.94 ± 3.99 |
11.00 - 32.00 |
|
Waist (cm) |
91.99 ± 8.92 |
60.96 - 106.00 |
|
Hip (cm) |
99.06 ± 8.04 |
71.12 - 121.92 |
|
WH ratio |
0.93 ± 0.08 |
0.71 - 1.05 |
|
Bicep (cm) |
3.83 ± 2.62 |
1.10 - 12.70 |
|
Tricep (cm) |
9.74 ± 5.14 |
1.50 - 25.20 |
|
Duration of dialysis (months) |
9.75 ± 14.7 |
0.5 – 88 |
Table- 1: Basic data of the study patients (N= 69)
Regarding biochemical parameters of the study patients, mean haemoglobin (Hb) level was 9.48 ± 1.77 g/dl. Mean serum creatinine and blood urea level was 9.32 ± 3.94 mg/dl and 86.8 ± 35.7 mg/dl. Mean high sensitive C-reactive protein (hsCRP) was 23.73 ± 26.67 mg/dl. Mean fasting blood sugar (FBS), serum triglyceride (TG), serum cholesterol and serum high-density lipoprotein cholesterol (S. HDL- C) levels were- 8.49 ± 4.53 mmol/L, 286.73 ± 247.04 mg/dl, 179.48 ± 112.78 mg/dl and 35.81 ± 7.53 mg/dl respectively. The mean serum albumin level was 3.11 ± 0.60 mg/dl and mean serum pre-albumin level was 0.30 ± 0.11 mg/dl. Mean serum calcium (S. Ca), serum phosphate (S. PO4) and serum uric acid levels were- 8.78 ± 1.80 mg/dl, 4.64 ± 1.90 mg/dl and 6.27 ± 1.33 mg/dl respectively. Mean total iron-binding capacity (TIBC) of the study patients was- 165.8 ± 59.5 mcg/dl (Table- 2).
|
Biochemical parameters |
Mean ± SD |
Minimum – maximum |
|
Hb (g/dl) |
9.48 ± 1.77 |
6.00 - 14.30 |
|
S Creatinine (mg/dl) |
9.32 ± 3.94 |
4.03 - 23.17 |
|
Blood urea (mg/dl) |
86.8 ± 35.7 |
31.2 – 240 |
|
hsCRP (mg/dl) |
23.73 ± 26.67 |
0.79 - 142.00 |
|
FBS (mmol/L) |
8.49 ± 4.53 |
3.80 - 22.50 |
|
TG (mg/dl) |
286.73 ± 247.04 |
38.40 - 1027.10 |
|
Cholesterol (mg/dl) |
179.48 ± 112.78 |
51.30 - 723.40 |
|
S. HDL- C (mg/dl) |
35.81 ± 7.53 |
17.30 - 57.50 |
|
Serum albumin (mg/dl) |
3.11 ± 0.60 |
2.00 - 4.50 |
|
Serum pre-albumin (mg/dl) |
0.30 ± 0.11 |
0.04 – 0.54 |
|
S. Ca (mg/dl) |
8.78 ± 1.80 |
4.27 - 13.46 |
|
S. PO4 (mg/dl) |
4.64 ± 1.90 |
1.85 - 11.63 |
|
Serum uric acid (mg/dl) |
6.27 ± 1.33 |
2.98 - 9.00 |
|
TIBC (mcg/dl) |
165.8 ± 59.5 |
24 – 298 |
Table- 2: Biochemical parameters of the study patients (N= 69)
Data analysis revealed that mean malnutrition inflammation score (MIS) of 69 study patients was 11.2 ± 4.7, mean normalized protein catabolic rate (nPCR) of the study patients was 1.93 ± 1.12 and mean Kt/V urea of the study patients was 1.36 ± 0.59 (Table- 3).
|
Variables |
Mean ± SD |
Minimum – maximum |
|
MIS |
11.2 ± 4.7 |
4 – 30 |
|
nPCR |
1.93 ± 1.12 |
0.30 – 6.69 |
|
Kt/V urea |
1.36 ± 0.59 |
0.64 – 4.04 |
Table- 3: Malnutrition inflammation score (MIS), normalized protein catabolic rate (nPCR) and Kt/V urea of the study patients (N= 69)
We compared demographic, anthropometric and biochemical parameters among well-nourished and malnourished CAPD patients. That revealed serum albumin and serum pre-albumin was found significantly lower in patients with higher MIS-score (>5) (Table- 4).
|
Variables |
MIS score <5 |
MIS score ≥5 |
p-value |
|
(n=15) |
(n=54) |
||
|
Mean ± SD |
Mean ± SD |
||
|
Age (year) |
52.73 ± 14.48 |
59.85 ± 13.28 |
0.076 |
|
BMI (kg/m2) |
27.79 ± 4.21 |
25.65 ± 3.87 |
0.067 |
|
Systolic BP (mmHg) |
134.67 ± 15.06 |
141.51 ± 22.22 |
0.267 |
|
Diastolic BP (mmHg) |
76.67 ± 13.97 |
84.81 ± 9.90 |
0.013 |
|
MAC (cm) |
27.43 ± 2.40 |
25.53 ± 4.25 |
0.102 |
|
Waist (cm) |
92.65 ± 10.61 |
91.81 ± 8.50 |
0.75 |
|
Hip (cm) |
101.46 ± 7.15 |
98.40 ± 8.21 |
0.195 |
|
WH ratio |
0.91 ± 0.10 |
0.93 ± 0.07 |
0.376 |
|
Bicep (cm) |
3.92 ± 1.57 |
3.80 ± 2.86 |
0.877 |
|
Tricep (cm) |
10.50 ± 3.26 |
9.53 ± 5.56 |
0.523 |
|
Duration of dialysis (Months) |
10.73 ± 10.37 |
9.46 ± 15.82 |
0.771 |
|
Serum albumin (mg/dl) |
3.74 ± 0.41 |
2.94 ± 0.52 |
<0.001s |
|
Serum pre-albumin (mg/dl) |
0.39 ± 0.10 |
0.28 ± 0.10 |
<0.001s |
|
CRP |
14.36 ± 11.66 |
26.33 ± 29.07 |
0.125 |
|
nPCR |
2.14 ± 1.19 |
1.88 ± 1.12 |
0.43 |
|
Total KT/V urea |
1.48 ± 0.67 |
1.34 ± 0.57 |
0.432 |
Unpaired t test was done, s= significant
Table- 4: Comparison of demographic, anthropometric and biochemical parameters among well-nourished and malnourished CAPD patients
Pearson’s correlation analysis revealed that; body mass index (BMI), serum albumin, serum pre-albumin, serum total iron binding capacity (TIBC) had showed significant negative correlation (p<0.001) with malnutrition inflammation score (MIS); but high sensitive C- Reactive protein (hsCRP) had showed significant positive correlation to malnutrition inflammation score (MIS) (p<0.001) (Table- 5).
|
Variables |
r value |
p value |
|
Body mass index (BMI) |
-0.487 |
<0.001s |
|
Serum albumin |
-0.447 |
<0.001s |
|
Serum pre-albumin |
-0.591 |
<0.001S |
|
Total iron binding capacity (TIBC) |
-0.6 |
<0.001S |
|
High sensitive C- Reactive protein (hsCRP) |
0.322 |
<0.001S |
|
Normalized protein catabolic rate (nPCR) |
-0.067 |
0.733 |
|
Serum creatinine |
-0.221 |
0.07 |
|
Haemoglobin (Hb) |
0.198 |
0.122 |
Pearson’s correlation test was performed, s= significant
Table-5: Correlation of malnutrition inflammation score (MIS) with BMI, serum albumin, serum pre-albumin, TIBC, hsCRP, nPCR, serum creatinine and haemoglobin (Hb) levels (N= 69)
In this study we observed that normalized protein catabolic rate (nPCR) had a significant positive correlation with total Kt/v urea (r= 0.356 and p= 0.003) (Figure- 4).
Here, r = 0.356 and p = 0.003
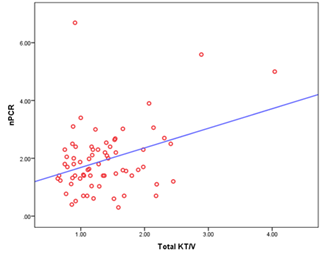
Figure- 4: Scatter diagram displaying the correlation of total KT/v urea with nPCR among the study patients (N=69)
But there was no significant correlation observed between KT/v urea with malnutrition inflammation score (MIS) in this study (r= 0.056 and p= 0.648) (Figure- 5).
Here, r= 0.056 and p= 0.648
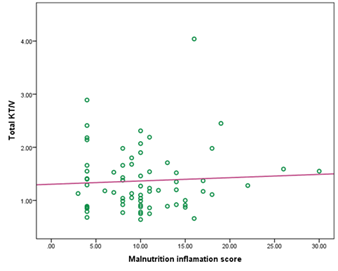
Figure- 5: Correlation of total KT/v urea with malnutrition inflammation score (MIS) among the study patients (N=69)
5. Discussion
Inflammation and inflammatory factors play an important role in malnutrition among patients with chronic kidney disease (CKD). The malnutrition inflammation score (MIS), a nutritional scoring system, has been verified in chronic kidney disease (CKD), particularly in dialysis patients [17-19]. We examined the nutritional status and inflammatory markers of sixty-nine (69) patients on continuous ambulatory peritoneal dialysis (CAPD) and observed the correlation of MIS with several nutritional variables and inflammatory markers like hsCRP, TIBC, pre-albumin, albumin and BMI. In this study, among 69 CAPD patients, 56.5% was male and 43.5% was female which indicates a male predominance. In accordance, Singh et at. also found male predominance in a related study [20].
In this study analyzing the primary causes of CKD revealed that; 49.3% study patients had diabetes mellitus (DM), 30.4% had glomerulonephritis (GN), 15.2% had hypertension (HTN), 2.9% had obstructive uropathy (OU) and 1.4% had polycystic kidney disease (PKD). In this series, one previous study showed primary causes of CKD were unknown in 41% patients, followed by DM 33%, HTN 11% and 14% was other causes [20]. So, there were more diabetic patients who developed CKD and ESRD underwent CAPD, even percentage of GN is also higher as primary disease in our study.
In this present study, we explored the possible cut-off score for defining malnutrition with the malnutrition inflammation score (MIS). MIS score of <5 obtained for all patients indicated normal nutritional status whilst scores of ≥5 indicated presence of malnutrition [19]. According to this cut-off value we found 78.3% of our study patients were malnourished. This finding was consistent with related previous studies [20, 21]. It was reported that prevalence of malnutrition among CAPD population was much higher (74%-90%) [17, 20, 21]. The plausible reason could be patients on chronic dialysis may experience malnutrition due to inadequate dietary intake, significant losses, elevated protein catabolism, and acidosis.
In this study the mean age of the patients was 58.3 ± 13.7 years, that was comparable with couple of related previous studies [20, 21, 22]. The mean body mass index (BMI) of the study patients was 26.1±4.0 kg/m2. This finding was supported by a similar previous study [22]. However, another stud y showed the mean BMI of the study patients was 21.8 ± 5.2, which was much lower than our study [21].
In this study the mean value of malnutrition inflammation score (MIS) was 11.2 ± 4.7. In this context, Afsar et al. reported that mean MIS of 50 CAPD patients was 8.1 ± 5.0 [22]. In comparison to that study our CAPD patients were higher MIS scorer. Normalized protein catabolic rate (nPCR) is a rate which correlates with normalized dietary protein intake. The mean normalized protein catabolic rate (nPCR) of the study patients was 1.93 ± 1.12, which was comparable with similar previous studies [21, 22]. The mean Kt/V urea of our study patients was 1.36 ± 0.59. In accordance Prasad et al. showed mean weekly Kt/V urea was 1.8 ± 0.5 in their study, which was consistent with our study [21].
In this current study, BMI showed significant negative correlation with MIS which was consistent with a related previous study [20]. High sensitive C-reactive protein (hsCRP), which is an inflammatory marker, we found mean hsCRP was 23.73 ± 26.67 mg/dl in this study and that had showed a significant positive correlation with MIS. In this context, Afsar et al. also found a similar result [22]; but Singh et al. found a negative correlation of CRP with MIS [20]. Albumin, which is a negative acute-phase protein, is an important marker of nutrition in the dialysis population. We found mean serum albumin level was 3.11 ± 0.60 mg/dl in our study patients, which had showed a negative correlation with MIS. This finding was in a line of similar previous studies [20-22]. Serum pre-albumin, like albumin, is also a negative acute-phase protein which reflects the presence of ongoing or resolving inflammatory condition that may influence the patient’s nutritional state. In this current study, the mean serum pre-albumin level was 0.30 ± 0.11 ng/ml, and we found a negative correlation between MIS and serum pre-albumin. In accordance Afsar et al. also showed similar results [22]. In this study, mean total iron-binding capacity (TIBC) level was 165.8 ± 59.5 mg/dl and that had showed a significant negative correlation with MIS. This finding was supported by a couple of previous study [20, 22].
It was reported that nPCR was significantly correlated with dialysis adequacy including Kt/V [23, 24]. Our study also showed a significant positive correlation between nPCR and Kt/V urea (r= 0.356, p= 0.003). But in this study, there was no significant correlation observed between MIS and Kt/V urea (r = 0.056 and p = 0.648), which means that to detect dialysis adequacy only solute clearance or Kt/V is not sufficient. MIS should be performed among patients on CAPD. Nutritional status and inflammatory markers are important indicators to assess adequacy and to predict the outcome of CAPD.
In this study different clinical and laboratory parameters were compared between well-nourished and malnourished patients like age, BMI, MAC, waist-hip ratio, biceps skin fold thickness, triceps skin fold thickness, duration of dialysis, serum albumin, pre-albumin, hsCRP, nPCR, Kt/V urea, out of which albumin and pre-albumin showed significant difference. Malnutrition inflammation score was higher among CAPD patients in our country. There was also a significant negative correlation of albumin, pre-albumin, BMI and TIBC with MIS. But hsCRP had a significant positive correlation with MIS. So, MIS can be used as a useful tool to detect malnutrition and inflammatory conditions among patients on CAPD.
5. Conclusion
This study demonstrated that malnutrition inflammation score (MIS) is significantly higher in patients on CAPD. Serum albumin, serum pre-albumin, BMI and TIBC has significant negative correlation with malnutrition inflammation score, whereas hsCRP have significant positive correlation with malnutrition inflammation score. Comparison to clinical and laboratory parameters with different variables in CAPD patients revealed that the malnutrition inflammation score (MIS) is related to inflammation in continuous ambulatory peritoneal dialysis (CAPD) patients. As MIS is a reliable tool to predict malnutrition and inflammation status in chronic dialysis patients, therefore MIS could be used for prevention of fatal outcomes among CAPD patients.
Limitations of the study
There were some limitations in this study. The sample size was relatively small, which may have a lower statistical power. The present study was conducted over a short period of time.
Recommendation
Further studies with larger sample size could be done over longer duration.
Conflict of interest
The authors declared no conflicts of interest related to the research, authorship or publication of this article.
References
- Jain AK, Blake P, Cordy P, et al. Global trends in rates of peritoneal dialysis. Journal of the American Society of Nephrology23(3) (2012):533-44.
- Krautzig, S., & Brunkhorst, R. Nephrology Dialysis Transplantation. Oxford University Press. September 11 9(1996): 1911–1912.
- Katirtzoglou A, Oreopoulos DG, Husdan H, et al. Reappraisal of protein losses in patients undergoing continuous ambulatory peritoneal dialysis. Nephron26(5) (1980):230-3.
- Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. American journal of kidney diseases37(1) (2001):S66-70.
- Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney international73(4) (2008):391-8.
- Heimburger O. Obesity on PD patients: causes and management. Contributions to Nephrology 140(2003):91-7.
- Avesani CM, Heimburger O, Stenvinkel P, et al. Nutritional aspects of adult patients treated with chronic peritoneal dialysis. J Bras Nefrol28(4) (2006):232-8.
- Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis35(2) (2000):S1-140.
- Lapidus L, Bengtsson C, Larsson BO, et al. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed)289(6454) (1984):1257-61.
- Heimbürger O, Qureshi AR, Blaner WS, et al. Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. American journal of kidney diseases36(6) (2000):1213-25.
- Pupim LB, Ikizler TA. Assessment and monitoring of uremic malnutrition. Journal of Renal Nutrition14(1) (2004):6-19.
- Cho Y, Hawley CM, Johnson DW. Clinical causes of inflammation in peritoneal dialysis patients. International journal of nephrology2014(2014).
- Hurst SM, Wilkinson TS, McLoughlin RM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity14(6) (2001):705-14.
- Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. American journal of kidney diseases38(6) (2001):1343-50.
- Stenvinkel P, Ketteler M, Johnson RJ, et al. central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney international67(4) (2005):1216-33.
- Hurst SM, Wilkinson TS, McLoughlin RM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity14(6) (2001):705-14.
- Naeeni AE, Poostiyan N, Teimouri Z, et al. Assessment of Severity of Malnutrition in Peritoneal Dialysis Patients via Malnutrition Inflammatory Score. Advanced Biomedical Research6(1) (2017):128.
- Kalantar-Zadeh K, Kopple JD, Block G, et al. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38 (2001):1251-63.
- Harvinder GS, Swee WC, Karupaiah T, et al. Dialysis malnutrition and malnutrition inflammation scores: Screening tools for prediction of dialysis-related protein-energy wasting in Malaysia. Asia Pacific journal of clinical nutrition25(1) (2016):26-33.
- Singh P, Shankar H, Singh TB, Singh S. NUTRITIONAL PARAMETERS IN CHRONIC KIDNEY DISEASE PATIENTS. Indian Journal of Preventive & Social Medicine47(1-2) (2016):7-17.
- Prasad N, Sinha A, Gupta A, et al. Validity of nutrition risk index as a malnutrition screening tool compared with subjective global assessment in end-stage renal disease patients on peritoneal dialysis. Indian Journal of Nephrology26(1) (2016):27.
- Afsar B, Sezer S, Ozdemir FN, et al. Malnutrition–inflammation score is a useful tool in peritoneal dialysis patients. Peritoneal Dialysis International26(6) (2006):705-11.
- Harty J, Faragher B, Venning M, et al. Urea kinetic modeling exaggerates the relationship between nutrition and dialysis in CAPD patients.(The hazards of cross-sectional analysis). Peritoneal dialysis international15(2) (1995):105-9.
- Combe C, Chauveau P, French Study Group Nutrition in Dialysis, et al. Influence of nutritional factors and hemodialysis adequacy on the survival of 1,610 French patients. American Journal of Kidney Diseases37(1) (2001):S81-8.
