Application of Plant-Base Fungicides to Control Aflatoxigenic Fungi Producing Mycotoxins in Stored Cowpea Seeds
Article Information
Oredoyin A Ogungbemile, Peter M Etaware*, Adegboyega C Odebode
1Department of Botany, Faculty of Science, University of Ibadan, Ibadan, Nigeria
*Corresponding author: Peter M Etaware, Department of Botany, Faculty of Science, University of Ibadan, Ibadan, Nigeria
Received: 26 December 2019; Accepted: 31 December 2019; Published: 03 January 2020
Citation:
Oredoyin A Ogungbemile, Peter M Etaware, Adegboyega C Odebode. Application of Plant-Base Fungicides to Control Aflatoxigenic Fungi Producing Mycotoxins in Stored Cowpea Seeds. Journal of Biotechnology and Biomedicine 3 (2020): 001-009.
View / Download Pdf Share at FacebookAbstract
The inhibition of aflatoxin production in stored grains and pulses was achieved by the use of chemicals. However, these chemicals constitute serious health hazards to humans and animals. It is neither environmentally friendly nor safe for the sustenance of the ecosystem. Therefore, the current study was designed to investigate the use of plant extracts in the eradication of seed- and air-borne aflatoxigenic species of fungi in line with the recommendations of World Health Organization [WHO]. Aflatoxin detoxification and mortality of aflatoxigenic fungal strains by plant-based fungicides were examined using rigorous laboratory procedures. The strains of Aspergillus flavus, A. Parasiticus and A. Fumigatus were totally killed by 1000mg/mL cons. of the phyto-fungicides (100% death recorded). Also, 100% aflatoxin detoxification was recorded by the application of 750 and 100mg/mL concentration of the plant-based fungicides used for this experiment. The use of plant extracts for control of fungal pests is safe and less expensive than synthetic pesticides. More so, they are environmentally friendly and constitute no health risk to humans and animals. This will help reduce pollution from chemical sources and also contribute to a sustainable development of the ecosystem.
Keywords
Aflatoxin production, Phyto-fungicides, Detoxification and mortality of aflatoxigenic fungal strains, Synthetic pesticides, Sustenance of the ecosystem
Aflatoxin production articles Aflatoxin production Research articles Aflatoxin production review articles Aflatoxin production PubMed articles Aflatoxin production PubMed Central articles Aflatoxin production 2023 articles Aflatoxin production 2024 articles Aflatoxin production Scopus articles Aflatoxin production impact factor journals Aflatoxin production Scopus journals Aflatoxin production PubMed journals Aflatoxin production medical journals Aflatoxin production free journals Aflatoxin production best journals Aflatoxin production top journals Aflatoxin production free medical journals Aflatoxin production famous journals Aflatoxin production Google Scholar indexed journals Phyto-fungicides articles Phyto-fungicides Research articles Phyto-fungicides review articles Phyto-fungicides PubMed articles Phyto-fungicides PubMed Central articles Phyto-fungicides 2023 articles Phyto-fungicides 2024 articles Phyto-fungicides Scopus articles Phyto-fungicides impact factor journals Phyto-fungicides Scopus journals Phyto-fungicides PubMed journals Phyto-fungicides medical journals Phyto-fungicides free journals Phyto-fungicides best journals Phyto-fungicides top journals Phyto-fungicides free medical journals Phyto-fungicides famous journals Phyto-fungicides Google Scholar indexed journals Detoxification and mortality of aflatoxigenic fungal strains articles Detoxification and mortality of aflatoxigenic fungal strains Research articles Detoxification and mortality of aflatoxigenic fungal strains review articles Detoxification and mortality of aflatoxigenic fungal strains PubMed articles Detoxification and mortality of aflatoxigenic fungal strains PubMed Central articles Detoxification and mortality of aflatoxigenic fungal strains 2023 articles Detoxification and mortality of aflatoxigenic fungal strains 2024 articles Detoxification and mortality of aflatoxigenic fungal strains Scopus articles Detoxification and mortality of aflatoxigenic fungal strains impact factor journals Detoxification and mortality of aflatoxigenic fungal strains Scopus journals Detoxification and mortality of aflatoxigenic fungal strains PubMed journals Detoxification and mortality of aflatoxigenic fungal strains medical journals Detoxification and mortality of aflatoxigenic fungal strains free journals Detoxification and mortality of aflatoxigenic fungal strains best journals Detoxification and mortality of aflatoxigenic fungal strains top journals Detoxification and mortality of aflatoxigenic fungal strains free medical journals Detoxification and mortality of aflatoxigenic fungal strains famous journals Detoxification and mortality of aflatoxigenic fungal strains Google Scholar indexed journals Synthetic pesticides articles Synthetic pesticides Research articles Synthetic pesticides review articles Synthetic pesticides PubMed articles Synthetic pesticides PubMed Central articles Synthetic pesticides 2023 articles Synthetic pesticides 2024 articles Synthetic pesticides Scopus articles Synthetic pesticides impact factor journals Synthetic pesticides Scopus journals Synthetic pesticides PubMed journals Synthetic pesticides medical journals Synthetic pesticides free journals Synthetic pesticides best journals Synthetic pesticides top journals Synthetic pesticides free medical journals Synthetic pesticides famous journals Synthetic pesticides Google Scholar indexed journals Sustenance of the ecosystem articles Sustenance of the ecosystem Research articles Sustenance of the ecosystem review articles Sustenance of the ecosystem PubMed articles Sustenance of the ecosystem PubMed Central articles Sustenance of the ecosystem 2023 articles Sustenance of the ecosystem 2024 articles Sustenance of the ecosystem Scopus articles Sustenance of the ecosystem impact factor journals Sustenance of the ecosystem Scopus journals Sustenance of the ecosystem PubMed journals Sustenance of the ecosystem medical journals Sustenance of the ecosystem free journals Sustenance of the ecosystem best journals Sustenance of the ecosystem top journals Sustenance of the ecosystem free medical journals Sustenance of the ecosystem famous journals Sustenance of the ecosystem Google Scholar indexed journals Biologically articles Biologically Research articles Biologically review articles Biologically PubMed articles Biologically PubMed Central articles Biologically 2023 articles Biologically 2024 articles Biologically Scopus articles Biologically impact factor journals Biologically Scopus journals Biologically PubMed journals Biologically medical journals Biologically free journals Biologically best journals Biologically top journals Biologically free medical journals Biologically famous journals Biologically Google Scholar indexed journals Azadirachta indica articles Azadirachta indica Research articles Azadirachta indica review articles Azadirachta indica PubMed articles Azadirachta indica PubMed Central articles Azadirachta indica 2023 articles Azadirachta indica 2024 articles Azadirachta indica Scopus articles Azadirachta indica impact factor journals Azadirachta indica Scopus journals Azadirachta indica PubMed journals Azadirachta indica medical journals Azadirachta indica free journals Azadirachta indica best journals Azadirachta indica top journals Azadirachta indica free medical journals Azadirachta indica famous journals Azadirachta indica Google Scholar indexed journals Fungal articles Fungal Research articles Fungal review articles Fungal PubMed articles Fungal PubMed Central articles Fungal 2023 articles Fungal 2024 articles Fungal Scopus articles Fungal impact factor journals Fungal Scopus journals Fungal PubMed journals Fungal medical journals Fungal free journals Fungal best journals Fungal top journals Fungal free medical journals Fungal famous journals Fungal Google Scholar indexed journals Petiveria alliacea articles Petiveria alliacea Research articles Petiveria alliacea review articles Petiveria alliacea PubMed articles Petiveria alliacea PubMed Central articles Petiveria alliacea 2023 articles Petiveria alliacea 2024 articles Petiveria alliacea Scopus articles Petiveria alliacea impact factor journals Petiveria alliacea Scopus journals Petiveria alliacea PubMed journals Petiveria alliacea medical journals Petiveria alliacea free journals Petiveria alliacea best journals Petiveria alliacea top journals Petiveria alliacea free medical journals Petiveria alliacea famous journals Petiveria alliacea Google Scholar indexed journals aflatoxigenic fungal articles aflatoxigenic fungal Research articles aflatoxigenic fungal review articles aflatoxigenic fungal PubMed articles aflatoxigenic fungal PubMed Central articles aflatoxigenic fungal 2023 articles aflatoxigenic fungal 2024 articles aflatoxigenic fungal Scopus articles aflatoxigenic fungal impact factor journals aflatoxigenic fungal Scopus journals aflatoxigenic fungal PubMed journals aflatoxigenic fungal medical journals aflatoxigenic fungal free journals aflatoxigenic fungal best journals aflatoxigenic fungal top journals aflatoxigenic fungal free medical journals aflatoxigenic fungal famous journals aflatoxigenic fungal Google Scholar indexed journals
Article Details
1. Introduction
Aflatoxin is a regular contaminant of stored cowpea seeds in Nigeria and around the globe, constituting health hazard to humans and animals, serious danger to some beneficial microbes within the environment and other endangered species (plants and animals inclusive). The inhibition of aflatoxin production in stored grain and pulses was achieved over time from antiquity till modern age by the use of chemicals, basically preservatives and pesticides [1]. Pesticides (especially fungicides and insecticides) inhibit the transmission of spores of mycotoxigenic fungi, reduce fungal growth and minimize insect infestation of crops [2]. Chemical control of plant disease has been of immense benefit in the production of viable seeds for consumption and cultivation, however, the use of chemicals constitute serious danger to human and animal health, because they are carcinogenic [3], and neither environmentally friendly nor safe [4]. Biologically active compounds have been proven to be abundant in medicinal plants [5]. Some essential compounds from plants possess antimicrobial, fungicidal and insecticidal activities [3]. The use of some phytochemicals as food preservatives (unlike synthetic fungicides) leave no toxic residue on treated produce, require no pre-harvest interval during application or dosage limitation and contain many bioactive metabolites which makes pathogens’ development of resistance to them less likely [6]. Several workers have affirmed their efficacy for managing field and storage disease of produce [7, 8, 4]. Fandohan et al. [9] reported significant inhibitory effects of essential oil from seeds of the neem tree (Azadirachta indica) on the growth of Fusarium verticillioides and fumonisin contamination in maize. Also, Suleiman et al. (2008) reported the effect of aqueous leaf extracts on Fusarium species isolated from cowpea. The leaves of Ageratum conyzoides was used as preservatives in cowpea and maize storage bins to repel insects and disallow storage fungi in traditional homesteads of Benin Republic [10]. Adegoke and Odesola [11] investigated the effects of essential oil of lemon grass in the control of seed mycoflora of cowpea and maize in storage. Many methods have been adopted to control aflatoxin in cowpea and other crops, these methods can be grouped into two which are; preventive approach and curative approach. Preventive approach aims at preventing the growth of aflatoxigenic fungi before harvest (pre-harvest) and after harvest (post-harvest). On the other hand curative approach involves the de-activation of already existing aflatoxin in cowpea and other crops. Generally, the best control of fungal toxins is usually the eradication of the fungi that produce them. Therefore, the current study was designed to investigate the use of plant extracts in the control of seed- and air-borne aflatoxigenic species of fungi below the disease threshold level in line with the recommendations of World Health Organization [WHO]. The outcome of this research will be pertinent to cowpea farmers and merchants as it will help to protect/sustain life and ensure the circulation of disease free and non- toxic cowpea seeds for consumption worldwide and to better ameliorate the problem of environmental pollution.
2. Methodology
2.1 Fungal pathogens
The fungal pathogens of cowpea seeds used for this experiment were obtained from the Department of Botany, Faculty of Science, University of Ibadan, Ibadan, Oyo State, Nigeria.
2.2 Preparation of plant-based fungicides
The procedure for preparation of plant extract as described by Okigbo and Ogbonnaya [12] was adopted with little modifications. Freshly collected leaves of Bryophyllum pinnatum and Petiveria alliacea was weighed, decontaminated and pretreated using 70% ethanol (to remove germs), rinsed in deionized water (to remove alcohol residue), pulverized and trickled using standard laboratory equipment.
The concentrations used were 500 mg/mL, 750 mg/mL and 1000 mg/mL. All extract was stored in the refrigerator at 40C in air tight bottles.
2.3 Aflatoxin detoxification and fungi mortality experiment
The plant-based fungicides (1mL) and Potato Dextrose Broth (9mL) were homogenized in sterile McCartney bottles in a laminar airflow chamber. Radial mycelia plug of 5mm diameter (7day old culture of each pathogen) was aseptically inoculated into each bottles in replicates of three (3). This was done for each of the extracts and at different concentrations as well as the control setup for the experiment (The control was setup in McCartney bottles containing PDB and the individual pathogens only). The inoculants were characterized according to the treatment applied, treatment concentration, and the pathogen’s identity. They were labeled appropriately and incubated at 25 ± 2oC for 7days. At the end of the experiment, the fungi mycelia were harvested and weighed to determine the fresh weight (M1), oven dried for 3 hours at 80oC and weighed again to determine the dry weight (M2). The mycelia weight for the control experiment was recorded too (Mo). The formula below was used to determine the percentage weight loss of the pathogen:
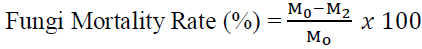
Where,
Mo = The dry mycelia weight of the control experiment (Untreated pathogens)
M2 = The dry mycelia weight of the treated pathogen(s)
2.4 Experimental layout for bio-treatment
A completely randomized design was used for the experimental set up for bio-treatment.
2.5 Data analysis
Data collected was organized and analyzed using Costat 6.451 statistical software and the homogeneity of means was determined using Duncan Multiple Range Test (DMRT). Data was represented as means and standard deviation.
3. Results
3.1 Fungal pathogens of stored cowpea seeds used for the experiment
- Aspergillus flavus
- Aspergillus parasiticus
- Aspergillus fumigatus
Note: All the pathogens used for this experiment were confirmed to be highly aflatoxigenic.
3.2 Fungi mortality rate (FMR) experiment
It was observed that 1000mg/mL of each treatment applied had 100% mortality effect on all the aflatoxigenic pathogens of cowpea seeds investigated in this research i.e. The strains of Aspergillus flavus were totally killed by 1000mg/mL concentration of Petiveria alliacea, while similar effects was recorded for A. parasiticus and A. fumigatus with 100% destruction recorded by applying 1000mg/mL concentration of Bryophyllum pinnatum (Table 1). Also, 750mg/mL of Plant-based fungicide obtained from Petiveria alliacea totally killed the strains of Aspergillus flavus only (100% mycelia mortality). Other concentrations of the treatment had appreciable phyto-fungitoxic effects on the aflatoxigenic pathogens too (Table 1).
3.3 Aflatoxin detoxification
100% aflatoxin detoxification was recorded by the application of 750 and 100mg/mL concentration of the plant-based fungicides used for this experiment (Table 2). Application of 500mg/mL concentration of each treatment was unable to detoxify or totally inhibit aflatoxin production (Table 2).
|
Treatment |
Location |
Aflatoxigenic Pathogen |
Fungi Mortality Rate (%) |
||
|
500mg/mL |
750mg/mL |
1000mg/mL |
|||
|
Petiveria alliacea |
Sasa |
A. flavus |
96 |
98 |
100 |
|
Bodija |
A. flavus |
17 |
100 |
83 |
|
|
Oja-Oba |
A. flavus |
33 |
67 |
83 |
|
|
Sasa |
A. parasiticus |
22 |
78 |
89 |
|
|
Bodija |
A. parasiticus |
25 |
75 |
75 |
|
|
Oja-Oba |
A. parasiticus |
14 |
71 |
71 |
|
|
Sasa |
A. fumigatus |
-17 |
67 |
83 |
|
|
Bodija |
A. fumigatus |
0 |
86 |
86 |
|
|
Bryophyllum pinnatum |
Sasa |
A. flavus |
96 |
96 |
96 |
|
Bodija |
A. flavus |
17 |
17 |
83 |
|
|
Oja-Oba |
A. flavus |
33 |
17 |
67 |
|
|
Sasa |
A. parasiticus |
33 |
44 |
89 |
|
|
Bodija |
A. parasiticus |
25 |
38 |
88 |
|
|
Oja-Oba |
A. parasiticus |
43 |
57 |
100 |
|
|
Sasa |
A. fumigatus |
33 |
17 |
100 |
|
|
Bodija |
A. fumigatus |
14 |
29 |
14 |
|
Table 1: The mortality rate of the treated fungal pathogens of store cowpea seeds.
|
Treatment |
Location |
Aflatoxigenic Pathogen |
Aflatoxin Detection |
||
|
500mg/mL |
750mg/mL |
1000mg/mL |
|||
|
Petiveria alliacea |
Sasa |
A. flavus |
- |
- |
- |
|
Bodija |
A. flavus |
+ |
- |
- |
|
|
Oja-Oba |
A. flavus |
+ |
- |
- |
|
|
Sasa |
A. parasiticus |
+ |
- |
- |
|
|
Bodija |
A. parasiticus |
+ |
- |
- |
|
|
Oja-Oba |
A. parasiticus |
+ |
- |
- |
|
|
Sasa |
A. fumigatus |
+ |
- |
- |
|
|
Bodija |
A. fumigatus |
+ |
- |
- |
|
|
Bryophyllum pinnatum |
Sasa |
A. flavus |
- |
- |
- |
|
Bodija |
A. flavus |
+ |
+ |
- |
|
|
Oja-Oba |
A. flavus |
+ |
+ |
- |
|
|
Sasa |
A. parasiticus |
+ |
+ |
- |
|
|
Bodija |
A. parasiticus |
+ |
+ |
- |
|
|
Oja-Oba |
A. parasiticus |
+ |
- |
- |
|
|
Sasa |
A. fumigatus |
+ |
+ |
- |
|
|
Bodija |
A. fumigatus |
+ |
+ |
+ |
|
Table 2: Aflatoxin detection in stored cowpea seeds after treatment.
3.4 Fresh weight loss
It was observed that application of 1000mg/ml of Petiveria alliacea decreased the fresh mycelia weights of Aspergillus flavus isolated from cowpea samples collected from Sasa [0.01g], Bodija [0.02g] and Oja-Oba [0.05g] markets, respectively, A. parasiticus (Isolated from Oja-Oba [0.05g], and Sasa [0.05g] market samples, respectively), and A. fumigatus (Isolated from Bodija [0.09g], and Sasa [0.07g] market samples, respectively). Also, Bryophyllum pinnatum was able to effectively reduce the fresh mycelia weight of A. flavus (isolated from Sasa [0.13g], Bodija [0.04g] and Oja-Oba [0.14g] markets, respectively), A. parasiticus (from Oja-Oba [0.03g], Sasa [0.04g], and Bodija [0.04g] markets, respectively), and finally, A. fumigatus (obtained from Sasa [0.03] only) compared to the untreated pathogens used as control for this experiment i.e. A. flavus (Sasa [0.14g], Oja-Oba [0.22g] and Bodija [0.21g], respectively), A. parasiticus (Oja-Oba [0.28g], Sasa [0.32g], and Bodija [0.29g], respectively), and A. fumigatus (Bodija [0.31g] and Sasa [0.29g], respectively) as recorded in Table 3. There was no significant difference in the fresh weight composition of the aflatoxigenic pathogens with 500 and 700mg/mL phyto-fungicides and the control set up for the experiment (P≤0. 05) as shown in Table 3.
3.5 Dry weight loss
It was observed that at 1000mg/mL conc., the dry mycelia weights of all the aflatoxigenic pathogens were significantly affected by the antifungal properties of Bryophyllum pinnatum and Petiveria alliacea (Table 4). 750mg/mL formulation of the plant extracts of Petiveria alliacea inhibited the production of mycelia mass of Aspergillus flavus isolated from cowpea samples from Sasa [0.01g], Bodija [0.00g] and Oja-Oba [0.02g], respectively, A. parasiticus (from Sasa [0.02g], Bodija [0.02g] and Oja-Oba [0.02g] respectively), and A. fumigatus (from Sasa [0.02g], and Bodija [0.01g], respectively). The bio-treatment application of Bryophyllum pinnatum was only effective in the control of A. flavus isolated from Sasa market only (0.02g). 500mg/mL formulation of the plant extracts of Bryophyllum pinnatum and Petiveria alliacea effectively reduced the dry matter of Aspergillus flavus (0.02g each from Sasa market) only, compared to the control experimental set up i.e. A. flavus (Sasa [0.50g], Bodija [0.06g] and Oja-Oba [0.06g] respectively), A. parasiticus (Sasa [0.09g], Bodija [0.08g] and Oja-Oba [0.07g] respectively), and A. fumigatus (Sasa [0.06g], and Bodija [0.07g] respectively) as recorded in Table 4.
|
Treatment |
Location |
Aflatoxigenic Pathogens |
Fresh Mycelia Weight (g) |
||
|
500mg/mL |
750mg/mL |
1000mg/mL |
|||
|
Petiveria alliacea |
Sasa |
A. flavus |
0.14 ± 0.12b |
0.09 ± 0.07cd |
0.01 ± 0.01h |
|
Bodija |
A. flavus |
0.24 ± 0.10ab |
0.04 ± 0.03d |
0.02 ± 0.00h |
|
|
Oja-Oba |
A. flavus |
0.21 ± 0.15b |
0.22 ± 0.02bcd |
0.05 ± 0.06fgh |
|
|
Sasa |
A. parasiticus |
0.29 ± 0.03ab |
0.17 ± 0.05bcd |
0.05 ± 0.06fgh |
|
|
Bodija |
A. parasiticus |
0.27 ± 0.04ab |
0.15 ± 0.04bcd |
0.15 ± 0.06cde |
|
|
Oja-Oba |
A. parasiticus |
0.28 ± 0.03ab |
0.09 ± 0.11cd |
0.14 ± 0.07cdef |
|
|
Sasa |
A. fumigatus |
0.25 ± 0.11ab |
0.19 ± 0.01bcd |
0.07 ± 0.09efgh |
|
|
Bodija |
A. fumigatus |
0.28 ± 0.04ab |
0.10 ± 0.13bcd |
0.09 ± 0.06efgh |
|
|
Bryophyllum pinnatum |
Sasa |
A. flavus |
0.08 ± 0.07b |
0.10 ± 0.12bcd |
0.13 ± 0.07defg |
|
Bodija |
A. flavus |
0.20 ± 0.10b |
0.52 ± 0.73ab |
0.04 ± 0.04gh |
|
|
Oja-Oba |
A. flavus |
0.26 ± 0.06ab |
0.86 ± 0.51a |
0.14 ± 0.11cdef |
|
|
Sasa |
A. parasiticus |
0.47 ± 0.49a |
0.53 ± 0.51ab |
0.04 ± 0.03gh |
|
|
Bodija |
A. parasiticus |
0.14 ± 0.00b |
0.18 ± 0.10bcd |
0.04 ± 0.03gh |
|
|
Oja-Oba |
A. parasiticus |
0.15 ± 0.11b |
0.17 ± 0.13bcd |
0.03 ± 0.03h |
|
|
Sasa |
A. fumigatus |
0.21 ± 0.16b |
0.30 ± 0.01bcd |
0.03 ± 0.04h |
|
|
Bodija |
A. fumigatus |
0.22 ± 0.02b |
0.20 ± 0.00bcd |
0.30 ± 0.07ab |
|
|
Control |
Sasa |
A. flavus |
0.14 ± 0.00b |
0.14 ± 0.00bcd |
0.14 ± 0.00cdef |
|
Bodija |
A. flavus |
0.21 ± 0.00b |
0.21 ± 0.00bcd |
0.21 ± 0.00bcd |
|
|
Oja-Oba |
A. flavus |
0.22 ± 0.00b |
0.22 ± 0.00bcd |
0.22 ± 0.00bc |
|
|
Sasa |
A. parasiticus |
0.32 ± 0.00ab |
0.32 ± 0.00bcd |
0.32 ± 0.00a |
|
|
Bodija |
A. parasiticus |
0.29 ± 0.00ab |
0.29 ± 0.00bcd |
0.29 ± 0.00ab |
|
|
Oja-Oba |
A. parasiticus |
0.28 ± 0.00ab |
0.28 ± 0.00bcd |
0.28 ± 0.00ab |
|
|
Sasa |
A. fumigatus |
0.29 ± 0.00ab |
0.29 ± 0.00bcd |
0.29 ± 0.00ab |
|
|
Bodija |
A. fumigatus |
0.31 ± 0.00ab |
0.31 ± 0.00bcd |
0.31 ± 0.00a |
|
Means with the same alphabets down the column are not significantly different at P≤0.05 using Duncan Multiple Range Test (DMRT) for separation of statistically significant means. Data collected were represented as “Means ± SD” only
Table 3: Fresh weight measurement of aflatoxigenic pathogens treated with phyto-fungicides.
|
Treatment |
Location |
Aflatoxigenic Pathogen |
Dry Mycelia Weight (g) |
||
|
500mg/mL |
750mg/mL |
1000mg/mL |
|||
|
Petiveria Alliacea |
Sasa |
A. flavus |
0.02 ± 0.03d |
0.01 ± 0.01ef |
0.00 ± 0.01c |
|
Bodija |
A. flavus |
0.05 ± 0.03bcd |
0.00 ± 0.01f |
0.01 ± 0.01c |
|
|
Oja-Oba |
A. flavus |
0.04 ± 0.03bcd |
0.02 ± 0.01def |
0.01 ± 0.01c |
|
|
Sasa |
A. parasiticus |
0.07 ± 0.01bcd |
0.02 ± 0.01def |
0.01 ± 0.00c |
|
|
Bodija |
A. parasiticus |
0.06 ± 0.02bcd |
0.02 ± 0.01def |
0.02 ± 0.01c |
|
|
Oja-Oba |
A. parasiticus |
0.06 ± 0.02bcd |
0.02 ± 0.01def |
0.02 ± 0.01c |
|
|
Sasa |
A. fumigatus |
0.07 ± 0.02bcd |
0.02 ± 0.01def |
0.01 ± 0.01c |
|
|
Bodija |
A. fumigatus |
0.07 ± 0.01bcd |
0.01 ± 0.02ef |
0.01 ± 0.01c |
|
|
Bryophyllum Pinnatum |
Sasa |
A. flavus |
0.02 ± 0.02d |
0.02 ± 0.03def |
0.02 ± 0.01c |
|
Bodija |
A. flavus |
0.05 ± 0.02bcd |
0.05 ± 0.04bcde |
0.01 ± 0.01c |
|
|
Oja-Oba |
A. flavus |
0.04 ± 0.00bcd |
0.05 ± 0.01bcde |
0.02 ± 0.01c |
|
|
Sasa |
A. parasiticus |
0.06 ± 0.01bcd |
0.05 ± 0.01bcde |
0.01 ± 0.01c |
|
|
Bodija |
A. parasiticus |
0.06 ± 0.01bcd |
0.05 ± 0.02bcde |
0.01 ± 0.01c |
|
|
Oja-Oba |
A. parasiticus |
0.04 ± 0.03bcd |
0.03 ± 0.02cdef |
0.00 ± 0.01c |
|
|
Sasa |
A. fumigatus |
0.04 ± 0.02bcd |
0.05 ± 0.01bcde |
0.00 ± 0.01c |
|
|
Bodija |
A. fumigatus |
0.06 ± 0.01bcd |
0.05 ± 0.01bcde |
0.06 ± 0.01b |
|
|
Control |
Sasa |
A. flavus |
0.50 ± 0.10bcd |
0.50 ± 0.10a |
0.50 ± 0.10a |
|
Bodija |
A. flavus |
0.06 ± 0.01bcd |
0.06 ± 0.01bcd |
0.06 ± 0.01b |
|
|
Oja-Oba |
A. flavus |
0.06 ± 0.01bcd |
0.06 ± 0.01bcd |
0.06 ± 0.01b |
|
|
Sasa |
A. parasiticus |
0.09 ± 0.01b |
0.09 ± 0.01b |
0.09 ± 0.01b |
|
|
Bodija |
A. parasiticus |
0.08 ± 0.01bc |
0.08 ± 0.01bc |
0.08 ± 0.01b |
|
|
Oja-Oba |
A. parasiticus |
0.07 ± 0.01bcd |
0.07 ± 0.01bc |
0.07 ± 0.01b |
|
|
Sasa |
A. fumigatus |
0.06 ± 0.01bcd |
0.06 ± 0.01bcd |
0.06 ± 0.01b |
|
|
Bodija |
A. fumigatus |
0.07 ± 0.01bcd |
0.07 ± 0.01bc |
0.07 ± 0.01b |
|
Means with the same alphabets down the column are not significantly different at P≤0.05 using Duncan Multiple Range Test (DMRT) for separation of statistically significant means. Data collected were represented as “Means ± SD” only
Table 4: Dry weight measurement of aflatoxigenic pathogens treated with phyto-fungicides.
4. Discussion
The research conducted showed that Petiveria alliacea and Bryophyllum pinnatum were very effective in detoxification and inhibition of aflatoxin production in stored cowpea seeds collected from local markets within Ibadan, Oyo State, Nigeria. Also, these phyto-fungicides showed a tremendous capacity for elimination of aflatoxigenic fungal pathogens of stored cowpea seeds too. The success achieved by the application of these plants-based fungicides can be attributed to the presence of high levels of antifungal substances present in the plant samples. A similar report was given by Silva et al., [13] who noted the ability of P. alliacea to inhibit the growth of aflatoxigenic fungal species and also Colletotrichum gloeosporioides. The dry mycelia weight of most of the isolated aflatoxigenic species was significantly reduced by the antifungal activity of P. alliacea and B. pinnatum. This was in connection with the research ideology of Alabi et al., [14] who initially investigated the fungitoxic and phytotoxic effects of aqueous extract of B. pinnatum on some fungal pathogens that induced wilting in cowpea grown in Ago-Iwoye, Ogun State, Nigeria. They also reported that the plant extracts significantly reduced the Disease Infection Rate (DIR) in treated plants.
5. Conclusion
The use of plant extracts for control of fungal pests is safe and less expensive than synthetic pesticides. There are no side effects to high dosages of botanical extracts applied for the treatment or management of plant diseases. Production of plant-based fungicides are very easy to setup, cheap, available and affordable, and there are no skills or precautions needed for application. More so, they are environmentally friendly and constitute no health risk to humans and animals. This will help reduce pollution from chemical sources and also contribute to a sustainable development of the ecosystem.
References
- Passone M A, Resnik S, Etcheverry M G. The Potential of Food Grade Antioxidants in the Control of Aspergillus Section Flavi, Interrelated Mycoflora and Aflatoxin B1 Accumulation on Peanut Grains. Food Control. 19 (2008): 364-371.
- Munkvold G P. Cultural and genetic approaches to managing mycotoxins in maize. Annual Review of Phytopathology 41 (2003): 99-116.
- Etaware P M, Etaware E U, Olaoluwa O O, Oyetunji O J, Aiyelaagbe O O, Odebode A C. The impact crude plant extracts: As potential biofertilizers and treatment against tomato plant infection. Journal of Plant Pathology and Microbiology 10 (2019): 481-491.
- Etaware P M. The effects of Calotropis procera, Adansonia digitata and Manihot esculenta in the remediation of soil-borne fungal diseases of tomato. Journal of Agricultural Research Advances 1 (2019): 28-37.
- Saadabi A. Antifungal activity of some Saudi plants used in traditional medicine. Asian Journal of Plant Science 5 (2006): 907-909.
- Enyiukwu D N, Awurum A N, Nwaneri J A. Efficacy of plant-derived pesticides in the control of myco-induced postharvest and storage rots of tubers and agricultural products: A review. Net Journal of Agricultural Sciences 2 (2014): 30-46.
- Amadioha A C. Reducing food losses through sustainable methods of plant disease management: an imperative for actualization of food security in Nigeria. A paper presented at the 13th inaugural lecture MOUAU (2012): 7.
- Awurum A N, Ucheagwu P O. Effects of duration of contact of seed extract of Piper guineense, Monodora myristica and Xylopia aethiopica on the germination and incidence of seed-borne fungi of stored cowpea (Vigna unguiculata L. Walp) seeds. Continental Journal of Biological Sciences 6(2013): 37-42.
- Fandohan P, Gnonfin B, Hell K, Marasas W F O, Wingfield MJ. Natural occurrence of Fusarium and subsequent fumonisin contaminantion in preharvest and stored maize in Benin, West Africa. Internationl Journal of Food Microbiology 99 (2003): 173-183.
- Adjou E S, Dahouenon-Ahoussi E, Degnon R, Soumanou M M, Sohounhloue D. Investigations on the bioactivity of essential oil of Ageratum conyzoides L. from Benin against the growth of fungi and aflatoxin production. International Journal of Pharmaceutical Science Review and Research 13 (2012): 143-148.
- Adegoke G O, Odesola B A. Storage of maize and cowpea and inhibition of microbial agents of biodeterioration using the powder and essential oil of lemon grass (Cymbopogon citratus). International Journal of Biodeterioration and Biodegradation 37 (1996): 81-84.
- Okigbo R N, Ogbonnaya U O. Antifungal effects of two tropical plant leaf extracts (Ocimum gratissimum and Aframomum melegueta) on postharvest yam (Dioscorea spp.) rot. African Journal of biotechnology 5 (2006): 727-731.
- Silva P A, Oliveira D F, Taironi do Prado N, Antonio de Carvaiho D, Aparecidade carvaiho G. Evaluation of the antifungal activity of plant extracts against Colletotrichum gloeosporioides Penz. Cienc. Agrotec. 32 (2008): 420-428.
- Alabi D A, Oyero I A, Jimoh, Amusa N A. Fungitoxic and Phytotoxic Effect of Vernonia amygdalina (L), Bryophyllum pinnantus Kurz Ocimum gratissimum (Closium) and Eucalyptna globules (Caliptos) Labill Water Extracts on Cowpea and Cowpea Seedling Pathogens in Ago Iwoye, South Western Nigeria. World Journal of Agricultural Sciences 1 (2005): 70-75.
