Applicability of Histopathological and Biochemical Data of Anthracyclines Cardiotoxicity in Animal Studies for Regulatory Purposes
Article Information
Nikolaos Avgeros1*, Nektaria Kompi1*, Georgia Papadimitriou1*, Konstantinos Tsarouhas3*, Christina Tsitsimpikou2, George EN Kass4, Jean-Lou CM Dorne5, Dimitrios Kouretas1#, Nikolaos Georgiadis5#
1Department of Biochemistry & Biotechnology, University of Thessaly, Larissa, Greece
2Directorate of Energy, Industrial & Chemical Products, General Chemical State Laboratory of Greece, Athens, Greece
3Department of Cardiology, University Hospital of Larissa, Larissa, Greece
4European Food Safety Authority, Parma, Italy
5European Chemicals Agency, Helsinki, Finland
*Authors have contributed equally
*Corresponding author 1: Nikolaos Georgiadis, MSC, PhD, ERT European Chemicals Agency, Helsinki, Finland, Helsinki, Finland
*Corresponding author 2: Professor Dimitrios Kouretas, Department of Biochemistry & Biotechnology, University of Thessaly, Larissa, Viopolis, 41500, Greece
Received: 15 April 2025; Accepted: 06 May 2025; Published: 03 July 2025
Citation: Nikolaos Avgeros, Nektaria Kompi, Georgia Papadimitriou, Konstantinos Tsarouhas, Christina Tsitsimpikou, George EN Kass, Jean- Lou CM Dorne, Dimitrios Kouretas, Nikolaos Georgiadis. Applicability of Histopathological and Biochemical Data of Anthracyclines Cardiotoxicity in Animal Studies for Regulatory Purposes. Journal of Biotechnology and Biomedicine. 8 (2025): 184-243.
View / Download Pdf Share at FacebookAbstract
Anthracyclines are commonly used anticancer drugs with important therapeutic applications and well-known and extensively studied cardiotoxic effects in humans. In the clinical setting guidelines for assessing and treating cardiotoxicity in humans are well established. Apart from pharmaceuticals, other everyday chemicals have lately been implicated in causing cardiotoxic effects in humans, as a side effect. In the current general toxicology regulatory framework, cardiotoxicity is not a distinct endpoint and no objective criteria or reference values exist in the regulations in order to uniformly characterize cardio-toxic adverse effects observed in animal models with relevance to humans. This in depth review uses cardiotoxicity caused by anthracyclines in rat as the gold standard model and focuses on the evaluation of the most common histopathological lesions reported and the alterations observed in biomarkers of oxidative stress and inflammation of the cardiovascular function in rat studies of anthracycline induced cardiotoxicity. The present review comes as a continuation of a previous study of our group that described the echocardiographic observations of the rat anthracycline cardiotoxicity model. All these range values and histopathology findings registered could be used to differentiate normal cardiac function in animals from pathological findings indicative of cardiotoxicity and could eventually be applied to recognize possible cardiotoxic effects of everyday chemicals. The analysis of the gathered data in this review gives promising results and creates prospects for defining cardiotoxicity reference values in animal species based on the anthracycline model and eventually developing potential guidelines for assessing cardiotoxicity as a separate hazard class of everyday chemicals for regulatory purposes.
Keywords
Anthracyclines; Histopathology; Inflammation; Oxidative stress; Rats
Anthracyclines articles; Histopathology articles; Inflammation articles; Oxidative stress articles; Rats articles
Article Details
Abbreviations: LAD: Left Anterior Descending Artery; IL-1β: Interleukin 1β; IL-6 :Inteleukin 6; TNF-α:Tumor Necrosis Factor alpha; cTnT: cardiac Troponin T; cTnI:cardiac Troponin I; ST elevation: refers to ST-segment from echocardiogram; βAR:beta Adrenergic Receptor; CV: Cardiovascular; MDA: Malondialdehyde; CAT: Catalase; GPx: Glutathione Peroxide; GSH: Glutathione; SOD: Superοxide Dismutase; GPX1-β: Isoenzyme of Glutathione Peroxide; ROS: Reactive Oxygen Species; EF: Ejection Fraction; FS: Fraction Shortening; NT-proBNP: N-terminal pro B-type Natriuretic Peptide; BNP: B-type Natriuretic Peptide; ANP: Atrial Νatriuretic Peptide; LV: Left Ventricle; CMR: Cardiovascular Magnetic Resonance; GLS : Global Longitudinal Strain; AAS : Anabolic Androjenicstreoids
Introduction
Cardiotoxicity caused by chemotherapeutic agents is a significant issue for clinicians managing various types of cancer, as it can limit treatment strategies and negatively impact patient survival. In preclinical animal studies designed to assess new anticancer compounds and potential cardioprotective agents, accurately determining the degree of cardiotoxicity is essential for evaluating the effectiveness of any intervention aimed at mitigating these adverse effects [1]. Anthracyclines are strong chemotherapy agents extracted from Streptomyces spp. used to treat a range of blood-related and solid cancers. The available types for treatment are daunorubicin, doxorubicin, epirubicin, idarubicin, mitoxantrone and valrubicin [2-3]. These compounds are used for the treatment of many cancers, like leukemia, lymphomas and breast, stomach, uterine, ovarian, lung and bladder cancers. Anthracyclines are widely recognized for their cardiotoxic properties, often leading to myocardial impairment in a significant portion of patients. In preclinical studies, they are commonly employed as a simple and cost-effective method to induce dilated cardiomyopathy in animal models [4]. This approach serves as an alternative to more invasive experimental models of cardiac infarction or ischemia, such as permanent ligation of the left anterior descending (LAD) artery or the use of a cryo-probe to create localized ischemic damage on the heart. The literature describes a variety of animal species and administration protocols, involving different dosages and delivery methods of anthracyclines, aimed at inducing cardiotoxicity [5]. These models are used not only to track the progression of cardiac damage but also to assess the effectiveness of various therapeutic agents and intervention strategies in-tended to reduce myocardial injury. For the evaluation of anthracycline-related cardiotoxicity, biochemical markers are used in addition to cardiac imaging [6]. At the same time, other pharmaceutical compounds have been linked to negative alterations in cardiac pathology, resulting in compromised heart function, while there is increasing scientific evidence that everyday chemicals, such as metals pesticides and ethanol, could cause acute or long-lasting cardiovascular impairment in humans [7].
Chemicals, through the various steps of their lifecycle, including production, handling, transport use and disposal, could represent an important risk for the environment and human health. People regardless their demographic characteristics are exposed to everyday chemicals, from pesticides to detergents and cosmetics, either intentionally or through passive exposure. Toxicity and risk for human health posed by chemicals are well controlled at a European level through a thoroughly developed regulatory network. However, in many regulatory frameworks, cardiotoxicity is not defined as a stand-alone hazard nor are there harmonized criteria for classifying substances as cardiotoxic. Identifying the hazard in advance (before adverse effects are observed in humans) and regulating chemical substances that could lead to cardiovascular toxicity would undoubtedly strengthen the national health systems. Although in the Global Harmonized System (GHS) under the auspices of United Nations, there is a generic hazard class (i.e. Specific target Organ Toxicity STOT, single or repeated exposure), which could potentially capture cardiotoxicity, the lack of established criteria still remains. Furthermore, cardiotoxicity, due to its strong relevance to human health, regardless of age, sex or other demographic characteristics, could represent a hazard of equivalent level of concern to CMRs (carcinogenic, mutagenic, reproductive) toxicants or endocrine disruptors. A recent publication [48] has outlined a roadmap for deriving regulatory criteria from animal studies to support legislation for classifying substances as cardiotoxic, and a related initiative has also received endorsement from the Organization for Economic Co-operation and Development (OECD). In a previous study [6], echocardiographic indices of anthracycline induced cardiotoxicity in rats have been reviewed, in the same context as described above, and encouraging results regarding establishing threshold values of ejection fraction (EF) and fractional shortening (FS) alterations in rats with cardiotoxicity induced by anthracyclines have been reported. In the present study, alterations of biomarker indices of oxidative stress and inflammation in rats exposed to anthracyclines are reviewed, together with the histopathological findings re-ported, in order to elucidate whether these three lines of evidence could be further used to identify cardiotoxicity in animal studies. Since cardiotoxicity is not a distinct endpoint recognized by OECD, no testing guidelines are available for animal models and animal studies. Therefore, there is no uniformity in animal models of cardiotoxicity induction and most importantly, not all studies describe the same decline of myocardial function, as it most often lies to the expert judgement of the study authors. Moreover, the echocardiographic assessment of myocardial function decline in many published animal studies claiming cardiotoxicity was not utilized. It is therefore a need to delineate if specific biochemical alterations and histopathological findings could serve as a stand-alone tool or in conjunction with echocardiography to define cardiotoxicity in animal studies. Since anthracycline induced cardiotoxicity is regarded as an established animal model, its applicability to serve the regulatory needs described above to identify cardiovascular implications of unknown origin possibly attributed to chemicals exposure, is explored. The rat is again chosen as the species of interest in accordance with previous studies in the same regulatory framework [6]. In the current in-depth review, the authors focus on the evaluation of the most common histopathological lesions reported and the alterations observed in biomarkers of oxidative stress and inflammation of the cardiovascular function in rat studies of anthracycline induced cardiotoxicity. Whether range or threshold values of these indices could differentiate normal animals from animals with anthracycline induced cardiotoxic effects is also discussed.
Materials and Methods
Figure 1 illustrates the chemical structures of the anthracyclines used in the studies. These compounds were administered, in the sequence presented, through various routes including intraperitoneal and intravenous injections, or orally via feed. Dosing frequency ranged from one to three times per week, with treatment durations spanning from one to ten weeks. In most cases, administration was halted upon confirmation of cardiotoxicity. The data indicate that there is no universal dosing threshold for inducing the effect; rather, it ap-pears to vary across studies and may be influenced by factors such as the animals’ age and overall health status. A systematic search of the PubMed database was conducted to identify all original research articles published between March 2020 and October 2023, following the guidelines of the Preferred Re-porting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [8].
Histopathological findings
PubMed Advanced Search Builder was used to search throughout all Fields and the utilized filters in separate searches were as follows:
1) “Cardiotoxicity” AND “rats” AND “anthracycline” AND “histopathology”
2) “Cardiotoxicity” AND “rats” AND “anthracycline” AND “lesion”
3) “Cardiotoxicity” AND “rats” AND “anthracycline” AND “staining”
4) “Cardiotoxicity” AND “rats” AND “doxorobucin” AND “histopathology”
5) “Cardiotoxicity” AND “rats” AND “doxorobucin” AND “lesion”
6) “Cardiotoxicity” AND “rats” AND “doxorobucin” AND “staining”
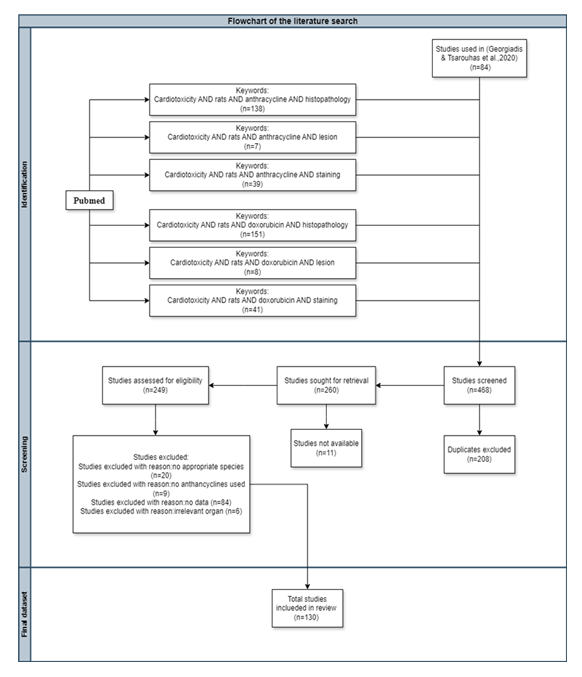
The list of studies obtained was additionally reviewed for the relevance of the subject and the eligibility by screening the titles and abstracts of the full paper. Only the original research studies were used and other particular filter was used. The cumulative count of articles retrieved from each of the afore-mentioned individual searches amounted to 384 articles, with 84 articles coming from a previous study [6] of the same research group targeting echocardiographic indices added to the list of studies in order to be used for comparison. From the pool of articles yielded from the above-mentioned search strategy of the present study, 130 were deemed suitable for inclusion in this study. 208 articles were excluded due to being duplicates, 11 articles were not available for various reasons, 20 articles due to inappropriate species tested, 9 articles due to no anthracycline used, 84 articles due to no histopathological data and at last 6 articles due to irrelevant organ tested. (Fig. 3).
Biomarkers of inflammation
The specific literature search strategy which used, was:
“*Cardiotoxicity*” AND “*rats*” AND “*anthracycline*” AND “*inflammation*”
“*Cardiotoxicity*” AND “*rats*” AND “*daunorubicin*” AND “*inflammation*”
“*Cardiotoxicity*” AND “*rats*” AND “*doxorubicin*” AND “*inflammation*”
“*Cardiotoxicity*” AND “*rats*” AND “*epirubicin*” AND “*inflammation*”
either in the Title, or in the Abstracts. The list of studies obtained was also re-viewed for the relevance of the subject and the eligibility by screening the titles and abstracts of the full paper. Only the original research studies were used.
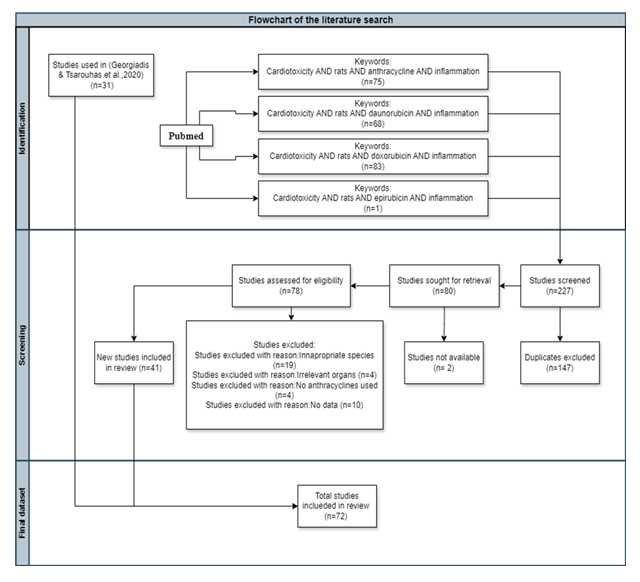
Only animal data retrieved from rat species were evaluated, as indicated from the search strategy, following the roadmap strategy identified in previous studies [6,48]. Various anthracyclines administration modes were applied in the studies screened in the present review (Table 2): Anthracyclines were administered either intraperitoneally, intravenously, or orally through feed. While a minority of studies employed acute administration, most involved prolonged treatment periods ranging from 2 to 10 weeks. Dosing frequencies varied—once, twice, or three times per week—with overall treatment durations spanning from one to ten weeks. All dosing protocols were deliberately designed to induce cardiotoxicity. In the majority of studies, treatment was discontinued upon confirmation of cardiotoxic effects, as documented by the respective authors. Previous findings based on echocardiographic measurements indicate that there is no universal dosing threshold that reliably triggers cardiotoxicity. Instead, the onset appears to be experiment-specific and influenced by factors such as the animals’ age, general health, and the method of administration. Notably, the mode of administration did not significantly impact the observed reduction in ejection fraction (%EF) and fractional shortening (%FS) [6].From the 227 studies screened, 147 were excluded as duplicates, 2 were excluded be-cause they were not available (no free access), 19 were excluded due to inappropriate species, 4 due to irrelevant organs, 4 because no anthracyclines were used, and 10 because there were no data for the inflammation biomarkers (IL-1β, IL-6, ΤΝF-α) along with cTnT, cTnI, representing cardiac muscle injury. Lastly, all types of citations other than the original research studies (e.g., review articles, etc.) were also excluded. In total, 31 published animal studies were used from a previous study of the same research group targeting echocardiographic indices [6] and 41 from the current one, as illustrated in the diagram (Fig. 4).
Cardiac Troponins T and I (cTnT and cTnI)
Cardiac Troponin T (cTnT) and cardiac Troponin I (cTnI) are cardiac regulatory proteins that regulate the calcium interaction between actin and myosin. Their forms are coded by specific genes and are mainly found in the myocardium [9]. They are sensitive and specific biomarkers of myocardial cell injury, acute myocarditis, myocardial infarction, early myocardial damage in non-ST elevation acute coronary syndromes and isoproterenol induced myocardial necrosis [10-11].
Tumor Necrosis Factor-α (TNFα)
Tumor Necrosis Factor-α (TNF-α) is a pro-inflammatory cytokine released by macrophages and monocytes in response to acute inflammatory stimuli. It regulates various signaling events leading to necrosis or apoptosis and therefore plays a role in resistance to infections and cancers [12]. TNF-α is a potential biomarker which is associated with heart failure [13]. Based on research, TNF-α mediates negative inotropic effect in vivo and in vitro, which indicates that the pro- inflammatory cytokine TNF-α is possible to cross talk with the be-ta-adrenergic receptor (βAR) system and underly the negative inotropic pheno-type observed in heart failure [14].
Interleukin (IL)-6
Interleukin (IL)-6 is a cytokine with pro-inflammatory and anti-inflammatory properties [15] with an important role in cardiovascular (CV) disease. Increased levels of cardiac IL-6 and IL-6 receptor mRNA have been linked to deteriorating hemodynamics in advanced heart failure [16].IL-6 is considered a crucial biomarker for heart failure, as pharmacological agents that target IL-6 signaling, such as tocilizumab, sarilumab, and siltuxumab, have already been developed and used in the treatment of various dis-eases [17].
Interleukin (IL)-1β
The Interleukin-1 (IL-1) family is one of the most important cytokine family related to acute and chronic inflammation. Within the IL-1 family, IL-1β has been proven to be a therapeutic target for many autoinflammatory diseases. Inhibition of IL-1β leads to prompt and persistent reduction in disease severity [18-19].
Biomarkers of oxidative stress
The research strategy included the following terms either in the title or in the abstracts: [AND (“*rats*” OR “*doxorubicin*” OR “*epirubicin*” OR “*daunorubicin*” OR “*anthracycline*” OR “*oxidative stress*”)]. Overall, a total of 105 published manuscripts concerning animal studies were included in the analysis. Following the exclusion of duplicates, the retrieved studies under-went additional assessment to ascertain their relevance to the subject matter and eligibility. Subsequently, a thorough examination was carried out to extract all relevant data. Only data from rat species was evaluated. Only original re-search studies were included. Furthermore, articles containing data from irrelevant organs (e.g., brain, kidney) were also excluded, as well as those without anthracyclines being the administered substances. The searches targeted oxidative stress, particularly focusing on malondialdehyde (MDA) as a lipid peroxidation marker in cardiac tissue as well as three endogenous enzymes-Superoxide dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPx)-and the antioxidant glutathione (GSH) in cardiac tissue. Incorporating the supplementary 40 studies from the preceding publication [6], the total number of articles increases to 145, as illustrated in the diagram (Fig. 5).
Malondialdehyde
Malondialdehyde (MDA) is an organic compound which is derived from the peroxidation of polyunsaturated fatty acids and arachidonic acid metabolism [20]. It is a highly reactive three-carbon dialdehyde mostly found in the enol form [21]. Lipid peroxidation plays a significant role in the development of various tissue injuries and MDA, as an important secondary product of lipid peroxidation, used as a marker of cell membrane injury. Elevated levels of lipid peroxidation products have been linked to various chronic diseases in both humans and model systems [20].
Glutathione Peroxidase
The glutathione peroxidase (GPX) family includes several isozymes (GPX1-8) located in various subcellular compartments and tissues. Among these, GPX1-4 and GPX6 are selenocysteine-containing proteins [22]. Their active site consists of a conserved tetramer that includes selenocysteine (Sec) residues, as well as glutamine (Gln), tryptophan, and asparagine. In contrast, the other three GPXs have cysteine as an active site [23]. The main function of GPX1-4 is to mitigate oxidative stress by catalyzing the reduction of H2O2 or organic hydroperoxides. Moreover, GPX1-4 inhibits inflammation and apoptosis, necroptosis, and pyroptosis [24-26]. They constitute an important mechanism of defense within the heart [27-28]. Besides, GPX2 and GPX3 demonstrate antioxidant effects in intestinal epithelial cells and plasma, respectively, while GPX5-8 lack significant antioxidant capacity [22].
Glutathione
Glutathione is a tripeptide of glutamate, cysteine, and glycine, found in all cells. Glutathione is important in regulating enzyme activity and maintaining the cellular oxidation-reduction (redox) status. GSH is crucial in the cardiovascular system as it functions as a key antioxi-dant, helping to restore intracellular redox balance and prevent the inactivation of nitric oxide produced by the endothelium. This action is particularly im-portant in preventing abnormal vasomotor responses in individuals with coro-nary spastic angina [29-30].
Superoxide dismutase
Superoxide dismutases (SODs) are a family of metalloenzymes that play a crucial role in protecting against reactive oxygen species (ROS)-induced damage. They catalyze the conversion of superoxide anion free radicals (O2•-) into molecular oxygen and hydrogen peroxide (H2O2), thereby reducing the levels of O2•- that can harm cells [31].
Catalase (CAT)
Hydrogen peroxide is a freely diffusible reactive species that acts as an oxidizing and reducing agent. It is the progenitor of many other ROS. It modifies glyceraldehyde-3-phosphate dehydrogenase by oxidation of the labile essential thiol groups at the active site of this enzyme [32].
Results
Evaluation of Results to Identify Classification Criteria
We display below the results of the three parallel searches that have been conducted, namely the histopathological data, the inflammation, and the oxidative stress indices.
Histopathological findings
A key factor, examined and reviewed using a weight-of-evidence approach, includes the results of histopathological analysis of heart tissue from animals exposed to known cardiotoxic agents like anthracyclines. Notable morphological alterations in the heart muscle observed during necropsy and/or microscopic examination, which indicate severe heart dysfunction and cell death, are con-sidered significant. Additionally, small changes in heart weight with no evidence of organ dysfunction could also provide useful information. Similar results re-ported in the literature, when other allegedly cardiotoxic substances are studied are also taken into consideration in order to evaluate histopathological findings: in rabbits exposed to anabolic steroids, local fibrosis as well as a mild chronic inflammation of cardiac tissue were observed [7, 33].
An interesting finding reported in pesticide studies is the persistence or accumulation of varying amounts of pesticides in cardiac tissues, indicating that heart muscle cells were directly exposed to these chemicals [17].
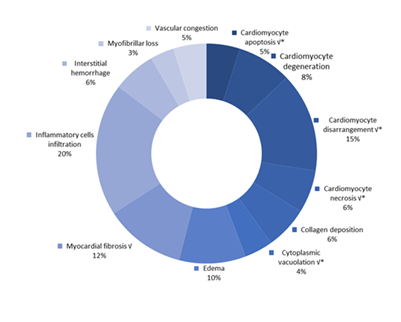
The histopathological findings are illustrated in Tables 1,2 & Figure 2. The findings were categorized into observations at the tissue level and the cellular level. When it refers to tissue level the major findings are reversible inflammatory cell infiltration (10.21% of the examined rats), cardiomyocyte disarrangements and disorganizations, and even loss of muscular striations (8.82% of the examined rats), fibrosis, myocardial and interstitial fibrosis (6.06% of the examined rats). Additionally, reversible edema and interstitial edema (4.67% of the examined rats) were observed, collagen deposition which is linked to fibrosis development (3.29%), interstitial hemorrhage, a non-specific finding concerning functional decline (3.11% of the examined rats). Vascular congestion (2.6% of the examined rats) was also found, as myofibrillar loss (1.73% of the examined rats), cardiomyofibrillar disruption (1.38% of the examined rats), perivascular fibrosis (1.21% of the examined rats) and vacuolization of the connective tissue, a finding non-indicative of functional decline and depicts cellular debris mainly (1.21% of the examined rats).
The most frequent findings related to the cellular level were cardiomyocyte degeneration (3.98% of the examined rats), cardiomyocyte necrosis (3.29% of the examined rats), cardiomyocyte apoptosis (2.42% of the examined rats) and cytoplasmic vacuolization (1.38% of the examined rats). Pyknotic nucleus formation (1.38% of the examined rats) was also found along with cardiomyocyte hypertrophy (1.21% of the examined rats), vacuolar degeneration (1.21% of the examined rats), and cardiomyocyte congestion (1.04% of the examined rats).
Figure 2: Frequency of occurence for the main histopathological findings. Symbol "√" represents five histopathological findings of upmost importance. Symbol " * " is used to indicate findings associated with cellular level damage. Findings with no annotation correspond to tissue level findings. Note that some findings are not represented in the current figure, as their occurrence is relatively infrequent. Namely: Tissue level findings a) perivascular fibrosis, b) vacuolization of the connective tissue, c) cardiomyofibrillar disruption and d) vascular congestionCellular level findings a) cardiomyocyte vacuolization, b) pyknotic nucleus formation, c) cardiomyocyte hypertrophy, d) vacuolar degeneration and e) cardiomyocyte congestion. Frequencyrepresents the number of publications included in the present study with relevant reported data.
Biomarkers of Inflammation
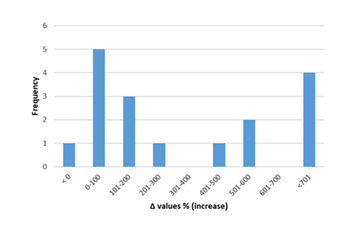
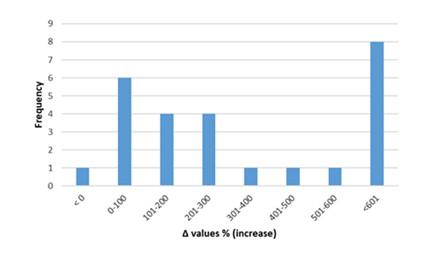
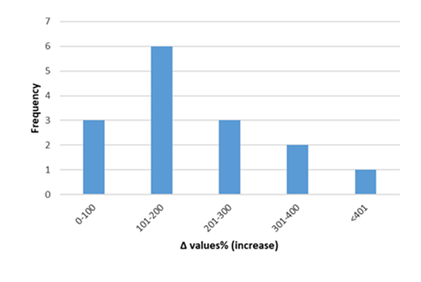
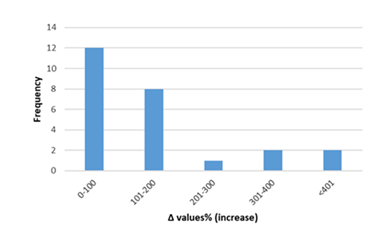
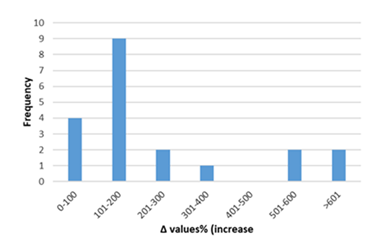
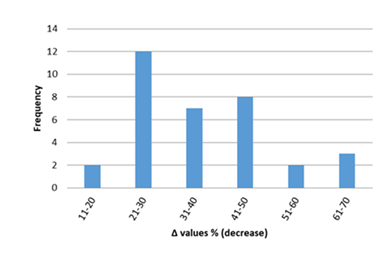
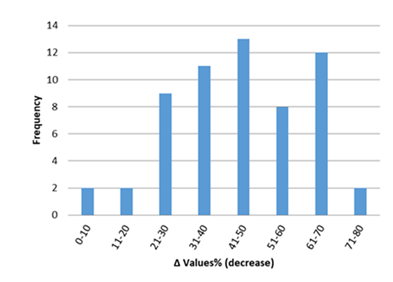
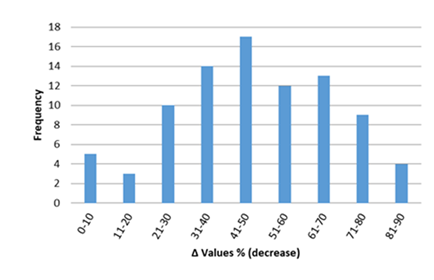
Inflammatory biomarkers evaluation findings are presented in Figures 6-11. The overall change in cardiac tissue inflammation biomarkers (i.e. cTnT, cTnI, TNFα, IL-6, IL-1β) was significant in rats with anthracyclines cardiotoxicity com-pared to their healthy counterparts. More specifically, serum cTnI increased significantly with 47.05% of the values ranging from 50 to 200%. For serum cTnT, the increase was of even greater magnitude ranging from 50 to 400%. Regarding the cardiac tissue TNFα content, a significant increase in cardiotoxicity evaluated rats was also observed with 53.58% of the values increase ranging from 50 to 200%. Serum TNFα content was also significantly augmented in rats with cardiotoxicity ranging from 50 to 600%. Interleukins also increased significantly in rats with cardiotoxicity (in-creases in cardiac tissues ranging from 50 to 500% regarding IL-6 and 50 to 200% for IL-1β, respectively).
Figure 6: Percentage increase in serum cTnT in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values = [(values of rats exposed to anthracyclines) - (values of control rats)]/ (values of control rats)] x 100. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature. Frequency represents the number of publications with reported data.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure 7: Percentage increase in serum cTnI in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values = [(values of rats exposed to anthracyclines) - (values of control rats)]/ (values of control rats)] x 100. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature. Frequency represents the number of publications with reported data.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure 8: Percentage increase in cardiac tissue TNF-α in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values = [(values of rats exposed to anthracyclines) - (values of control rats)]/ (values of control rats)] x 100. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature. Frequency represents the number of publications with reported data. The final bar (<601) represents two percentage change (Δ%) values of TNF-α at 1250 and 1412.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure 9: Percentage increase in serum TNF-α in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values = [(values of rats exposed to anthracyclines) - (values of control rats)]/ (values of control rats)] x 100. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature. Frequency represents the number of publications with reported data. The final bar (<401) represents one percentage change (Δ%) values of TNF-α at 2700.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure 10: Percentage increase in cardiac tissue IL-6 in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values = [(values of rats exposed to anthracyclines) - (values of control rats)]/ (values of control rats)] x 100. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature. Frequency represents the number of publications with reported data. The final bar (<401) represents two percentage change (Δ%) values of IL-6 at 700 and 3746.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure 11: Percentage increase in cardiac tissue IL-1β in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values = [(values of rats exposed to anthracyclines) - (values of control rats)]/ (values of control rats)] x 100. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature. Frequency represents the number of publications with reported data. The final bar (<601) represents two percentage change (Δ%) values of IL-1β at 720 and 1043.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Biomarkers of oxidative stress
Exposure to anthracyclines suppressed CAT, SOD, GSH and GSH-Px indices and increased MDA values in the 145 studies reviewed in the present report.
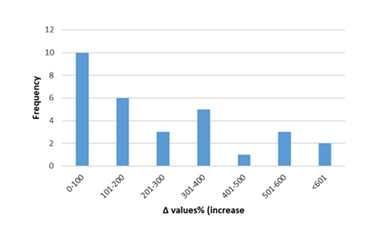
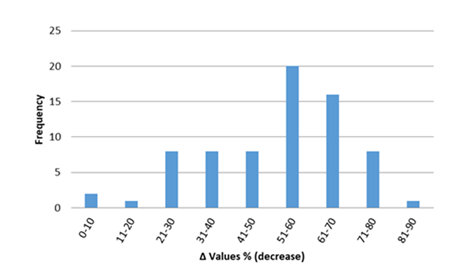
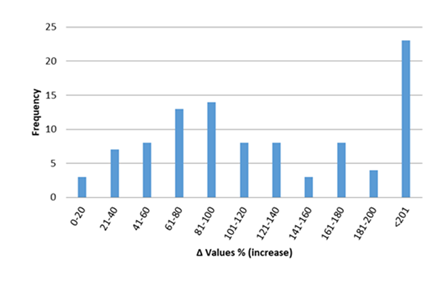
In figures 12-17, the overall change in biomarkers of oxidative stress (i.e. GSH, GSH-Px, CAT, SOD, and MDA) in cardiac tissue of rats with anthracyclines cardiotoxicity compared to their healthy counterpart are presented. More specifically, MDA increases ranged mainly from 20 to 200%. For GSH-Px, decreases ranged from 10 to 70%, and for GSH decreases ranged from 20 to 80%. Furthermore, decreases from 30 to 80% were observed for SOD and from 20 to 70% for CAT, respectively.
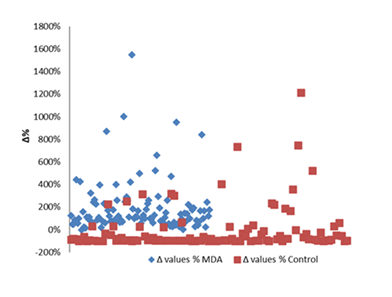
Especially for MDA (figure 17), there is a clear separation of values when we compared MDA values found in anthracycline-exposed rats’ cardiac tissues and the mean values of healthy rats.
There is a clear dependence of all the oxidative stress markers monitored in rats’ cardiac tissue, mentioned above, upon anthracycline exposure. A pathophysiological link between anthracycline exposure and cardiac dysfunction is implied, with oxidative stress being involved. It is very encouraging that there is a concurrent deviation from normal values of the oxidative stress markers when the rats develop cardiotoxicity, a finding that can provide supportive lines of evidence in a weight-of-evidence approach for assessing cardiotoxicity in a regulatory framework.
Figure 12: Percentage decrease in cardiac tissue GSH (%Δ values [(values of rats exposed to anthracyclines) - (values of control rats)/ (values of control rats)] x 100) in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure_13. Percentage decrease in cardiac tissue GSH-Px (%Δ values [(values of rats exposed to anthracyclines) - (values of control rats)/ (values of control rats)] x 100) in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure 14: Percentage decrease in cardiac tissue CAT (%Δ values [(values of rats exposed to anthracyclines) - (values of control rats)/ (values of control rats)] x 100) in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure 15: Percentage decrease in cardiac tissue SOD (%Δ values [(values of rats exposed to anthracyclines) - (values of control rats)/ (values of control rats)] x 100) in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature.Frequencyrepresents the number of publications included in the present study with relevant reported data.
Figure 16: Percentage increase in cardiac tissue MDA in rats exposed to anthracyclines compared to control animals, as reported in the reviewed studies. %Δ values = {(values of rats exposed to anthracyclines) - (values of control rats)]/ (values of control rats)} x 100. The maximum increase observed reaches 540%. %Δ values were calculated in order to overcome the diversity of measuring units used in the literature. Frequency represents number of publications with reported data.
Figure 17: Difference between the Δ% observed between the control and MDA Δ% values of the reviewed studies (blue points) compared to the Δ% values of the control values and the mean control of all the reviewed studies (red values).Frequencyrepresents the number of publications included in the present study with relevant reported data.
Table1: Frequency of occurrence of the five most common histopathological findings (cardiomyocyte necrosis, cardiomyocyte disarrangement, cytoplasmic vacuolation, and cardiomyocyte apoptosis) in correlation with changes inbiochemical markers monitoredin the publications included in the present study.
% of publications in which a finding is observed from a total of 130 publicationsincluded in the present study
% of studies where no concurrent biochemichal effect is reported based on the frequency of the occurence of the histopathological effect.
There are changes in additional biochemical markers concurrent to histopahtological effects at lower frequencies and are not included in the present Table.
Table 2: Summary of all relevantinformationon each animal study design along with results dataextracted from the publications screenedin the present study,including concurrent to histology pathophysiological findings, such as biochemical markers and echocardiography data alterations. Less common observations are listed as footnotes. The cummulative dose for each animal study, which is of paramount clinical importance to humans, has not been calculate, since it was not often provided by the study authors.
Footnotes
a.Refer to Figure 3 for a clearer understanding of the data retrieval process.
- Moreover, LVEDD and LVESD decreased after DOX challenge.Echocardiographic examination demonstrated that DOX treatment markedly reduced CO and the E/A ratio,an index used to reflect diastolic function.
- The doxorubicin control group exhibited signifcant prolongation of QT (p = 0.003), QTc (p = 0.026), and PR (p=0.044) interval and reduction in QRS complex amplitude (p = 0.001) compared to normal control rats.
- We observed the echocardiography of rats and found that when the cumulative dose of DOX reached 15 mg/kg, compared with the control group, the DOX group had significantly reduced heart rate, LVEF, LVFS, SV and CO, and DXXK had a significant improvement effect on the above indicators.
- ECG charts from DOX control rats confirmed a significant broadening of the T waves. In some of the examined ECG charts, the P waves were almost absent. Moreover, the baseline was not straight and spiked. A significant ST segment elevation was evident, QT and PR intervals, amplitude of both QRS complex and T wave, and T wave duration significantly increased by approximately 9.8-, 1.4-, 1.2-, 1.5-,2.5-, and 2.1-fold, respectively, compared with the control (Fig. 2B-E). Heart rate significantly decreased by 20 % in the DOX control compared with the CTRL.
- The results showed that DOX significantly induced an abnormally prolonged QRS complex and QT interval compared to the control group (P < 0.05 and P < 0.001, respectively).
- Secondary findings: 3 rats severe, 2 moderate, 2 mild
- LVEF and LVSV index (LVSVi) in the third subgroup (week 8, 3 weeks after the sixth DOX injection) and the fourth subgroup (week 10, 5 weeks after the sixth DOX injection) significantly declined compared with those in the control.In addition, LVEDV index (LVEDVi) in the third DOX subgroup was significantly lower than that in the control group. LV mass index in each DOX subgroups was significantly lower than that in the control group.
- Rats treated with DOX alone (2 mg/kg) showed several ECG changes including increased RRI, STI, and QRS duration and decreased HR compared to the saline group (p < .001). LVDP decreased in rats that received DOX.
- Secondary findings: Grade 3 characteristics (severe disruption of cardiac muscle architecture, severe loos of muscular striations, severe vascular congestion and hemosederin, severe inflammatory cell infiltration, severe nuclear pyknosis)
- Revealed that exposure to DOX seriously impaired left ventricular (LV) function involving in parameters decrease of EF, FS, CO, LVID and LVAW.
- The acquired results displayed substantial variations in the ECG of doxorubicin-administered rats displaying significant (p < 0.05) prolongation of QT, QRS intervals and ST elevation compared to the control animals.The Dox control group revealed significant (p < 0.05) bradycardia (261 ± 15.60 beat/min) compared to control rats (379.33 ± 24.54 beat/min)
- Accordingly, the heart rate reduced by 24% in the DOX group compared to the control group (p<0.01).
- Compared to control rats, the doxorubicin-treated rats showed a signifcantly lower Hct (58.8±2.0% vs.43.3±12.0%, P=0.001), cardiac output (0.15±0.01 l/min vs.0.12±0.03 l/min, P=0.009) and a higher LV end-systolic volume (LVESV) (0.18±0.02 ml vs. 0.24±0.05 ml,P=0.05). LV mass and heart rate were not signifcantlydiferent between the controls and doxorubicin-treated rats. In the subgroup analysis, the LVEF signifcantly decreased in 12-week treated rats (73±4% vs. 59±9%, P=0.01)
- Secondary findings: Group 1= 2 deaths, Group 4 & 5: 1 death
- The results of the Echocardiographic M-mode tracings showed that the rats in the DOX group experienced a decrease in FS index, and DOX markedly decreased cardiac function indicator of HR, CO, and EF (p <0.05, p < 0.01).
- The electrocardiographic report showed some abnormalities associated with DOX cardiotoxicity,including increased QT and QTc interval duration, bradycardia, and R-R interval prolongation.
- There was a significant increase in PR interval(p=0.003), QT interval (p<0.001), QTc interval (p<0.001) and decrease in QRS complex amplitude (p<0.001) among DOX control animals in comparison with normal control animals.
- Rats in the DOX group showed signifcantly increased duration of QRS complex (p<0.001), PR interval (p<0.01), QT interval (p<0.001), and QTc interval (p<0.001) compared to the control group
- DOX intoxication induced a significant impairment in electrical conduction within the myocardium with a significant augmentation in ST segment elevation, QRS and T wave amplitudes, and OT and PR intervals by approximately 7.5-, 1.4-, 1.8-, 10.8-, and 1.33-fold, respectively, compared with the normal control.
- The intra-peritoneal injection of DOX induced bradycardia with elongation of R-R interval, accompanied by elongation of corrected QTc, elevation of ST height and shortening of T amplitude as compared with normal group. More specific: heart rate (bpm) :-23.44%, RR:27.8%, QTc duration(s):72.7%, P amplitude(mV): 16.07%, ST Height (mV): 108.5%, T amplitude: -72.0%
- RR was increaced (33,0%), HR was decreased (-29%), QT interval was increased (38,8%) and ST elevation was also increased (258,3%).
- Rats treated with DOX had almost three-fold rise in the ST-height, DOX-induced QT prolongation.
- Secondary findings: 1st group: 1 rat died, 2nd group: 4 rats dies, 3rd group:2 rats died before completion of study (hair loss, abdominal distention, ascites and pericardial effusion)
- J-point elevation and heart rate were obviously raised in the DOX rats.
- Secondary findings: Two deaths in 2nd group.
- Secondary findings: Increased eye secretion, erected back hair and 1 death noticed.
- t LVSP and +dp/dtmax decreased significantly in the DOX group, while LVEDP and −dp/dtmax increased substantially in the DOX group.
- A)No pathologic arrhythmia was seen during the ECG in both times (T0 and TF) in the diferent groups. DOXO-treated rats however presented increases in the HR (378.8±17.75 bpm), P-wave duration (43.8±2.48 ms), duration of PR interval (62.8±3.56 ms), and T-wave amplitude (54.6±12.79 mV) in comparison to the other groups at TF. Other parameters such as P-wave amplitude, QRS complex duration, duration of QT interval, and amplitude of R wave remained unchanged B)Troponin I (cTnI) qualitative test was negative in all evaluated animals.
- When heart tissues of rats in control and GA groups were examined, TNF-a and Cox-2 expressions were found to be negative
- A)The authors did not evaluate troponin and N-terminal pro B-type natriuretic peptide (NT-proBNP) levels for model verification since literature data indicate the ambiguity of the relationship between these criteria and the severity of myocardial damage under the action of doxorubicin B)an increase in TNF-α was revealed in the group of animals treated with 15 mg/kg of the drug, which indicates there are persisting inflammatory processes in the heart muscle.
- Secondary findings: 1st group: all rats dead,2nd group: 30% of rats dead,3rd group: no deaths
- DOX injections to the rats caused a significant decline in the HR, SP, DP, and MAP when compared to the normal control group.
- Secondary findings: During 4-6 week: activity decreased, eyelid secretion increased, food & water intake decreased, depilation, respiration accelerated, diarrhea. In the 2nd group only 8 rats survived and 9 rats in the others.
- Secondary findings: Gradually developed poor diet, decreased activity, dull hair, yellowing, occasional diarrhea, and significant weight loss.
- Secondary findings: 2 rats died, scruffy appearance, significant body weight loss, heart weight raised.
- Intergroup differences in the indices of RR, QT, and QRS intervals. Difference in mean 95% CI, one-way ANOVA, post-hoc Tukey test. * p = 0.05–0.01 ** p = 0.01–0.001, **** p < 0.0001. N = 80
- Secondary findings: 33% of rats died, minor body weight loss, significantly lower heart weight, rats with scruffy fur with pink tinge, red exudates around eyes and nose, soft watery feces and necrosis
- Secondary findings: At the beginning of week 4, the rats became lethargic, had decreased food and water intake, slow movement, loss of hair luster, decreased activity, and symptoms of eyeball hyperemia and diarrhea. One rat died on each of days 35, 47, and 70 of the experiment in the ADR group, and the survival rate was 70%.
- The LVIDd, and LVIDs in these two groups were significantly higher than those in the control group (P<0.05). The LVEF and LVFS in these two groups were significantly lower than those in the control group (P<0.05), whereas the LVEDP was higher.
- The LVIDd and LVIDs of the DOX-CM group that were injected with DOX for 6 weeks and did not receive any treatment, showed great increase compared with the control group (P<0.05), while LVEF and LVFS were significantly decreased (P<0.05).
- LV end-systolic diameter was marginally larger in the DXR group compared to controls.
- Secondary findings: 19% mortality by the end of DXR injection protocol
- LVIDd and LVIDs were increased, LVEDV had doubled and LVEF decreased.
- Decrease in IVSTs, EF, and FS values and the increase in LVESD were significant.
- LV posterior wall thickness, LV end-diastolic and end-systolic dimensions, as well as relative wall thickness and percentage fractional shortening and ejection fraction were increased significantly.
- Secondary findings: 9 rats died throughout experiment
- Significantly increased the LV internal dimensions, evident from LVIDD (1.4 fold higher, p < 0.001) and LVIDS (1.3 fold higher, p < 0.01) as well as thinning of LV as assessed by decrease in LVPWD (0.7 fold lower, p < 0.05), LVPWS (0.8 fold lower, p < 0.05), IVSS (0.8 fold lower, p < 0.01) and IVSD (1.2 fold lower, p < 0.01), decreased E wave and relatively increased A wave.
- Secondary findings :5/6 rats with grade 1 and 1/6 rats with grade 2
- Secondary findings :"body hair was ruffled and alopecia occurred, physical activity was reduced, and the rats ingested less food. (11/50) of the rats died in the Dox 1 group, while 48% (24/50) of rats died in the Dox 2 group 22% (11/50) of the rats died in the Dox 1 group, while 48% (24/50) of rats died in the Dox 2 group (acute heart failure, sudden cardiac death)"
- Secondary findings: 6 mg/kg: 1 rat :perivascular fibrosis(very slight), interstitial fibrosis (moderate), myocyte vacuolisation and degeneration, 7.5 mg/kg: perivacular fibrosis (2/6 rats very slight, 1/6 rat slight, 3/6 rats moderate), interstitial fibrosis (2/6 rats very slight), myocyte vacuolisation and degeneration (2/6 rats very slight), 12 mg/kg: perivascular fibrosis (6/11 rats very slight, 4/11 rats slight, 1/11 rats moderate), interstitial fibrosis (5/11 rats very slight, 2/11 rats slight, 1/11 rats moderate), myocyte vacuolisation& degeneration (3/11 very slight, 1/11 rats slight)
- End-diastolic and end-systolic LV diameters/body weight (BW) significantly increased, whereas LV fractional shortening was significantly decreased after 9 weeks of treatment in the DOX group.
aaa. Secondary findings: 12/30 rats died in the Dox group.
bbb. LVD was significantly increased in the DOX group.
ccc. Secondary findings: 23 rats died, scruffy, light yellowish fur, diarrhea and red exudates around the eyes with grossly enlarged abdomen and ascites.
ddd. The echocardiograph results demonstrated that IVSTs significantly decreased in the DOX-treated group when compared with control rats (1.64±0.15 vs. 2.03±0.1 mm; P<0.05; Fig. 2B). However, the decrease in IVSTd exhibited by DOX-treated rats was not significantly different when compared with the controls (0.99±0.11 vs. 1.15±0.12 mm; P=0.13; Fig. 2C).
eee. Significant decrease in cardiac function, as quantified by depressed fractional shortening of the LV.
fff. ECG changes including QT and RR interval prolongation, significantly prolonged QRS segment.
ggg. Decrease in the P wave and QRS complex, indicative of cardiac dysfunction, but the QT and RR intervals and ST segment were increased.
hhh. Lower heart rate, decreased R wave voltage, and prolonged QT interval.
iii. LVEDP was 7.24 ± 1.24 mmHg in the control group, and it was 49.94 ± 5.32 mmHg in DOXO treated group, respectively, BP increased from (147.18 ± 27.21 versus 93.56 ± 9.25 mmHg, respectively, Figure 2A), indicating LV overload.
jjj. Secondary findings: 3 rats died
kkk. DOX treatment significantly impaired cardiac function (means ± SDs of −15.1 ± 14.3 mil/min for ΔCO and −16.3 ± 39.0 μl for ΔSV).
Discussion and Conclusions
In order to substantiate the concern of cardiotoxicity as an emerging hazard for everyday chemicals, and after having identified a gap analysis in the ability of existing toxicity testing methods to identify cardiotoxic hazard, anthracycline induced cardiotoxicity in rats has been selected as a preliminary reference chemical exposed animal model, with the ultimate aim to develop reference parameters monitored and threshold levels for criteria based on effects in vivo. These outcomes will be eventually be included into the regulation in order to classify chemicals for this new emerging hazard class of cardiotoxicity. In the present pilot scale review study, more than 500 different histopathological findings of anthracycline cardiotoxicity are reported in the literature, currently reviewed. In Figure 2 the most frequently reported (>1%) are summarized. Histopathological effects both at the cellular level and tissue level are recognized. In addition, some of the reported effects could be regarded as more significant than others, concerning functional decline of the cardiac muscle.
Myocardial fibrosis seems to be a key event in anthracycline cardiotoxicity both in animals and humans. Interstitial and replacement fibrosis were evident in patients receiving cancer therapy as shown in a recent study evaluating biopsy and autopsy material [34]. Myocardial tissue disorganization and de-arrangement, probably as the result of myocardial cell necrosis, is another key event. Myocardial architecture preservation is of paramount importance and its loss is connected to the functional decline of the left ventricle in patients presenting cancer-related cardiotoxicity. Cardiomyocyte necrosis and replacement fibrosis work hand in hand, however, it is believed that another crucial step could interfere in the changes induced in the heart cytoskeleton, which crucially alters the mechanical properties of the heart [35].
In the cellular level cardiomyocyte necrosis and apoptosis are the hallmarks of cancer-related cardiotoxicity along with cell vacuolization, when the tissue samples are histopathologically examined [36]. These irreversible cellular changes are connected to the well-known effects of anthracycline cardiotoxicity and justify the perception of the permanent insults of anthracyclines justifying the struggle for the early detection of cardiotoxicity at the clinical level. Thus, the histopathological findings of the anthracycline cardiotoxicity in connection to the functional decline of the myocardium could be arbitrarily separated into crucial findings highly predictive of a consequent loss of function and findings not firmly connected to them. Myocardial necrosis and vacuolization in histopathological evaluation should be considered significant findings especially if observed in high frequency. Myocardial disorganization and fibrosis, both replacement and interstitial, should be considered key events closely linked to functional decline especially when the myocardial cytoskeleton is affected too. Anthracycline cardiotoxicity has received scientific and clinician attention since the 70s. Histopathology played an important role in the quest to define the specific type of cardiotoxicity. However, despite the considerable efforts, neither the biochemical pathways nor the specific histopathology lesions were firmly connected to functional decline in humans and the relevant animal models. In humans, additional parameters beyond the administered scheme, chemical substance, cumulative dose, and duration of exposure as comorbidities including hypertension and renal failure, concomitant medication, and age impact the extent of functional decline.
Amongst the histopathological findings, cardiomyocyte vacuolization represents an early stage of doxorubicin cardiotoxicity in which the histopathological alterations could be potentially reversible and this is a field of active research with the use of magnetic resonance imaging [274]. Later findings include interstitial fibrosis along with cardiomyocyte necrosis when the disease is thought to enter a more advanced state. At this point, myocardial functional decline is evident through echocardiography and its hallmark systolic function suppression, measured by myocardial ejection fraction and fractional shortening, decreases. In animal models, focal fibrosis occurs late in the cardiotoxicity progression, usually along with cardiomyocyte necrosis, and it represents a time point where echocardiography could meet prominent histopathological lesions [275]. A temporal gap was found between histopathological changes and functional de-cline in animal models of cardiotoxicity possibly representing the importance of cytoskeleton alterations in the occurrence of myocardial ejection fraction de-cline [276]. Myocardial cytoskeleton and ultrastructural alterations are of par-amount importance in the study of heart failure and those lesions are thought to be critically important in cardiac mechanics. Among the most recognized factors affecting anthracycline cardiotoxicity, characterized widely as the main pathophysiological hypothesis is oxidative stress. Reactive oxygen species and lipid peroxidation of cardiomyocyte cell membranes pave the way for myocardial functional decline [277]. Mitochondrial dysfunction and genetic and epigenetic changes are also induced by the central role of molecules as Topoisomerase (Top) 2b [278]. Oxidative stress occurs early in the continuum of anthracycline cardiotoxicity, it acts primarily at the subcellular (molecular) level and the myocardial functional decline depends on a function of oxidative stress exposure and time [279]. Thus, pinpointing actual oxidative stress levels where oxidative stress meets overt cardiotoxicity even in animal models is extremely difficult. This review summarizes the range of key biochemical markers related to inflammation and oxidative stress, including cTnT, cTnI, TNFα, IL-6, IL-1β, GSH, GSH-Px, CAT, SOD, and MDA, as well as histopathological findings used to de-scribe anthracycline-induced cardiotoxicity in rats. The review also presents the normal values for these markers as reported in the respective studies. The graphical representations indicate a clear distinction between the normal values and those suppressed due to anthracycline administration. This finding is significant and promising, as it suggests that, when combined with echocardiographic and histopathological data, it could greatly reduce uncertainty and enhance the reliability of weight-of-evidence assessments for potential cardiotoxicity in hu-mans caused by chemicals. Consequently, the results highlight the need for more centralized research, ideally coordinated by a regulatory agency, to effectively establish a set of criteria for classifying cardiotoxicity. Currently, in the evaluation of chemical toxicity, cardiotoxicity is not designated as a separate hazard class for chemical substances under international regulations. Therefore, the detected effects in animal studies, are considered if detected mainly on the tissue level. Hence, chemicals other than pharmaceutical agents are classified as cardiotoxic after having manifested severe effects on humans, based on epidemiological studies. In a previous review of our research team [38], the cardi-ac pathology and function impairment due to exposure to pesticides revealed that several cardiovascular complications have been reported in animal models including electrocardiogram abnormalities, myocardial infarction, impaired systolic and diastolic performance and histopathological findings, such as haemorrhage, vacuolization, signs of apoptosis and degeneration [37]. In addition, there is evidence that short and/or long term exposure to anabolic andro-genic steroids is linked to a variety of cardiovascular complications which could be identified by using echocardiography or biochemical markers [7,38,39]. In the context of recognizing all chemicals that cause cardiotoxicity, studies have also assessed the impact of indole-3-acetic acid (IAA), revealing hypertrophic and fibrotic responses in cardiac tissues, increased oxidative stress, and im-paired cardiac function, supported by biomarker alterations, echocardiographic impairment, and severe histopathological changes [268,269]. The available data clearly indicate the necessity of establishing regulatory criteria to assess cardiotoxicity as an intrinsic property of chemical substances in advance. This would allow for the characterization of exposure risks via a robust regulatory frame-work based on animal models—similar to the approach taken for other human health hazards, such as carcinogenicity. The establishment of regulatory criteria will enable international organizations to promptly detect cardiotoxic effects and classify chemicals, thereby pre-venting long-term cardiovascular complications. Specific classification criteria should be developed based on anatomical, histopathological, echocardiographical, and biochemical findings in animals in a way that could exclude confounding factors in the evaluation of the observed cardiotoxicity. The results of the present study are promising in identifying biochemical and histopathological criteria which could further enhance the validity of echocardiography in evaluating cardiotoxicity. Further studies and meta-analyses are needed to gather additional evidence from other species, commonly used in research, and set values for biochemistry and histopathology for the diagnosis of cardiotoxicity in animal experiments, thus adding significantly to scientific knowledge and the quest for early diagnosis of not-so-known forms of cardiotoxicity beyond anthracyclines and cancer treatment. The diagnostic methods discussed so far in this manuscript have been frequently used for several years. However, it must be noted that in the past years, novel biomarkers of target organ toxicity have been widely used with significant applicability. More specifically, tumor suppressive and oncogenic pathways have been found to involve microRNAs (miRNAs) [40]—microRNAs that are noncoding RNAs that repress the expression of tar-get mRNAs in a post transcriptional way, including apoptosis, differentiation and cancer [41]. Measuring plasma miRNAs and messenger RNAs (mRNAs) reveals the on-going physiological processes in cells and tissues that package and release miRNAs into the extracellular space. Additionally, miRNA assessment is non- or minimally invasive, thereby enhancing animal welfare. Technologically, these molecules are ideal for quantitative analysis due to their rapid standardization and robust performance [42]. Notably, these markers exhibit significant and early changes—reflecting tissue-specific expression—after being released into the plasma during toxic events [43, 44, 45]. These advantages have heightened research interest in circulating miRNAs as promising biomarker candidates.
Relatively novel biomarker as these mentioned above, although of high scientific relevance, their applicability for regulatory purposes is limited due to various reasons, such as restricted availability of data and narrow experience in hazard assessment. Therefore, this review has chosen to concentrate on the most commonly used and traditional biomarkers—those extensively documented in the literature and for which ample data exist. Additionally, the experience gained from risk assessments in the newly established hazard classes for endocrine disruptors indicates that, particularly for regulatory purposes, assessors tend to rely on older data to uphold animal welfare considerations.
In conclusion, both researchers and regulators must intensify their efforts to further support the weight-of-evidence approach and delineate cardiotoxicity as a toxicological hazard class relevant to humans, recognized by regulatory agencies. Specifically, the updated roadmap [48] proposed in this manuscript for de-riving regulatory criteria from animal studies includes the following steps:
- A meta-analysis should be conducted for each line of scientific evidence—such as histopathological, biochemical, and echocardiographic indices—observed in animal species after exposure to established human cardio-toxicants (e.g., anthracyclines). This analysis aims to identify threshold values or ranges that distinguish between normal and altered states resulting from exposure;
- Validation of the previously described evidence in animals that exposed to different alleged cardiotoxic substances (e.g., AAS and pesticides);
- Establish the mechanisms of action by analyzing data from both confirmed and suspected cardiotoxicants, and correlate these with the evidence-based parameters used in developing classification criteria.
- Introduction of novel biomarkers and computational (in silico) methodologies.
Author Contributions
All authors have read and approved the final version of this manuscript. NA, NEK, GP and KT have equally contributed. NA, NEK and GP conducted the review in the context of the master thesis and KT was the main cardiologist of the team. NA, NEK, GP, NG: Performing of the research, collecting data, writing of the research article. KT, CT: Conceptualization of the project, setting criteria for the research, verification of the results, reviewing the manuscript, the statistics and the reference list, overall project management. GENK, J-LD: Data extraction, evaluation of the results, statistical analysis. DK, NG, CT: Overall project overview, data assessment, evaluation of the results, evaluation of the applicability of the findings, reviewing and writing of the re-search article and plan assessment.
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Acknowledgments
Not applicable
Conflicts of Interest
The authors declare no conflict of interest. The positions and opinions presented in this article are those of the authors (Nikolaos Georgiadis, Jean-Lou C. M. Dorne, George E. N. Kass) alone and are not intended to represent the views or any official position or scientific works of the European Agencies EFSA and ECHA.
References
- Park CJ, Branch ME, Vasu S, et al. The Role of Cardiac MRI in Animal Models of Cardiotoxicity: Hopes and Challenges. Journal of Cardiovascular Translational Research Online ahead of print (2020).
- Venkatesh P, Kasi A. Anthracyclines. StatPearls. StatPearls Publishing (2023)
- Johnson M, Keyes D. Anthracycline Toxicity. StatPearls. StatPearls Publishing (2024)
- Schwarz ER, Pollick C, Dow J, et al. A Small Animal Model of Non-Ischemic Cardiomyopathy and Its Evaluation by Transthoracic Echocardiography. Cardiovascular Research 39 (1998): 216-223.
- Robert J. Preclinical Assessment of Anthracycline Cardiotoxicity in Laboratory Animals: Predictiveness and Pitfalls. Cell Biology and Toxicology 23 (2007): 27-37.
- Georgiadis N, Tsarouhas K, Rezaee R, et al. What Is Considered Cardiotoxicity of Anthracyclines in Animal Studies. Oncology Reports 44 (2020): 798-818.
- Germanakis I, Tsarouhas K, Fragkiadaki P, et al. Oxidative Stress and Myocardial Dysfunction in Young Rabbits After Short Term Anabolic Steroids Administration. Food and Chemical Toxicology 61 (2013): 101-105.
- Moher D, Shamseer L, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Systematic Reviews 4 (2015): 1.
- Sharma S, Jackson PG, Makan J. Cardiac Troponins. Journal of Clinical Pathology 57 (2004): 1025-1026.
- Adams JE, Bodor GS, Dávila-Román VG, et al. Cardiac Troponin I. A Marker With High Specificity for Cardiac Injury. Circulation 88 (1993): 101-106.
- Bertinchant JP, Polge A, Juan JM, et al. Evaluation of Cardiac Troponin I and T Levels as Markers of Myocardial Damage in Doxorubicin-Induced Cardiomyopathy Rats, and Their Relationship With Echocardiographic and Histological Findings. Clinica Chimica Acta 329 (2003): 39-51.
- Idriss HT, Naismith JH. TNF Alpha and the TNF Receptor Superfamily: Structure-Function Relationship(s). Microscopy Research and Technique 50 (2000): 184-195.
- Ueland T, Gullestad L, Nymo SH, et al. Inflammatory Cytokines as Biomarkers in Heart Failure. Clinica Chimica Acta 443 (2015): 71-77.
- Schumacher SM, Naga Prasad SV. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Current Cardiology Reports 20 (2018): 117.
- Rose-John S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. International Journal of Biological Sciences 8 (2012): 1237-1247.
- Plenz G, Song ZF, Tjan TD, et al. Activation of the Cardiac Interleukin-6 System in Advanced Heart Failure. European Journal of Heart Failure 3 (2001): 415-421.
- Garbers C, Heink S, Korn T, et al. Interleukin-6: Designing Specific Therapeutics for a Complex Cytokine. Nature Reviews Drug Discovery 17 (2018): 395-412.
- Szekely Y, Arbel Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiology and Therapy 7 (2018): 25-44.
- Dinarello CA. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 117 (2011): 3720-3732.
- Dzoyem JP, Kuete V, Eloff JN. Biochemical Parameters in Toxicological Studies in Africa: Significance, Principle of Methods, Data Interpretation, and Use in Plant Screenings. Toxicological Survey of African Medicinal Plants (2014): 659-715.
- Cordiano R, Di Gioacchino M, Mangifesta R, et al. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 28 (2023): 5979.
- Tan M, Yin Y, Ma X, et al. Glutathione System Enhancement for Cardiac Protection: Pharmacological Options Against Oxidative Stress and Ferroptosis. Cell Death and Disease 14 (2023): 131.
- Brigelius-Flohé R, Maiorino M. Glutathione Peroxidases. Biochimica et Biophysica Acta 1830 (2013): 3289-3303.
- Brigelius-Flohé R, Flohé L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxidants and Redox Signaling 33 (2020): 498-516.
- Chen X, Li J, Kang R, et al. Ferroptosis: Machinery and Regulation. Autophagy 17 (2021): 2054-2081.
- Zhu M, Wang H, Chen J, et al. Sinomenine Improve Diabetic Nephropathy by Inhibiting Fibrosis and Regulating the JAK2/STAT3/SOCS1 Pathway in Streptozotocin-Induced Diabetic Rats. Life Sciences 265 (2021): 118855.
- D’Oria R, Schipani R, Leonardini A, et al. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxidative Medicine and Cellular Longevity 2020 (2020): 5732956.
- Dubois-Deruy E, Peugnet V, Turkieh A, et al. Oxidative Stress in Cardiovascular Diseases. Antioxidants 9 (2020): 864.
- Kugiyama K, Motoyama T, Hirashima O, et al. Vitamin C Attenuates Abnormal Vasomotor Reactivity in Spasm Coronary Arteries in Patients With Coronary Spastic Angina. Journal of the American College of Cardiology 32 (1998): 103-109.
- Kugiyama K, Sugiyama S, Soejima H, et al. Increase in Plasma Levels of Oxidized Low-Density Lipoproteins in Patients With Coronary Spastic Angina. Atherosclerosis 154 (2001): 463-467.
- Younus H. Therapeutic Potentials of Superoxide Dismutase. International Journal of Health Sciences 12 (2018): 88-93.
- Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine, Chapter 2. Oxidative Stress: Adaptation, Damage, Repair and Death. Oxford Science Publications, New York (1999): 36-104.
- Chang E, Lee M, Onwuanyi A. Monitoring of Chemotherapy Induced Left Ventricular Systolic Dysfunction in Breast Cancer Patients. Journal of the American College of Cardiology 77 Suppl 1 (2021): 3326.
- Terada CI, Onoue K, Fujii T, et al. Histopathological and Epigenetic Changes in Myocardium Associated With Cancer Therapy-Related Cardiac Dysfunction. ESC Heart Failure 9 (2022): 3031-3043.
- O’Connell JL, Romano MMD, Pulici ECC, et al. Short-Term and Long-Term Models of Doxorubicin-Induced Cardiomyopathy in Rats: A Comparison of Functional and Histopathological Changes. Experimental and Toxicologic Pathology 69 (2017): 213-219.
- Zhang J, Li X, Liu J, et al. Early and Dynamic Detection of Doxorubicin Induced Cardiotoxicity by Myocardial Contrast Echocardiography Combined With Two-Dimensional Speckle Tracking Echocardiography in Rats. Frontiers in Cardiovascular Medicine 9 (2023).
- Georgiadis N, Tsarouhas K, Tsitsimpikou C, et al. Pesticides and Cardiotoxicity. Where Do We Stand? Toxicology and Applied Pharmacology 353 (2018): 1-14.
- Germanakis I, Tsarouhas K, Fragkiadaki P, et al. Oxidative Stress and Myocardial Dysfunction in Young Rabbits After Short Term Anabolic Steroids Administration. Food and Chemical Toxicology 61 (2013): 101-105.
- Vasilaki F, Tsitsimpikou C, Tsarouhas K, et al. Cardiotoxicity in Rabbits After Long-Term Nandrolone Decanoate Administration. Toxicology Letters 241 (2016): 143-151.
- Achar S, Rostamian A, Narayan SM. Cardiac and Metabolic Effects of Anabolic-Androgenic Steroid Abuse on Lipids, Blood Pressure, Left Ventricular Dimensions, and Rhythm. American Journal of Cardiology 106 (2010): 893-901.
- Jackstadt R, Hermeking H. MicroRNAs as Regulators and Mediators of c-MYC Function. Biochimica et Biophysica Acta Gene Regulatory Mechanisms 1849 (2015): 544-553.
- Gomez de Cedron M, Ramirez de Molina A. Microtargeting Cancer Metabolism: Opening New Therapeutic Windows Based on Lipid Metabolism. Journal of Lipid Research 57 (2016): 193-206.
- Schraml E, Hackl M, Grillari J. MicroRNAs and Toxicology: A Love Marriage. Toxicology Reports 4 (2017): 634-636.
- Mikaelian I, Scicchitano M, Mendes O, et al. Frontiers in Preclinical Safety Biomarkers: MicroRNAs and Messenger RNAs. Toxicologic Pathology 41 (2013): 18-31.
- French D, Wu AHB. Cardiac Markers. In: Wild D, ed. The Immunoassay Handbook. 4th ed. Elsevier, Amsterdam (2013): 817-831.
- Yuan M, Zang L, Xu A, et al. Dynamic Changes of Serum Heart Type-Fatty Acid Binding Protein in Cancer Patients Treated With Immune Checkpoint Inhibitors. Frontiers in Pharmacology 12 (2021): 748677.
- Manrique C, Park M, Tiwari N, et al. Diagnostic Strategies for Early Recognition of Cancer Therapeutics-Related Cardiac Dysfunction. Clinical Medicine Insights: Cardiology 11 (2017): 1179546817697983.
- Georgiadis N, Tsarouhas K, Dorne JLCM, et al. Cardiotoxicity of Chemical Substances: An Emerging Hazard Class. Journal of Cardiovascular Development and Disease 9 (2022): 226.
- de Vries JE. Immunosuppressive and Anti-Inflammatory Properties of Interleukin 10. Annals of Medicine 27 (1995): 537-541.
- Von Der Thüsen JH, Kuiper J, Fekkes ML, et al. Attenuation of Atherogenesis by Systemic and Local Adenovirus-Mediated Gene Transfer of Interleukin-10 in LDLr-/- Mice. FASEB Journal 15 (2001): 2730-2732.
- Madeddu C, Deidda M, Piras A, et al. Pathophysiology of Cardiotoxicity Induced by Nonanthracycline Chemotherapy. Journal of Cardiovascular Medicine 17 Suppl 1 (2016): e12-e18.
- Abdelatty A, Ahmed MS, Abdel-Kareem MA, et al. Acute and Delayed Doxorubicin-Induced Myocardiotoxicity Associated With Elevation of Cardiac Biomarkers, Depletion of Cellular Antioxidant Enzymes, and Several Histopathological and Ultrastructural Changes. Life 11 (2021): 880.
- Abdelrahman AM, Al Suleimani YM, Manoj P, et al. Effect of Infliximab, a Tumor Necrosis Factor-Alpha Inhibitor, on Doxorubicin-Induced Nephrotoxicity in Rats. Naunyn-Schmiedeberg's Archives of Pharmacology 393 (2020): 121-130.
- Aljumaily SA, Demir M, Elbe H, et al. Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects of Crocin Against Doxorubicin-Induced Myocardial Toxicity in Rats. Environmental Science and Pollution Research International 28 (2021): 65802-65813.
- Adeyemi DH, Obembe OO, Hamed MA, et al. Sodium Acetate Ameliorates Doxorubicin-Induced Cardiac Injury via Upregulation of Nrf2/HO-1 Signaling and Downregulation of NFkB-Mediated Apoptotic Signaling in Wistar Rats. Naunyn-Schmiedeberg's Archives of Pharmacology 397 (2024): 423-435.
- Adiyaman MS, Adiyaman ÖA, Dagl? AF, et al. Prevention of Doxorubicin-Induced Experimental Cardiotoxicity by Nigella Sativa in Rats. Revista Portuguesa de Cardiologia 41 (2022): 99-105.
- Adiyaman MS, Adiyaman ÖA, Dagli AF, et al. Effects of Grapeseed Extract on Doxorubicin-Induced Cardiotoxicity in Rats. Herz 46 Suppl 1 (2021): 103-108.
- Ahmed AZ, Mumbrekar KD, Satyam SM, et al. Chia Seed Oil Ameliorates Doxorubicin-Induced Cardiotoxicity in Female Wistar Rats: An Electrocardiographic, Biochemical and Histopathological Approach. Cardiovascular Toxicology 21 (2021): 533-542.
- Ahmed AZ, Satyam SM, Shetty P, et al. Methyl Gallate Attenuates Doxorubicin-Induced Cardiotoxicity in Rats by Suppressing Oxidative Stress. Scientifica 2021 (2021): 6694340.
- Ahmed LA, Abdou FY, El Fiky AA, et al. Bradykinin-Potentiating Activity of a Gamma-Irradiated Bioactive Fraction Isolated From Scorpion (Leiurus Quinquestriatus) Venom in Rats With Doxorubicin-Induced Acute Cardiotoxicity: Favorable Modulation of Oxidative Stress and Inflammatory, Fibrogenic and Apoptotic Pathways. Cardiovascular Toxicology 21 (2021): 127-141.
- Aktay I, Bitirim CV, Olgar Y, et al. Cardioprotective Role of a Magnolol and Honokiol Complex in the Prevention of Doxorubicin-Mediated Cardiotoxicity in Adult Rats. Molecular and Cellular Biochemistry (2023): Advance online publication.
- Alanazi AM, Fadda L, Alhusaini A, et al. Liposomal Resveratrol and/or Carvedilol Attenuate Doxorubicin-Induced Cardiotoxicity by Modulating Inflammation, Oxidative Stress and S100A1 in Rats. Antioxidants 9 (2020): 159.
- Alhazzani K, Alotaibi MR, Alotaibi FN, et al. Protective Effect of Valsartan Against Doxorubicin-Induced Cardiotoxicity: Histopathology and Metabolomics In Vivo Study. Journal of Biochemical and Molecular Toxicology 35 (2021): e22842.
- Al-Kenany SA, Al-Shawi NN. Protective Effect of Cafestol Against Doxorubicin-Induced Cardiotoxicity in Rats by Activating the Nrf2 Pathway. Frontiers in Pharmacology 14 (2023): 1206782.
- Alkhanjaf AAM, Athar MT, Ullah Z, et al. Farnesol Protects Against Cardiotoxicity Caused by Doxorubicin-Induced Stress, Inflammation, and Cell Death: An In Vivo Study in Wistar Rats. Molecules 27 (2022): 8589.
- Al-Taee H, Azimullah S, Meeran MFN, et al. β-Caryophyllene, a Dietary Phytocannabinoid Attenuates Oxidative Stress, Inflammation, Apoptosis and Prevents Structural Alterations of the Myocardium Against Doxorubicin-Induced Acute Cardiotoxicity in Rats: An In Vitro and In Vivo Study. European Journal of Pharmacology 858 (2019): 172467.
- Alyasiry E, Janabi A, Hadi N. Dipyridamole Ameliorates Doxorubicin-Induced Cardiotoxicity. Journal of Medicine and Life 15 (2022): 1184-1190.
- Amin FM, Sharawy MH, Amin MN, El-Sherbiny M, et al. Nifuroxazide Mitigates Doxorubicin-Induced Cardiovascular Injury: Insight Into Oxidative/NLRP3/GSDMD-Mediated Pyroptotic Signaling Modulation. Life Sciences 314 (2023): 121311.
- Andreadou I, Mikros E, Ioannidis K, et al. Oleuropein Prevents Doxorubicin-Induced Cardiomyopathy Interfering With Signaling Molecules and Cardiomyocyte Metabolism. Journal of Molecular and Cellular Cardiology 69 (2014): 4-16.
- Argun M, Üzüm K, Sönmez MF, et al. Cardioprotective Effect of Metformin Against Doxorubicin Cardiotoxicity in Rats. Anatolian Journal of Cardiology 16 (2016): 234-241.
- Arinno A, Maneechote C, Khuanjing T, et al. Cardioprotective Effects of Melatonin and Metformin Against Doxorubicin-Induced Cardiotoxicity in Rats Are Through Preserving Mitochondrial Function and Dynamics. Biochemical Pharmacology 192 (2021): 114743.
- Arozal W, Watanabe K, Veeraveedu PT, et al. Effect of Telmisartan in Limiting the Cardiotoxic Effect of Daunorubicin in Rats. Journal of Pharmacy and Pharmacology 62 (2010): 1776-1783.
- Arunachalam S, Nagoor Meeran MF, Azimullah S, et al. Nerolidol Attenuates Oxidative Stress, Inflammation, and Apoptosis by Modulating Nrf2/MAPK Signaling Pathways in Doxorubicin-Induced Acute Cardiotoxicity in Rats. Antioxidants 10 (2021): 984.
- Avagimyan A, Sheibani M, Pogosova N, et al. Possibilities of Dapagliflozin-Induced Cardioprotection on Doxorubicin + Cyclophosphamide Mode of Chemotherapy-Induced Cardiomyopathy. International Journal of Cardiology 391 (2023): 131331.
- Awad HH, El-Derany MO, Mantawy EM, et al. Comparative Study on Beneficial Effects of Vitamins B and D in Attenuating Doxorubicin Induced Cardiotoxicity in Rats: Emphasis on Calcium Homeostasis. Biomedicine and Pharmacotherapy 140 (2021): 111679.
- Aykan DA, Yaman S, Eser N, et al. Bisoprolol and Linagliptin Ameliorated Electrical and Mechanical Isometric Myocardial Contractions in Doxorubicin-Induced Cardiomyopathy in Rats. Pharmacological Reports 72 (2020): 867-876.
- Aziz MM, Abd El Fattah MA, Ahmed KA, et al. Protective Effects of Olmesartan and L-Carnitine on Doxorubicin-Induced Cardiotoxicity in Rats. Canadian Journal of Physiology and Pharmacology 98 (2020): 183-193.
- Babaei H, Razmaraii N, Assadnassab G, et al. Ultrastructural and Echocardiographic Assessment of Chronic Doxorubicin-Induced Cardiotoxicity in Rats. Archives of Razi Institute 75 (2020): 55-62.
- Babaei-Kouchaki S, Babapour V, Panahi N, et al. Effect of Troxerutin on Oxidative Stress and Expression of Genes Regulating Mitochondrial Biogenesis in Doxorubicin-Induced Myocardial Injury in Rats. Naunyn-Schmiedeberg's Archives of Pharmacology 393 (2020): 1187-1195.
- Baniahmad B, Safaeian L, Vaseghi G, et al. Cardioprotective Effect of Vanillic Acid Against Doxorubicin-Induced Cardiotoxicity in Rat. Research in Pharmaceutical Sciences 15 (2020): 87-96.
- Baris VÖ, Dinçsoy AB, Gedikli E, et al. Empagliflozin Significantly Prevents the Doxorubicin-Induced Acute Cardiotoxicity via Non-Antioxidant Pathways. Cardiovascular Toxicology 21 (2021): 747-758.
- Belhan S, Özkaraca M, Özdek U, et al. Protective Role of Chrysin on Doxorubicin-Induced Oxidative Stress and DNA Damage in Rat Testes. Andrologia 52 (2020): e13747.
- Bin Jardan YA, Ansari MA, Raish M, et al. Sinapic Acid Ameliorates Oxidative Stress, Inflammation, and Apoptosis in Acute Doxorubicin-Induced Cardiotoxicity via the NF-κB-Mediated Pathway. BioMed Research International 2020 (2020): 3921796.
- Birari L, Wagh S, Patil KR, et al. Aloin Alleviates Doxorubicin-Induced Cardiotoxicity in Rats by Abrogating Oxidative Stress and Pro-Inflammatory Cytokines. Cancer Chemotherapy and Pharmacology 86 (2020): 419-426.
- Botelho AFM, Lempek MR, Branco SEMT, et al. Coenzyme Q10 Cardioprotective Effects Against Doxorubicin-Induced Cardiotoxicity in Wistar Rat. Cardiovascular Toxicology 20 (2020): 222-234.
- Bradic J, Andjic M, Novakovic J, et al. Lady’s Bedstraw as a Powerful Antioxidant for Attenuation of Doxorubicin-Induced Cardiotoxicity. Antioxidants 12 (2023): 1277.
- Carresi C, Musolino V, Gliozzi M, et al. Anti-Oxidant Effect of Bergamot Polyphenolic Fraction Counteracts Doxorubicin-Induced Cardiomyopathy: Role of Autophagy and c-kit+CD45-CD31- Cardiac Stem Cell Activation. Journal of Molecular and Cellular Cardiology 119 (2018): 10-18.
- Carvalho PB, Gonçalves AF, Alegre PH, et al. Pamidronate Attenuates Oxidative Stress and Energetic Metabolism Changes but Worsens Functional Outcomes in Acute Doxorubicin-Induced Cardiotoxicity in Rats. Cellular Physiology and Biochemistry 40 (2016): 431-442.
- Chaudhary R, Singh R, Verma R, et al. Investigation on Protective Effect of Terminalia bellirica (Roxb.) Against Drugs Induced Cardiotoxicity in Wistar Albino Rats. Journal of Ethnopharmacology 261 (2020): 113080.
- Chen M. Empagliflozin Attenuates Doxorubicin-Induced Cardiotoxicity by Activating AMPK/SIRT-1/PGC-1α-Mediated Mitochondrial Biogenesis. Toxicology Research 12 (2023): 216-223.
- Chen FX, Wan Q, Li QL, et al. Substance P Prevents Doxorubicin Induced Cardiomyocyte Injury by Regulating Apoptosis and Autophagy: In Vitro and In Vivo Evidence. Molecular Medicine Reports 25 (2022): 50.
- Chen J, Zhang S, Pan G, et al. Modulatory Effect of Metformin on Cardiotoxicity Induced by Doxorubicin via the MAPK and AMPK Pathways. Life Sciences 249 (2020): 117498.
- Chen Y, Wang L, Liu T, et al. Inhibitory Effects of Panax ginseng Glycoproteins in Models of Doxorubicin-Induced Cardiac Toxicity In Vivo and In Vitro. Food and Function 12 (2021): 10862-10874.
- Cheng D, Chen L, Tu W, et al. Protective Effects of Valsartan Administration on Doxorubicin Induced Myocardial Injury in Rats and the Role of Oxidative Stress and NOX2/NOX4 Signaling. Molecular Medicine Reports 22 (2020): 4151-4162.
- Chu X, Zhang Y, Xue Y, et al. Crocin Protects Against Cardiotoxicity Induced by Doxorubicin Through TLR-2/NF-κB Signal Pathway In Vivo and In Vitro. International Immunopharmacology 84 (2020): 106548.
- Chunchai T, Arinno A, Ongnok B, et al. Ranolazine Alleviated Cardiac/Brain Dysfunction in Doxorubicin-Treated Rats. Experimental and Molecular Pathology 127 (2022): 104818.
- Cicek B, Hacimuftuoglu A, Yeni Y, et al. Chlorogenic Acid Attenuates Doxorubicin-Induced Oxidative Stress and Markers of Apoptosis in Cardiomyocytes via Nrf2/HO-1 and Dityrosine Signaling. Journal of Personalized Medicine 13 (2023): 649.
- da Cunha Menezes Souza L, Fernandes FH, Presti PT, et al. Effect of Doxorubicin on Cardiac Lipid Metabolism-Related Transcriptome and the Protective Activity of Alda-1. European Journal of Pharmacology 898 (2021): 173955.
- Dantas D, Pereira AG, Fujimori ASS, et al. Doxycycline Attenuates Doxorubicin-Induced Cardiotoxicity by Improving Myocardial Energy Metabolism in Rats. Journal of Cardiovascular Development and Disease 9 (2022): 254.
- Devi Sampath P, Vijayaraghavan K. Cardioprotective Effect of Alpha-Mangostin, a Xanthone Derivative From Mangosteen on Tissue Defense System Against Isoproterenol-Induced Myocardial Infarction in Rats. Journal of Biochemical and Molecular Toxicology 21 (2007): 336-339.
- Dorostkar H, Haghiralsadat BF, Hemati M, et al. Reduction of Doxorubicin-Induced Cardiotoxicity by Co-Administration of Smart Liposomal Doxorubicin and Free Quercetin: In Vitro and In Vivo Studies. Pharmaceutics 15 (2023): 1920.
- Du XY, Xiang DC, Gao P, et al. Inhibition of (Pro)renin Receptor-Mediated Oxidative Stress Alleviates Doxorubicin-Induced Heart Failure. Frontiers in Oncology 12 (2022): 874852.
- Dulf PL, Mocan M, Coada CA, et al. Doxorubicin-Induced Acute Cardiotoxicity Is Associated With Increased Oxidative Stress, Autophagy, and Inflammation in a Murine Model. Naunyn-Schmiedeberg's Archives of Pharmacology 396 (2023): 1105-1115.
- Durdagi G, Pehlivan DY, Oyar EO, et al. Effects of Melatonin and Adrenomedullin in Reducing the Cardiotoxic Effects of Doxorubicin in Rats. Cardiovascular Toxicology 21 (2021): 354-364.
- Efentakis P, Varela A, Chavdoula E, et al. Levosimendan Prevents Doxorubicin-Induced Cardiotoxicity in Time- and Dose-Dependent Manner: Implications for Inotropy. Cardiovascular Research 116 (2020): 576-591.
- Eid BG, El-Shitany NAE, Neamatallah T. Trimetazidine Improved Adriamycin-Induced Cardiomyopathy by Downregulating TNF-α, BAX, and VEGF Immunoexpression via an Antioxidant Mechanism. Environmental Toxicology 36 (2021): 1217-1225.
- Eisvand F, Imenshahidi M, Ghasemzadeh Rahbardar M, et al. Cardioprotective Effects of Alpha-Mangostin on Doxorubicin-Induced Cardiotoxicity in Rats. Phytotherapy Research 36 (2022): 506-524.
- Ekinci Akdemir FN, Yildirim S, Kandemir FM, et al. Protective Effects of Gallic Acid on Doxorubicin-Induced Cardiotoxicity: An Experimental Study. Archives of Physiology and Biochemistry 127 (2021): 258-265.
- Elblehi SS, El-Sayed YS, Soliman MM, et al. Date Palm Pollen Extract Averts Doxorubicin-Induced Cardiomyopathy Fibrosis and Associated Oxidative/Nitrosative Stress, Inflammatory Cascade, and Apoptosis-Targeting Bax/Bcl-2 and Caspase-3 Signaling Pathways. Animals 11 (2021): 886.
- Elmorsi RM, Kabel AM, El Saadany AA, et al. The Protective Effects of Topiramate and Spirulina Against Doxorubicin-Induced Cardiotoxicity in Rats. Human and Experimental Toxicology 42 (2023): 9603271231198624.
- Elnoury HA, Elgendy SA, Baloza SH, et al. Synergistic Impacts of Montelukast and Klotho Against Doxorubicin-Induced Cardiac Toxicity in Rats. Toxicology Research 11 (2022): 592-604.
- Fan Y, Liang L, Tang X, et al. Changes in the Gut Microbiota Structure and Function in Rats With Doxorubicin-Induced Heart Failure. Frontiers in Cellular and Infection Microbiology 13 (2023): 1135428.
- Feitosa LAS, Carvalho JDS, Dantas CO, et al. Resistance Training Improves Cardiac Function and Cardiovascular Autonomic Control in Doxorubicin-Induced Cardiotoxicity. Cardiovascular Toxicology 21 (2021): 365-374.
- Feng P, Yang Y, Liu N, et al. Baicalin Regulates TLR4/IκBα/NFκB Signaling Pathway to Alleviate Inflammation in Doxorubicin Related Cardiotoxicity. Biochemical and Biophysical Research Communications 637 (2022): 1-8.
- Freiwan M, Kovács MG, Kovács ZZA, et al. Investigation of the Antiremodeling Effects of Losartan, Mirabegron and Their Combination on the Development of Doxorubicin-Induced Chronic Cardiotoxicity in a Rat Model. International Journal of Molecular Sciences 23 (2022): 2201.
- Furcea DM, Agrigoroaie L, Mihai CT, et al. 18F-FDG PET/MRI Imaging in a Preclinical Rat Model of Cardiorenal Syndrome—An Exploratory Study. International Journal of Molecular Sciences 23 (2022): 15409.
- Gao Y, Yang H, Fan Y, et al. Hydrogen-Rich Saline Attenuates Cardiac and Hepatic Injury in Doxorubicin Rat Model by Inhibiting Inflammation and Apoptosis. Mediators of Inflammation 2016 (2016): 1320365.
- Gao Y, Yang H, Fan Y, et al. Corrigendum to “Hydrogen-Rich Saline Attenuates Cardiac and Hepatic Injury in Doxorubicin Rat Model by Inhibiting Inflammation and Apoptosis”. Mediators of Inflammation 2017 (2017): 3675910.
- Ghaleb Z, Rizij FA, Hadi NR. Potential Role of Vitamin D3 in Ameliorating Doxorubicin Induced Cardiotoxicity in Male Rats. Wiadomosci Lekarskie 74 (2021): 3152-3155.
- Hafez HM, Hassanein H. Montelukast Ameliorates Doxorubicin-Induced Cardiotoxicity via Modulation of P-Glycoprotein and Inhibition of ROS-Mediated TNF-α/NF-κB Pathways. Drug and Chemical Toxicology 45 (2022): 548-559.
- Hanna M, Seddiek H, Aboulhoda BE, et al. Synergistic Cardioprotective Effects of Melatonin and Deferoxamine Through the Improvement of Ferritinophagy in Doxorubicin-Induced Acute Cardiotoxicity. Frontiers in Physiology 13 (2022): 1050598.
- Hassan MQ, Akhtar MS, Afzal O, et al. Edaravone and Benidipine Protect Myocardial Damage by Regulating Mitochondrial Stress, Apoptosis Signalling and Cardiac Biomarkers Against Doxorubicin-Induced Cardiotoxicity. Clinical and Experimental Hypertension 42 (2020): 381-392.
- Hekmat AS, Navabi Z, Alipanah H, et al. Alamandine Significantly Reduces Doxorubicin-Induced Cardiotoxicity in Rats. Human and Experimental Toxicology 40 (2021): 1781-1795.
- Hiona A, Lee AS, Nagendran J, et al. Pretreatment With Angiotensin-Converting Enzyme Inhibitor Improves Doxorubicin-Induced Cardiomyopathy via Preservation of Mitochondrial Function. Journal of Thoracic and Cardiovascular Surgery 142 (2011): 396-403.e3.
- Hosseini A, Safari MK, Rajabian A, et al. Cardioprotective Effect of Rheum turkestanicum Against Doxorubicin-Induced Toxicity in Rats. Frontiers in Pharmacology 13 (2022): 909079.
- Hu X, Li C, Wang Q, et al. Dimethyl Fumarate Ameliorates Doxorubicin-Induced Cardiotoxicity by Activating the Nrf2 Pathway. Frontiers in Pharmacology 13 (2022): 872057.
- Huang J, Lei Y, Lei S, et al. Cardioprotective Effects of Corilagin on Doxorubicin-Induced Cardiotoxicity via PI3K/Akt and NF-κB Signaling Pathways in a Rat Model. Toxicology Mechanisms and Methods 32 (2022): 79-86.
- Hung YC, Wang PW, Lin TY, et al. Functional Redox Proteomics Reveal That Salvia miltiorrhiza Aqueous Extract Alleviates Adriamycin-Induced Cardiomyopathy via Inhibiting ROS-Dependent Apoptosis. Oxidative Medicine and Cellular Longevity 2020 (2020): 5136934.
- Ibrahim Fouad G, Ahmed KA. Curcumin Ameliorates Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity via Suppressing Oxidative Stress and Modulating iNOS, NF-κB, and TNF-α in Rats. Cardiovascular Toxicology 22 (2022): 152-166.
- Ikewuchi JC, Ikewuchi CC, Ifeanacho MO, et al. Attenuation of Doxorubicin-Induced Cardiotoxicity in Wistar Rats by Aqueous Leaf-Extracts of Chromolaena odorata and Tridax procumbens. Journal of Ethnopharmacology 274 (2021): 114004.
- Jang YJ, Lee D, Hossain MA, et al. Korean Red Ginseng Enhances Cardiac Hemodynamics on Doxorubicin-Induced Toxicity in Rats. Journal of Ginseng Research 44 (2020): 483-489.
- Jayasinghe AN, Hewawasam RP, Jayatilaka KAPW, et al. Cardioprotective Potential of Murraya koenigii (L.) Spreng. Leaf Extract Against Doxorubicin-Induced Cardiotoxicity in Rats. Evidence-Based Complementary and Alternative Medicine 2020 (2020): 6023737.
- Kabel AM, Salama SA, Adwas AA, et al. Targeting Oxidative Stress, NLRP3 Inflammasome, and Autophagy by Fraxetin to Combat Doxorubicin-Induced Cardiotoxicity. Pharmaceuticals 14 (2021): 1188.
- Karabulut D, Ozturk E, Kaymak E, et al. Thymoquinone Attenuates Doxorubicin-Cardiotoxicity in Rats. Journal of Biochemical and Molecular Toxicology 35 (2021): e22618.
- Karakuyu NF, Savran M, Candan IA, et al. Investigation of Cardioprotective Effect of Lercanidipine on Doxorubicin-Induced Cardiotoxicity. Naunyn-Schmiedeberg's Archives of Pharmacology 396 (2023): 3635-3645.
- Khajavi Rad A, Entezari Heravi N, Kamkar-Del Y, et al. A Standardized Extract of Ziziphus jujuba Mill Protects Against Adriamycin-Induced Liver, Heart, and Brain Toxicity: An Oxidative Stress and Biochemical Approach. Journal of Food Biochemistry 45 (2021): e13698.
- Khosroshahi AJ, Mokhtari B, Badalzadeh R. Combination of Nicotinamide Mononucleotide and Troxerutin Induces Full Protection Against Doxorubicin-Induced Cardiotoxicity by Modulating Mitochondrial Biogenesis and Inflammatory Response. Molecular Biology Reports 49 (2022): 8209-8218.
- Khuanjing T, Ongnok B, Maneechote C, et al. Acetylcholinesterase Inhibitor Ameliorates Doxorubicin-Induced Cardiotoxicity Through Reducing RIP1-Mediated Necroptosis. Pharmacological Research 173 (2021): 105882.
- Kihara M, Kaiya H, Hirai Y, et al. Salmon Acyl-Ghrelin Increases Food Intake and Reduces Doxorubicin-Induced Myocardial Apoptosis in Rats, Likely by Anti-Oxidative Activity. Peptides 137 (2021): 170471.
- Kondru SK, Potnuri AG, Allakonda L, et al. Histamine 2 Receptor Antagonism Elicits Protection Against Doxorubicin-Induced Cardiotoxicity in Rodent Model. Molecular and Cellular Biochemistry 441 (2018): 77-88.
- Lai Y, Zhou X, Guo F, et al. Non-Invasive Transcutaneous Vagal Nerve Stimulation Improves Myocardial Performance in Doxorubicin-Induced Cardiotoxicity. Cardiovascular Research 118 (2022): 1821-1834.
- Li H, Xia B, Chen W, et al. Nimbolide Prevents Myocardial Damage by Regulating Cardiac Biomarkers, Antioxidant Level, and Apoptosis Signaling Against Doxorubicin-Induced Cardiotoxicity in Rats. Journal of Biochemical and Molecular Toxicology 34 (2020): e22543.
- Li L, Wu B, Zhao Q, et al. Attenuation of Doxorubicin-Induced Cardiotoxicity by Cryptotanshinone Detected Through Association Analysis of Transcriptomic Profiling and KEGG Pathway. Aging 12 (2020): 9585-9603.
- Li Z, Chinnathambi A, Ali Alharbi S, et al. Plumbagin Protects the Myocardial Damage by Modulating the Cardiac Biomarkers, Antioxidants, and Apoptosis Signaling in the Doxorubicin-Induced Cardiotoxicity in Rats. Environmental Toxicology 35 (2020): 1374-1385.
- Liao W, Rao Z, Wu L, et al. Cariporide Attenuates Doxorubicin-Induced Cardiotoxicity in Rats by Inhibiting Oxidative Stress, Inflammation and Apoptosis Partly Through Regulation of Akt/GSK-3β and Sirt1 Signaling Pathway. Frontiers in Pharmacology 13 (2022): 850053.
- Lin H, Meng L, Sun Z, et al. Yellow Wine Polyphenolic Compound Protects Against Doxorubicin-Induced Cardiotoxicity by Modulating the Composition and Metabolic Function of the Gut Microbiota. Circulation: Heart Failure 14 (2021): e008220.
- Liu X, Li D, Pi W, et al. LCZ696 Protects Against Doxorubicin-Induced Cardiotoxicity by Inhibiting Ferroptosis via AKT/SIRT3/SOD2 Signaling Pathway Activation. International Immunopharmacology 113 (2022): 109379.
- Liu X, Qiu Y, Huang N, et al. Citronellal Alleviates Doxorubicin-Induced Cardiotoxicity by Suppressing Oxidative Stress and Apoptosis via Na+/H+ Exchanger-1 Inhibition. Journal of Biochemical and Molecular Toxicology 36 (2022): e22971.
- Lu XL, Tong YF, Liu Y, et al. Gαq Protein Carboxyl Terminus Imitation Polypeptide GCIP-27 Improves Cardiac Function in Chronic Heart Failure Rats. PLoS ONE 10 (2015): e0121007.
- Lv X, Zhu Y, Deng Y, et al. Glycyrrhizin Improved Autophagy Flux via HMGB1-Dependent Akt/mTOR Signaling Pathway to Prevent Doxorubicin-Induced Cardiotoxicity. Toxicology 441 (2020): 152508.
- Ma H, Kong J, Wang YL, et al. Angiotensin-Converting Enzyme 2 Overexpression Protects Against Doxorubicin-Induced Cardiomyopathy by Multiple Mechanisms in Rats. Oncotarget 8 (2017): 24548-24563.
- Ma T, Yang L, Zhang B, et al. Hydrogen Inhalation Enhances Autophagy via the AMPK/mTOR Pathway, Thereby Attenuating Doxorubicin-Induced Cardiac Injury. International Immunopharmacology 119 (2023): 110071.
- Majhi S, Singh L, Yasir M. Evaluation of Ameliorative Effect of Quercetin and Candesartan in Doxorubicin-Induced Cardiotoxicity. Vascular Health and Risk Management 18 (2022): 857-866.
- Maleki F, Rabbani S, Shirkoohi R, et al. Allogeneic Mitochondrial Transplantation Ameliorates Cardiac Dysfunction Due to Doxorubicin: An In Vivo Study. Biomedicine and Pharmacotherapy 168 (2023): 115651.
- Maleki F, Salimi M, Shirkoohi R, et al. Mitotherapy in Doxorubicin-Induced Cardiotoxicity: A Promising Strategy to Reduce the Complications of Treatment. Life Sciences 304 (2022): 120701.
- Malik A, Khan A, Mahmood Q, et al. In Vivo and In Silico Assessment of the Cardioprotective Effect of Thymus linearis Extract Against Ischemic Myocardial Injury. ACS Omega 7 (2022): 43635-43646.
- Manna P, Dewanjee S, Joardar S, et al. Carnosic Acid Attenuates Doxorubicin-Induced Cardiotoxicity by Decreasing Oxidative Stress and Its Concomitant Pathological Consequences. Food and Chemical Toxicology 166 (2022): 113205.
- Mao M, Zheng W, Deng B, et al. Cinnamaldehyde Alleviates Doxorubicin-Induced Cardiotoxicity by Decreasing Oxidative Stress and Ferroptosis in Cardiomyocytes. PLoS ONE 18 (2023): e0292124.
- Mathias LMBS, Alegre PHC, Dos Santos IOF, et al. Euterpe oleracea Mart. (Açai) Supplementation Attenuates Acute Doxorubicin-Induced Cardiotoxicity in Rats. Cellular Physiology and Biochemistry 53 (2019): 388-399.
- Meeran MFN, Azimullah S, Mamoudh HH, et al. Nerolidol, a Sesquiterpene From the Essential Oils of Aromatic Plants, Attenuates Doxorubicin-Induced Chronic Cardiotoxicity in Rats. Journal of Agricultural and Food Chemistry 69 (2021): 7334-7343.
- Meicong C. Empagliflozin Attenuates Doxorubicin-Induced Cardiotoxicity by Activating AMPK/SIRT-1/PGC-1α-Mediated Mitochondrial Biogenesis. Toxicology Research 12 (2023): 216-223.
- Miyoshi T, Nakamura K, Amioka N, et al. LCZ696 Ameliorates Doxorubicin-Induced Cardiomyocyte Toxicity in Rats. Scientific Reports 12 (2022): 4930.
- Modesto PN, Polegato BF, Dos Santos PP, et al. Green Tea (Camellia sinensis) Extract Increased Topoisomerase IIβ, Improved Antioxidant Defense, and Attenuated Cardiac Remodeling in an Acute Doxorubicin Toxicity Model. Oxidative Medicine and Cellular Longevity 2021 (2021): 8898919.
- Mohammed HS, Hosny EN, Khadrawy YA, et al. Protective Effect of Curcumin Nanoparticles Against Cardiotoxicity Induced by Doxorubicin in Rat. Biochimica et Biophysica Acta: Molecular Basis of Disease 1866 (2020): 165665.
- Montalvo RN, Doerr V, Kwon OS, et al. Protection Against Doxorubicin-Induced Cardiac Dysfunction Is Not Maintained Following Prolonged Autophagy Inhibition. International Journal of Molecular Sciences 21 (2020): 8105.
- Moriyama T, Kemi M, Horie T. Elevated Cardiac 3-Deoxyglucosone, a Highly Reactive Intermediate in Glycation Reaction, in Doxorubicin-Induced Cardiotoxicity in Rats. Pathophysiology 23 (2016): 237-242.
- Morsy MA, Abdel-Gaber SA, Mokhemer SA, et al. Pregnenolone Inhibits Doxorubicin-Induced Cardiac Oxidative Stress, Inflammation, and Apoptosis—Role of Matrix Metalloproteinase 2 and NADPH Oxidase 1. Pharmaceuticals 16 (2023): 665.
- Mota F, Pell VR, Singh N, et al. A Reactivity-Based 18F-Labeled Probe for PET Imaging of Oxidative Stress in Chemotherapy-Induced Cardiotoxicity. Molecular Pharmaceutics 19 (2022): 18-25.
- Motlagh PE, Novin AG, Ghahari F, et al. Evaluation of the Effect of Crocin on Doxorubicin-Induced Cardiotoxicity. Advances in Experimental Medicine and Biology 1328 (2021): 143-153.
- Naderi Y, Khosraviani S, Nasiri S, et al. Cardioprotective Effects of Minocycline Against Doxorubicin-Induced Cardiotoxicity. Biomedicine and Pharmacotherapy 158 (2023): 114055.
- Nagoor Meeran MF, Arunachalam S, Azimullah S, et al. α-Bisabolol, a Dietary Sesquiterpene, Attenuates Doxorubicin-Induced Acute Cardiotoxicity in Rats by Inhibiting Cellular Signaling Pathways, Nrf2/Keap-1/HO-1, Akt/mTOR/GSK-3β, NF-κB/p38/MAPK, and NLRP3 Inflammasomes. International Journal of Molecular Sciences 24 (2023): 14013.
- Nordgren KKS, Wallace KB. Disruption of the Keap1/Nrf2-Antioxidant Response System After Chronic Doxorubicin Exposure In Vivo. Cardiovascular Toxicology 20 (2020): 557-570.
- Ogonowski N, Rukavina Mikusic NL, Kouyoumdzian NM, et al. Cardiotoxic Effects of the Antineoplastic Doxorubicin in a Model of Metabolic Syndrome: Oxidative Stress and Transporter Expression in the Heart. Journal of Cardiovascular Pharmacology 78 (2021): 784-791.
- Olorundare OE, Adeneye AA, Akinsola AO, et al. Clerodendrum volubile Ethanol Leaf Extract: A Potential Antidote to Doxorubicin-Induced Cardiotoxicity in Rats. Journal of Toxicology 2020 (2020): 8859716.
- Olorundare O, Adeneye A, Akinsola A, et al. Irvingia gabonensis Seed Extract: An Effective Attenuator of Doxorubicin-Mediated Cardiotoxicity in Wistar Rats. Oxidative Medicine and Cellular Longevity 2020 (2020): 1602816.
- Owumi S, Arunsi U, Otunla M, et al. 3-Indolepropionic Acid Mitigates Sub-Acute Toxicity in the Cardiomyocytes of Epirubicin-Treated Female Rats. Naunyn-Schmiedeberg's Archives of Pharmacology 397 (2024): 507-520.
- Park HS, Hong YJ, Han K, et al. Ultrahigh-Field Cardiovascular Magnetic Resonance T1 and T2 Mapping for the Assessment of Anthracycline-Induced Cardiotoxicity in Rat Models: Validation Against Histopathologic Changes. Journal of Cardiovascular Magnetic Resonance 23 (2021): 76.
- Patintingan CG, Louisa M, Juniantito V, et al. Moringa oleifera Leaves Extract Ameliorates Doxorubicin-Induced Cardiotoxicity via Its Mitochondrial Biogenesis Modulatory Activity in Rats. Journal of Experimental Pharmacology 15 (2023): 307-319.
- Pereira TCR, Fidale TM, Guimarães LC, et al. Cardioprotective Effects of the 4-Week Aerobic Running Exercises Before Treatment with Doxorubicin in Rats. Cardiovascular Toxicology 23 (2023): 265-277.
- Phungphong S, Kijtawornrat A, Kampaengsri T, et l. Comparison of Exercise Training and Estrogen Supplementation on Mast Cell-Mediated Doxorubicin-Induced Cardiotoxicity. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 318 (2020): R829-R842.
- Piegari E, Cozzolino A, Ciuffreda LP, et al. Cardioprotective Effects of miR-34a Silencing in a Rat Model of Doxorubicin Toxicity. Scientific Reports 10 (2020): 12250.
- Podyacheva E, Shmakova T, Kushnareva E, et al. Modeling Doxorubicin-Induced Cardiomyopathy with Fibrotic Myocardial Damage in Wistar Rats. Cardiology Research 13 (2022): 339-356.
- Prathumsap N, Ongnok B, Khuanjing T, et al. Acetylcholine Receptor Agonists Provide Cardioprotection in Doxorubicin-Induced Cardiotoxicity via Modulating Muscarinic M2 and α7 Nicotinic Receptor Expression. Translational Research 243 (2022): 33-51.
- Prathumsap N, Ongnok B, Khuanjing T, et al. Vagus Nerve Stimulation Exerts Cardioprotection Against Doxorubicin-Induced Cardiotoxicity Through Inhibition of Programmed Cell Death Pathways. Cellular and Molecular Life Sciences 80 (2022): 21.
- Qin Y, Lv C, Zhang X, et al. Protective Effect of Qiliqiangxin Against Doxorubicin-Induced Cardiomyopathy by Suppressing Excessive Autophagy and Apoptosis. Cardiovascular Therapeutics 2022 (2022): 9926635.
- Qurat-Ul-Ain S, Rukhsana A, Tariq SI, et al. Berberis lyceum Root Bark Extract Attenuates Anticancer Drugs Induced Neurotoxicity and Cardiotoxicity in Rats. African Health Sciences 22 (2022): 192-210.
- Radeva-Ilieva MP, Georgiev KD, Hvarchanova NR, et al. Protective Effect of Methylxanthine Fractions Isolated from Bancha Tea Leaves Against Doxorubicin-Induced Cardio- and Nephrotoxicities in Rats. BioMed Research International 2020 (2020): 4018412.
- Radonjic T, Rankovic M, Ravic M, et al. The Effects of Thiamine Hydrochloride on Cardiac Function, Redox Status and Morphometric Alterations in Doxorubicin-Treated Rats. Cardiovascular Toxicology 20 (2020): 111-120.
- Rahbardar MG, Eisvand F, Rameshrad M, et al. In Vivo and In Vitro Protective Effects of Rosmarinic Acid Against Doxorubicin-Induced Cardiotoxicity. Nutrition and Cancer 74 (2022): 747-760.
- Rahimi O, Kirby J, Varagic J, et al. Angiotensin-(1-7) Reduces Doxorubicin-Induced Cardiac Dysfunction in Male and Female Sprague-Dawley Rats Through Antioxidant Mechanisms. American Journal of Physiology. Heart and Circulatory Physiology 318 (2020): H883-H894.
- Rahmanifard M, Vessal M, Noorafshan A, et al. The Protective Effects of Coenzyme Q10 and Lisinopril Against Doxorubicin-Induced Cardiotoxicity in Rats: A Stereological and Electrocardiogram Study. Cardiovascular Toxicology 21 (2021): 936-946.
- Rajangam J, Krishnan N, Palei NN, et al. Ameliorative Potential of Rosuvastatin on Doxorubicin-Induced Cardiotoxicity by Modulating Oxidative Damage in Rats. Turkish Journal of Pharmaceutical Sciences 19 (2022): 28-34.
- Rankovic M, Draginic N, Jeremic J, et al. Protective Role of Vitamin B1 in Doxorubicin-Induced Cardiotoxicity in Rats: Focus on Hemodynamic, Redox, and Apoptotic Markers in Heart. Frontiers in Physiology 12 (2021): 690619.
- Refaie MMM, El-Hussieny M, Abdel-Hakeem EA, et al. Phosphodiesterase Inhibitor, Vinpocetine, Guards Against Doxorubicin-Induced Cardiotoxicity via Modulation of HIF/VEGF and cGMP/cAMP/SIRT Signaling Pathways. Human & Experimental Toxicology 41 (2022): 9603271221136209.
- Refaie MMM, Shehata S, Ibrahim RA, et al. Dose-Dependent Cardioprotective Effect of Hemin in Doxorubicin-Induced Cardiotoxicity via Nrf-2/HO-1 and TLR-5/NF-κB/TNF-α Signaling Pathways. Cardiovascular Toxicology 21 (2021): 1033-1044.
- Renu K, Vinayagam S, Madhyastha H, et al. Exploring the Pattern of Metabolic Alterations Causing Energy Imbalance via PPARα Dysregulation in Cardiac Muscle During Doxorubicin Treatment. Cardiovascular Toxicology 22 (2022): 436-461.
- Ribeiro APD, Pereira AG, Todo MC, et al. Pera Orange (Citrus sinensis) and Moro Orange (Citrus sinensis (L.) Osbeck) Juices Attenuate Left Ventricular Dysfunction and Oxidative Stress and Improve Myocardial Energy Metabolism in Acute Doxorubicin-Induced Cardiotoxicity in Rats. Nutrition 91-92 (2021): 111350.
- Saad S, Ahmad I, Kawish SM, et al. Improved Cardioprotective Effects of Hesperidin Solid Lipid Nanoparticles Prepared by Supercritical Antisolvent Technology. Colloids and Surfaces B: Biointerfaces 187 (2020): 110628.
- Sadek KM, Mahmoud SFE, Zeweil MF, et al. Proanthocyanidin Alleviates Doxorubicin-Induced Cardiac Injury by Inhibiting NF-kB Pathway and Modulating Oxidative Stress, Cell Cycle, and Fibrogenesis. Journal of Biochemical and Molecular Toxicology 35 (2021): e22716.
- Sahyon HA, Al-Harbi SA. Chemoprotective Role of an Extract of the Heart of the Phoenix dactylifera Tree on Adriamycin-Induced Cardiotoxicity and Nephrotoxicity by Regulating Apoptosis, Oxidative Stress and PD-1 Suppression. Food and Chemical Toxicology 135 (2020): 111045.
- Saleh Ahmed AS. Potential Protective Effect of Catechin on Doxorubicin-Induced Cardiotoxicity in Adult Male Albino Rats. Toxicology Mechanisms and Methods 32 (2022): 97-105.
- Saleh D, Abdelbaset M, Hassan A, et al. Omega-3 Fatty Acids Ameliorate Doxorubicin-Induced Cardiorenal Toxicity: In-Vivo Regulation of Oxidative Stress, Apoptosis and Renal Nox4, and In-Vitro Preservation of the Cytotoxic Efficacy. PLoS One 15 (2020): e0242175.
- Samir R, Hassan EA, Saber AA, et al. Seaweed Sargassum aquifolium Extract Ameliorates Cardiotoxicity Induced by Doxorubicin in Rats. Environmental Science and Pollution Research 30 (2023): 58226-58242.
- Samra YA, Amin MN, Said E. Cardio-Protective Impact of Gabapentin Against Doxorubicin-Induced Myocardial Toxicity in Rats; Emphasis on Modulation of Inflammatory-Apoptotic Signaling. International Immunopharmacology 90 (2021): 107125.
- Sandamali JAN, Hewawasam RP, Jayatilaka KAPW, et al. Cinnamomum zeylanicum Blume (Ceylon cinnamon) bark extract attenuates doxorubicin induced cardiotoxicity in Wistar rats. Saudi Pharmaceutical Journal 29 (2021): 820–832.
- Sandamali JAN, Hewawasam RP, Jayatilaka KAPW, et al. Nauclea orientalis (L.) Bark Extract Protects Rat Cardiomyocytes from Doxorubicin-Induced Oxidative Stress, Inflammation, Apoptosis, and DNA Fragmentation. Oxidative Medicine and Cellular Longevity 2022 (2022): 1714841.
- Sandamali JAN, Hewawasam RP, Jayatilaka KAPW, et al. Cardioprotective Potential of Murrayakoenigii (L.) Spreng. Leaf Extract against Doxorubicin-Induced Cardiotoxicity in Rats. Evidence-Based Complementary and Alternative Medicine 2020 (2020): 6023737.
- Satyam SM, Bairy LK, Shetty P, et al. Metformin and Dapagliflozin Attenuate Doxorubicin-Induced Acute Cardiotoxicity in Wistar Rats. Cardiovascular Toxicology 23 (2023): 107–119.
- Sergazy S, Shulgau Z, Fedotovskikh G, et al. Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Scientific Reports 10 (2020): 14720.
- Shabaan DA, Mostafa N, El-Desoky MM, et al. Coenzyme Q10 protects against doxorubicin-induced cardiomyopathy via antioxidant and anti-apoptotic pathway. Tissue Barriers 11 (2023): 2019504.
- Sharifiaghdam Z, Amini SM, Dalouchi F, et al. Apigenin-coated gold nanoparticles as a cardioprotective strategy against doxorubicin-induced cardiotoxicity in male rats. Heliyon 9 (2023): e14024.
- Sharma A, Parikh M, Shah H, et al. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon 6 (2020): e03803.
- Sheibani M, Nezamoleslami S, Faghir-Ghanesefat H, et al. Cardioprotective effects of dapsone against doxorubicin-induced cardiotoxicity in rats. Cancer Chemotherapy and Pharmacology 85 (2020): 563–571.
- Shekari M, Gortany NK, Khalilzadeh M, et al. Cardioprotective effects of sodium thiosulfate against doxorubicin-induced cardiotoxicity in male rats. BMC Pharmacology & Toxicology 23 (2022): 32.
- Shen LJ, Lu S, Zhou YH, et al. Developing a rat model of dilated cardiomyopathy with improved survival. Journal of Zhejiang University Science B 17 (2016): 975–983.
- Shi H, Tang H, Ai W, et al. Schisandrin B Antagonizes Cardiotoxicity Induced by Pirarubicin by Inhibiting Mitochondrial Permeability Transition Pore (mPTP) Opening and Decreasing Cardiomyocyte Apoptosis. Frontiers in Pharmacology 12 (2021): 733805.
- Shi H, Zeng Q, Wei Y, et al. Canagliflozin is a potential cardioprotective drug but exerts no significant effects on pirarubicin induced cardiotoxicity in rats. Molecular Medicine Reports 24 (2021): 703.
- Shoukry HS, Ammar HI, Rashed LA, et al. Prophylactic supplementation of resveratrol is more effective than its therapeutic use against doxorubicin induced cardiotoxicity. PLoS ONE 12 (2017): e0181535.
- Singh M, Khan MA, YT K, et al. Effect of Nardostachys jatamansi DC. on Apoptosis, Inflammation and Oxidative Stress Induced by Doxorubicin in Wistar Rats. Plants 9 (2020): 1579.
- Sirwi A, Shaik RA, Alamoudi AJ, et al. Mokko Lactone Alleviates Doxorubicin-Induced Cardiotoxicity in Rats via Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Nutrients 14 (2022): 733.
- Soliman NA, Abo El Gheit RE, Abdel Ghafar MT, et al. Unraveling the biomechanistic role of Rac1/TWEAK/Fn14/NF-κB network in doxorubicin-induced cardiotoxicity in rats: The role of curcumin. Journal of Biochemical and Molecular Toxicology 35 (2021): e22829.
- Sun XP, Wan LL, Yang QJ, et al. Scutellarin protects against doxorubicin-induced acute cardiotoxicity and regulates its accumulation in the heart. Archives of Pharmacal Research 40 (2017): 875–883.
- Sun X, Chen G, Xie Y, et al. Qiliqiangxin improves cardiac function and attenuates cardiac remodeling in doxorubicin-induced heart failure rats. Pharmaceutical Biology 58 (2020): 417–426.
- Sun X, Sun P, Zhen D, et al. Melatonin alleviates doxorubicin-induced mitochondrial oxidative damage and ferroptosis in cardiomyocytes by regulating YAP expression. Toxicology and Applied Pharmacology 437 (2022): 115902.
- Sun X, Zhou L, Han Y, et al. Scutellarin Attenuates Doxorubicin-Induced Cardiotoxicity by Inhibiting Myocardial Fibrosis, Apoptosis and Autophagy in Rats. Chemistry & Biodiversity 20 (2023): e202200450.
- Sun Z, Lu W, Lin N, et al. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochemical Pharmacology 175 (2020): 113888.
- Syahputra RA, Harahap U, Harahap Y, et al. Vernonia amygdalina Ethanol Extract Protects against Doxorubicin-Induced Cardiotoxicity via TGFβ, Cytochrome c, and Apoptosis. Molecules 28 (2023): 4305.
- Tan C, Zeng J, Wu G, et al. Xinshuitong Capsule extract attenuates doxorubicin-induced myocardial edema via regulation of cardiac aquaporins in chronic heart failure rats. Biomedicine & Pharmacotherapy 144 (2021): 112261.
- Tang DX, Zhao HP, Pan CS, et al. QiShenYiQi Pills ameliorates doxorubicin-induced myocardial structure damage and cardiac dysfunction in rats. Evidence-Based Complementary and Alternative Medicine 2013 (2013): 480597.
- Tatlidede E, Sehirli O, Velioglu-Ogünc A, et al. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radical Research 43 (2009): 195–205.
- Teng LL, Shao L, Zhao YT, et al. The beneficial effect of n-3 polyunsaturated fatty acids on doxorubicin-induced chronic heart failure in rats. Journal of International Medical Research 38 (2010): 940–948.
- Tian XQ, Ni XW, Xu HL, et al. Prevention of doxorubicin-induced cardiomyopathy using targeted MaFGF nanoparticles combined with ultrasound-targeted MB destruction. International Journal of Nanomedicine 12 (2017): 7103–7119.
- Tian Y, Lv W, Lu C, et al. Galectin-3 inhibition attenuates doxorubicin-induced cardiac dysfunction by upregulating peroxiredoxin-4. Canadian Journal of Physiology and Pharmacology 98 (2020): 700–707.
- Todorova VK, Siegel ER, Kaufmann Y, et al. Dantrolene Attenuates Cardiotoxicity of Doxorubicin Without Reducing its Antitumor Efficacy in a Breast Cancer Model. Translational Oncology 13 (2020): 471–480.
- Vasic M, Loncar-Turukalo T, Tasic T, et al. Cardiovascular variability and β-ARs gene expression at two stages of doxorubicin - Induced cardiomyopathy. Toxicology and Applied Pharmacology 362 (2019): 43–51.
- Wan Y, He B, Zhu D, et al. Nicorandil Ameliorates Doxorubicin-Induced Cardiotoxicity in Rats, as Evaluated by 7 T Cardiovascular Magnetic Resonance Imaging. Cardiovascular Drugs and Therapy 37 (2023): 39–51.
- Wan Y, He B, Zhu D, et al. Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Archives of Biochemistry and Biophysics 712 (2021): 109050.
- Wan Y, Zhu D, He B, et al. Protective effect of a chronic hypobaric hypoxic environment at high altitude on cardiotoxicity induced by doxorubicin in rats: a 7 T magnetic resonance study. Quantitative Imaging in Medicine and Surgery 12 (2022): 711–725.
- Wang C, Hu L, Guo S, et al. Phosphocreatine attenuates doxorubicin-induced cardiotoxicity by inhibiting oxidative stress and activating TAK1 to promote myocardial survival in vivo and in vitro. Toxicology 460 (2021): 152881.
- Wang F, Wang L, Jiao Y, et al. QishenHuanwu capsule reduces pirarubicin-induced cardiotoxicity in rats by activating the PI3K/Akt/mTOR pathway. Annals of Palliative Medicine 9 (2020): 3453–3461.
- Wang S, Zhu T, Wang C, et al. Identifying early stages of doxorubicin-induced cardiotoxicity in rat model by 7.0 tesla cardiovascular magnetic resonance combining hematological and pathological parameters. Magnetic Resonance Imaging 90 (2022): 17–25.
- Wang T, Yuan C, Liu J, et al. Targeting Energy Protection as a Novel Strategy to Disclose Di'aoXinxuekang against the Cardiotoxicity Caused by Doxorubicin. International Journal of Molecular Sciences 24 (2023): 897.
- Wang X, Chen L, Wang T, et al. Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine 22 (2015): 875–884.
- Wang Y, Liao J, Luo Y, et al. Berberine Alleviates Doxorubicin-Induced Myocardial Injury and Fibrosis by Eliminating Oxidative Stress and Mitochondrial Damage via Promoting Nrf-2 Pathway Activation. International Journal of Molecular Sciences 24 (2023): 3257.
- Waz S, Matouk AI. Cardioprotective effect of allyl isothiocyanate in a rat model of doxorubicin acute toxicity. Toxicology Mechanisms and Methods 32 (2022): 194–203.
- Wei Y, Zhao J, Xiong J, et al. Wogonin reduces cardiomyocyte apoptosis from mitochondrial release of cytochrome c to improve doxorubicin-induced cardiotoxicity. Experimental and Therapeutic Medicine 23 (2022): 205.
- Wen J, Zhang L, Wang J, et al. Therapeutic effects of higenamine combined with [6]-gingerol on chronic heart failure induced by doxorubicin via ameliorating mitochondrial function. Journal of Cellular and Molecular Medicine 24 (2020): 4036–4050.
- Wu J, Li K, Liu Y, et al. Daidzein ameliorates doxorubicin-induced cardiac injury by inhibiting autophagy and apoptosis in rats. Food & Function 14 (2023): 934–945.
- Wu YP, Zhang S, Xin YF, et al. Evidences for the mechanism of Shenmai injection antagonizing doxorubicin-induced cardiotoxicity. Phytomedicine 88 (2021): 153597.
- Wu Z, Zhao X, Miyamoto A, et al. Effects of steroidal saponins extract from Ophiopogon japonicus root ameliorates doxorubicin-induced chronic heart failure by inhibiting oxidative stress and inflammatory response. Pharmaceutical Biology 57 (2019): 176–183.
- Xing W, Wen C, Wang D, et al. Cardiorenal Protective Effect of Costunolide against Doxorubicin-Induced Toxicity in Rats by Modulating Oxidative Stress, Inflammation and Apoptosis. Molecules 27 (2022): 2122.
- Xu A, Deng F, Chen Y, et al. NF-κB pathway activation during endothelial-to-mesenchymal transition in a rat model of doxorubicin-induced cardiotoxicity. Biomedicine & Pharmacotherapy 130 (2020): 110525.
- Yildizhan K, Huyut Z, Alt?ndag F. Involvement of TRPM2 Channel on Doxorubicin-Induced Experimental Cardiotoxicity Model: Protective Role of Selenium. Biological Trace Element Research 201 (2023): 2458–2469.
- Yin Y, Niu Q, Hou H, et al. PAE ameliorates doxorubicin-induced cardiotoxicity via suppressing NHE1 phosphorylation and stimulating PI3K/AKT phosphorylation. International Immunopharmacology 113 (2022): 109274.
- Younis NS, Elsewedy HS, Soliman WE, et al. Geraniol isolated from lemon grass to mitigate doxorubicin-induced cardiotoxicity through Nrf2 and NF-κB signaling. Chemico-Biological Interactions 347 (2021): 109599.
- Bin Jardan YA, Ansari MA, Raish M, et al. Sinapic Acid Ameliorates Oxidative Stress, Inflammation, and Apoptosis in Acute Doxorubicin-Induced Cardiotoxicity via the NF-κB-Mediated Pathway. BioMed Research International 2020 (10): 3921796.
- Hsieh DJY, Islam MN, Kuo WW, et al. A combination of isoliquiritigenin with Artemisia argyi and Ohwia caudata water extracts attenuates oxidative stress, inflammation, and apoptosis by modulating Nrf2/Ho-1 signaling pathways in SD rats with doxorubicin-induced acute cardiotoxicity. Environmental Toxicology 38 (2023): 3026–3042.
- Zhang H, Lu X, Liu Z, et al. Rosuvastatin reduces the pro-inflammatory effects of adriamycin on the expression of HMGB1 and RAGE in rats. International Journal of Molecular Medicine 42 (2018): 3415–3423.
- Zhang L, Jiang Q, Wang X, et al. Boesenbergia rotunda displayed anti-inflammatory, antioxidant and anti-apoptotic efficacy in doxorubicin-induced cardiotoxicity in rats. Scientific Reports 13 (2023): 11398.
- Zhang XJ, Cao XQ, Zhang CS, et al. 17β-estradiol protects against doxorubicin-induced cardiotoxicity in male Sprague-Dawley rats by regulating NADPH oxidase and apoptosis genes. Molecular Medicine Reports 15 (2017): 2695–2702.
- Zhang X, Lv S, Zhang W, et al. Shenmai injection improves doxorubicin cardiotoxicity via miR-30a/Beclin 1. Biomedicine & Pharmacotherapy 139 (2021): 111582.
- Zhang Y, Ma C, Liu C, et al. Luteolin attenuates doxorubicin-induced cardiotoxicity by modulating the PHLPP1/AKT/Bcl-2 signalling pathway. PeerJ 8 (2020): e8845.
- Zhang Y, Ma J, Liu S, et al. Ginsenoside F1 attenuates pirarubicin-induced cardiotoxicity by modulating Nrf2 and AKT/Bcl-2 signaling pathways. Journal of Ginseng Research 47 (2023): 106–116.
- Zhou P, Gao G, Zhao CC, et al. In vivo and in vitro protective effects of shengmai injection against doxorubicin-induced cardiotoxicity. Pharmaceutical Biology 60 (2022): 638–651.
- Swamy AV, Gulliaya S, Thippeswamy A, et al. Cardioprotective effect of curcumin against doxorubicin-induced myocardial toxicity in albino rats. Indian Journal of Pharmacology 44 (2012): 73–77.
- Razmaraii N, Babaei H, MohajjelNayebi A, et al. Cardioprotective effect of grape seed extract on chronic doxorubicin-induced cardiac toxicity in Wistar rats. Advanced Pharmaceutical Bulletin 6 (2016): 423–433.
- Nayak SPRR, Boopathi S, Chandrasekar M, et al. Indole-3-acetic acid exposure leads to cardiovascular inflammation and fibrosis in chronic kidney disease rat model. Food and Chemical Toxicology 192 (2024): 114917.
- Nayak SPRR, Boopathi S, Chandrasekar M, et al. Indole-3-acetic acid induced cardiac hypertrophy in Wistar albino rats. Toxicology and Applied Pharmacology 486 (2024): 116917.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11296583, Pirarubicin. (2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 31703, Doxorubicin. (2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 30323, Daunorubicin. (2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 41867, Epirubicin. (2024).
- Galán-Arriola C, Lobo M, Vílchez-Tschischke JP, et al. Serial Magnetic Resonance Imaging to Identify Early Stages of Anthracycline-Induced Cardiotoxicity. Journal of the American College of Cardiology 73 (2019): 779–791.
- Ha JW, Kang SM, Pyun WB, et al. Serial assessment of myocardial properties using cyclic variation of integrated backscatter in an adriamycin-induced cardiomyopathy rat model. Yonsei Medical Journal 46 (2005): 73–77.
- O'Connell JL, Romano MM, Campos Pulici EC, et al. Short-term and long-term models of doxorubicin-induced cardiomyopathy in rats: A comparison of functional and histopathological changes. Experimental and Toxicologic Pathology 69 (2017): 213–219.
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity. European Heart Journal 37 (2016): 2768–2801.
- Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. Journal of the American College of Cardiology 64 (2014): 938–945.
- Carrasco R, Castillo RL, Gormaz JG, et al. Role of Oxidative Stress in the Mechanisms of Anthracycline-Induced Cardiotoxicity: Effects of Preventive Strategies. Oxidative Medicine and Cellular Longevity 2021 (2021): 8863789.
Journals List
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved