Aortic Valve Stenosis: Diagnostic Approaches and Recommendations of the 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease –A Review of the Literature
Article Information
Mukaram Rana*
Department of Cardiology, Angiology, and Intensive Care Unit, Fulda Clinic, University Medicine Marburg- Campus Fulda, Germany
*Corresponding author: Mukaram Rana, Department of Cardiology (Medical Clinic I), Fulda Clinic, University Medicine Marburg, Campus Fulda, Pacelliallee 4, 36043 Fulda, Germany
Received: 16 June 2022; Accepted: 21 June 2022; Published: 27 June 2022
Citation:
Mukaram Rana. Aortic Valve Stenosis: Diagnostic Approaches and Recommendations of the 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease –A Review of the Literature. Cardiology and Cardiovascular Medicine 6 (2022): 315-324.
View / Download Pdf Share at FacebookAbstract
Aortic stenosis (AS) is the most common valvular heart disease in Europe and North America requiring a surgical or interventional treatment. Due to demographic changes with an aging population the burden of valvular heart diseases and especially the importance of aortic stenosis (AS) will be growing in future. As the onset of symptoms is associated with a decrease in life expectancy appropriate and early diagnosis are of utmost importance. However, insights of clinical practice underline diagnostic challenges which may lead to a delayed initiation of treatment with an adverse effect on the prognosis. The aim of this review is to display different diagnostic approaches that may be helpful in detecting patients with aortic valve stenosis. This review will focus on both non-invasive and invasive diagnostic approaches that can be implemented in clinical routine. Further-more, we will especially highlight recommendations of the 2021 European guidelines for the management of valvular heart disease.
Methods: For this review a selective literature research on the databases PubMed and Google Scholar was conducted. Original articles, reviews and meta-analyses were included when meeting our search criteria. Following terms were used in different combinations: Aortic valve stenosis; Aortic stenosis; diagnosis of aortic stenosis; ESC Guidelines
Keywords
Aortic Valve Stenosis; Aortic Stenosis; Diagnosis of Aortic Stenosis; Guidelines for the Management of Valvular Heart Disease
Article Details
1. Background
1.1 Epidemiology and etiology
Aortic stenosis (AS) is the leading primary valvular heart disease in Europe and North America and the second most common cardiovascular disease after arterial hypertension and coronary artery disease [1]. The prevalence increases with age varying from 0.2 % in the age group 50-59 years to 9.8 % among 80-89 years [2]. Congenital and acquired etiologies are known as driving factors in the development of AS. Degenerative calcific aortic stenosis remains the most common type of acquired AS [3]. Both the development of calcific AS and atherosclerosis show pivotal histopathological similarities in the initial phase of the disease [4, 5]. Endothelial damages caused by mechanical stress lead to lipid deposition and infiltration of the subendothelial tissue [6]. Infiltrating lipids undergo a process of oxidation which is promoted by reactive oxygen species (ROS). Consequently, they develop the capability of promoting further cytotoxic and pro-inflammatory effects [7, 8]. The uptake of oxidized low-density lipoproteins (LDL) by monocytes leads to the development of foam cells which play a key role in the development of atherosclerosis [9]. The production of pro-inflammatory cytokines accelerates further immunological cascades which subsequently result in fibrosing and calcifying processes leading to a limitation of cusp mobility [10]. Rheumatic aortic stenosis is the second common type of acquired AS and is considered the result of rheumatic fever occurring after an infection with group A streptococcus and leading to an immunological response with the production of autoantibodies [3, 11]. The mitral valve is affected more frequently than the aortic valve [12]. Most cases can be observed in children between 5 - 14 years [13, 14]. Advances in health care systems and the provided medical treatment in Western Europe and Northern America led to a noticeable decline in the incidence so that the disease is primarily prevalent in developing countries [15, 16].
1.2 Pathophysiology
In adults the aortic valve area (AVA) measures 3.0 to 4.0 cm2. A significant reduction in the aortic valve orifice area under 1.5 cm2 consequently leads to an increase of transvalvular pressure gradient with a subsequent decrease of blood flow from the left ventricle in the systemic circulation [17]. Furthermore, the pressure overload causes a compensatory left ventricle hypertrophy to maintain a sufficient cardiac output [18]. The reduced compliance resulting from the ventricle hypertrophy leads to an increased end-diastolic pressure contributing to the destruction of cardiomyocytes and thus facilitating a left ventricular dysfunction which may be associated with signs of heart failure [19]. Moreover, an impairment of the coronary flow reserve following from an inappropriate myocardial hypertrophy and an increased wall stress may lead to an imbalance of oxygen demand and oxygen supply causing the symptom of angina pectoris [20, 21].
1.3 Clinical features
An aortic valve stenosis can remain asymptomatic in the initial phase of the disease due to sufficient compensatory mechanisms. However, the 5-year risk of developing symptoms remains high [22, 23]. The onset of symptoms is associated with a poor outcome if there is no causal treatment [24, 25]. Cardinal symptoms of aortic stenosis are anginal chest pain, loss of consciousness, dyspnea, and signs of heart failure [26, 27].
1.4 Aim
Due to contemporary trends in demographic changes with an aging population diagnosis and therapy possibilities of AS will attract growing attention in the future. However, diagnostic uncertainties may lead to delayed diagnosis of AS which can be of prognostic relevance since the onset of symptoms is associated with a decrease in life expectancy. We therefore aimed to conduct a review of the contemporary literature and provide invasive and non-invasive diagnostic approaches on the basis of the latest European guidelines that may be beneficial for detecting aortic valve stenosis in daily clinical practice.
2. Diagnosis
2.1 Physical examination
Physical examination is an integral part of any primary doctor-patient contact and can play a key role for bedside diagnosis of suspected valvular heart disease. Auscultation of the heart as one element of the physical exanimation can be used to detect abnormal heart sounds and maybe helpful to decide whether further investigations are needed. Aortic valve stenosis can be characterized by a systolic murmur that is audible with its maximum intensity in the second right intercostal space. A radiation to the external carotid artery can be detected additionally [28-30]. In some cases, the high frequency component of the systolic murmur can be detected at the cardiac apex. This clinical sign is called Gallavardin phenomenon [31]. Although the stethoscope is one of the most useful diagnostic tools for clinicians conducting physical examinations it has its natural limitations. Auscultat-ion of heart sounds and the capability of distinguishing between different heart murmurs is highly experience-dependent which can lead to inconclusive findings among doctors with different levels of experience. Limited specificity and low sensitivity suggest the need of alternative diagnostic strategies [32]. In this context the current European guidelines do not recommend auscultation as a reliable diagnostic tool for the diagnosis of aortic valve stenosis.
2.2 Chest X-ray
Insights from the clinical routine affirm the observation that the chest x-ray is one of the initial imaging diagnostics in symptomatic patients with an undiagnosed aortic valve stenosis as these patients usually present with symptoms such as angina pectoris or dyspnea which need to be further investigated. The radiological findings are dependent on the stage of the disease and may be missing in the initial phase due to sufficient compensatory mechanisms. However, in an advanced stage of the disease cardiomegaly with an enlargement of the cardiac silhouette resulting from a left ventricle hypertrophy due to an increased afterload and an enlargement of the left atrium on the antero-posterior view may be detected. Furthermore, signs of heart failure, such as pulmonary venous congestion can be present. Although there are numerous signs that may indicate the presence of a relevant AS the chest X-ray remains a nonspecific tool in the diagnosis of AS and therefore plays a subordinate role. The current ESC guidelines therefore do not suggest the use of a chest X-ray as a sufficient diagnostic tool.
2.3 Electrocardiography (ECG)
In some cases, patients with AS may present electrocardiographic findings such as a left axis deviation as well as a positive Sokolow-Lyon index (Sv1 + RV5 or 6 > 3.5 mV) as an expression of a left ventricular hypertrophy. Further optional signs such as left branch bundle blocks, T-wave negativations and ST depressions can also be present [28, 33, 34]. However, the ECG is not a sufficient diagnostic tool to detect or quantify any AS which highlights its subordinate role in the diagnosis of valvular heart diseases. Nevertheless, it must be considered that this limitation does not weaken the indication of an extensive use in the clinical routine since relevant comorbidities such as coronary heart diseases are usually present in these patient groups. As far as the current guidelines are concerned no proposal or explicit recommendations have been made by the authors.
2.4 Echocardiography
Echocardiography is the tool of choice for confirming the diagnosis of aortic valve stenosis. It is highly valuable for assessing the left ventricular ejection fraction, the valve morphology, the opening movement of the valve and the wall thickness. Furthermore, a semi-quantitative assessment of the extent of valve calcification can be performed as. The severity of aortic valve stenosis is quantified with the help of doppler measurement [17, 35, 36]. Three parameters are needed to classify the aortic valve stenosis: peak transvalvular velocity (Vmax), mean pressure gradient (Pmean) and aortic valve area (AVA). Low flow is defined by a stroke volume index 35 ml/m2. According to the current guidelines AS can be classified in four groups (Table 1) [37]:
|
High-gradient AS |
Low-flow low-gradient AS with reduced ejection function |
Low-flow low-gradient AS with preserved ejection fraction |
Normal-flow low-gradient AS with preserved ejection fraction |
|
AVA ≤ 1cm2 |
AVA ≤ 1cm2 |
AVA ≤ 1cm2 |
AVA ≤ 1cm2 |
|
Pmean ≥ 40mmHg |
Pmean < 40 mmHg |
Pmean < 40 mmHg |
Pmean < 40 mmHg |
|
Peak velocity ≥ 4.0m/s |
SVi 35 ml/m2 |
SVi 35 ml/m2 |
SVi > 35 ml/m2 |
|
LVEF < 50% |
LVEF 50% |
LVEF 50% |
Abbreviations: AS = aortic valve stenosis; AVA = aortic valve area; LVEF = left ventricular ejection fraction; Pmean = mean pressure gradient; SVi= stroke volume index.
Table 1: Categories of aortic valve stenosis (AS).
2.4.1 Peak transvalvular velocity (Vmax): Peak transvalvular velocity (Vmax) is the systolic velocity across the aortic valve. It can be measured using continuous-wave (CW) Doppler ultrasound (CW doppler sonography) [38]. It must be considered that velocity measurements require an optimal patient and transducer positioning. The ultrasound beam needs to be aligned parallel to the aortic jet. The so-called velocity-time integral (VTI) and the mean pressure gradient (Pmean) are measured by tracing the velocity curve [39]. It must be noted that at least three consecutive beats should be averaged in sinus rhythm and at least five consecutive beats with irregular rhythms, for example atrial fibrillation.
2.4.2 Mean pressure gradient (Pmean): Mean pressure gradient (Pmean) is another parameter which is needed to assess aortic stenosis severity. It represents the pressure gradient between the left ventricle and the aorta during the entire systole. The mean pressure gradient is calculated based on Bernoulli's equation [40]. However, for clinical use the so-called simplified Bernoulli equation is used:
Δp = 4 V2
Formula 1: Simplified Bernoulli equation. Δp = mean pressure gradient (mmHg); V = velocity (m/s).
2.4.3 Aortic valve area (AVA): The calculation of the aortic valve area (AVA) is performed using the continuity equation. The continuity equation is based on the consideration that the stroke volume (SV) moving over the left ventricular outflow tract (LVOT) corresponds to the stroke volume (SV) passing through the aortic valve. Three parameters are needed to calculate the aortic valve area (AVA) [41]:
- Diameter of the LVOT
- Flow velocity in the LVOT (determined by pulsed-wave Doppler sonography)
- Flow velocity across the aortic valve (determination by continuous wave Doppler sonography)
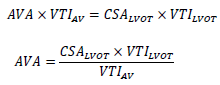
Formula 2: Continuity equation. AV = Aortic Valve; AVA= Aortic Valve Area (cm2); CSA= Cross Sectional Area (cm2); LVOT= Left ventricular outflow tract; VTI = Velocity Time Integral (m/s).
2.4.4 Grading of Aortic stenosis (AS) severity
|
Mild AS |
Moderate AS |
Severe AS |
|
|
Peak velocity (m/s) |
2,6 - 2,9 |
3,0 - 4,0 |
≥4,0 |
|
Mean gradient (mmHg) |
< 20 |
20 - 40 |
≥40 |
|
Aortic valve area (AVA) (cm2) |
> 1,5 |
1,0 - 1,5 |
< 1,0 |
|
Indexed AVA (cm2/m2 ) |
> 0,85 |
0,60 - 0,85 |
< 0,6 |
|
Velocity ratio |
> 0,5 |
0,25 - 0,5 |
< 0,25 |
Table 2: Grading of Aortic stenosis [39].
2.5 Cardial computer tomography (CCT)
The role of cardial computer tomography (CCT) for the diagnosis of AS and for the pre-interventional planning is crucial. CT imaging can provide information about the extent of valvular calcification which has been identified as a predictor for the progression and prognosis of aortic stenosis [42, 43]. Furthermore, aortic valve calcification scores measured by CCT can help assessing the severity of aortic stenosis which then would suggest further investigations [44]. According to the current ESC guidelines for the management of valvular heart diseases the measured calcification scores can be used to predict the probability for the presence of a severe aortic valve stenosis. A severe AS can be highly likely (men > 3000, women > 1600 Agatston units), likely (men > 2000, women > 1200 Agastson units) and unlikely (men < 1600, women < 800 Agatston units) [37]. Apart from this, CCT is used to for preinterventional planning. It provides information about vascular and valve calcification, aortic valve anatomy, annular size and shape, aortic root dimensions, distance of coronary ostia to the aortic valve plane which are necessary to avoid complications such as aortic root injuries or coronary occlusion [37, 45]. In this context CCT can be considered as an important diagnostic tool providing adjunctive information for the assessment of AS that is not only used in terms of uncertainties but also plays a crucial role for the preinterventional planning.
2.6 Dobutamine stress echocardiography (DSE)
Dobutamine stress echocardiography (DSE) is an additional diagnostic tool that should be considered according to the current guidelines whenever results remain inconclusive, especially in patients with low-flow low-gradient AS with reduced ejection fraction. Dobutamine stress echocardiography may help distinguishing between true severe AS and pseudo-severe AS in patients with [46]. This diagnostic approach assumes that AVA and Pmean are flow-dependent and the mismatch of a lower than expected transvalvular gradient is due to reduced transvalvular flow. Pharmalogical stress testing with dobutamine is used for further distinctions. The dobutamine infusion is usually started at 5 g/kg/min and can be titrated to maximum 20 g/kg/min. During titration Pmean and AVA should be measured regularly. True severe AS is diagnosed when AVA is 1 cm2 and Pmean 40 mmHg [47]. From this point of view DSE is highly valuable diagnostic tool which does not only provide information about the severity of AS but also about the contractile reserve which is of prognostic relevance [48].
2.7 Cardiac catheterization
In case of discrepant or inconclusive diagnostic findings right- and left-sided heart catheterization should be performed. Cardiac output is measured based on thermodilution or Fick equation using a Swan-Ganz catheter. Transvalvular gradient is obtained by simultaneous measurement of the pressure in the left ventricle and in the ascending aorta which can be performed with the help of a single dual-lumen catheter or with two separate catheters. Using the so called Gorlin equation the aortic valve area can be measured [49, 50].
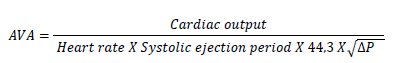
Formula 3: Gorlin equation. Abbreviation: AVA = aortic valve area (cm2); DP = mean gradient (mmHg).
3. Conclusion
This review aimed to highlight the importance of various non-invasive and invasive diagnostic approaches for detecting AS in clinical practice. We could show that the current ESC 2021 guidelines for the management of valvular heart diseases consider echocardiography as the tool of choice for confirming the diagnosis of aortic valve stenosis. However, in cases of inconclusive results and measurements additional diagnostic approaches using cardial computer tomography (CCT), dobutamine stress echocardiography (DSE) and right- and left-sided heart catheterization should be considered. Auscultation, chest X-ray and ECG play a subordinate role due to their natural limitations and their low specificity and sensitivity for the diagnosis of AS.
Declarations
Funding
No funding was received for conducting this study
Conflicts of interest
The author states that he has no conflicts of interest
References
- Brian R Lindman, Marie-Annick Clavel , Patrick Mathie, et al. Calcific aortic stenosis. Nat Rev Dis Primers 2 (2016): 16006.
- Gry Wisthus Eveborn, Henrik Schirmer, Geir Heggelund, et al. The evolving epidemiology of valvular aortic stenosis. the Tromso study. Heart 99 (2013): 396-400.
- Pompilio Faggiano, Francesco Antonini-Canterin, Ferdinando Baldessin, et al. Epidemiology and cardiovascular risk factors of aortic stenosis. Cardiovasc Ultrasound 4 (2006): 27.
- Michel Pompeu Barros de Oliveira Sá, Luiz Rafael P Cavalcanti, Álvaro M Perazzo, et al. Calcific Aortic Valve Stenosis and Atherosclerotic Calcification. Curr Atheroscler Rep 22 (2020): 2.
- Konstantinos Dean Boudoulas, Brian Wolfe, Yazhini Ravi, et al. The aortic stenosis complex: aortic valve, atherosclerosis, aortopathy. J Cardiol 65 (2015): 377-382.
- Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol 60 (2012): 1854-1863.
- Dania Mohty, Philippe Pibarot, Jean-Pierre Després, et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol 28 (2008): 187-193.
- Romain Capoulade, Kwan L Chan, Calvin Yeang, et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J Am Coll Cardiol 66 (2015): 1236-1246.
- Anja Schwarz, Gabriel A Bonaterra, Hans Schwarzbach, et al. Oxidized LDL-induced JAB1 influences NF-kappaB independent inflammatory signaling in human macrophages during foam cell formation. J Biomed Sci 24 (2017): 12.
- Jens J Kaden, Carl-Erik Dempfle, Rainer Grobholz, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 170 (2003): 205-211.
- Jonathan R Carapetis, Andrea Beaton, Madeleine W Cunningham, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers 2 (2016): 15084.
- Webb RH, Grant C, Harnden A. Acute rheumatic fever. BMJ 351 (2015): h3443.
- Jaine R, Baker M, Venugopal K. Epidemiology of acute rheumatic fever in New Zealand 1996-2005. J Paediatr Child Health 44 (2008): 564-571.
- Joanna G Lawrence, Jonathan R Carapetis, Kalinda Griffiths, et al. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation 128(2013): 492-501.
- Michael H Gewitz, Robert S Baltimore, Lloyd Y Tani, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation 131 (2015): 1806-1818.
- Tibazarwa KB, Volmink JA, Mayosi BM. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart, 94 (2008): 1534-1540.
- Helmut Baumgartner, Judy Hung, Javier Bermejo, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 10 (2009): 1-25.
- Florian Rader, Esha Sachdev, Reza Arsanjani, et al. Left ventricular hypertrophy in valvular aortic stenosis: mechanisms and clinical implications. Am J Med 128 (4): 344-352.
- Vasileios Kamperidis, Victoria Delgado, Nicolas M van Mieghem, et al. Diagnosis and management of aortic valve stenosis in patients with heart failure. Eur J Heart Fail 18 (2016): 469-481.
- Julius BK, Spillmann M, Vassalli G, et al. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation 95 (1997): 892-898.
- Aronow WS. A review of the pathophysiology, diagnosis, and treatment of aortic valve stenosis in elderly patients. Hosp Pract (1995) 41 (2013): 66-77.
- Jessica Joseph, Syed Yaseen Naqvi, Jay Giri, et al. Aortic Stenosis: Pathophysiology, Diagnosis, and Therapy. Am J Med 130 (2017): 253-263.
- Patricia A Pellikka, Maurice E Sarano, Rick A Nishimura, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 111 (2005): 3290-3295.
- Raphael Rosenhek, Robert Zilberszac, Michael Schemper, et al. Natural history of very severe aortic stenosis. Circulation 121 (2010): 151-156.
- Carabello BA. Introduction to aortic stenosis. Circ Res 113 (2013): 179-185.
- Manning WJ. Asymptomatic aortic stenosis in the elderly: a clinical review. JAMA 310 (2013): 1490-1497.
- Lindman BR, Bonow RO, Otto CM. Current management of calcific aortic stenosis. Circ Res 113 (2013): 223-237.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 373 (2009): 956-966.
- Vogelgesang A, Hasenfuss G, Jacobshagen C. Diagnosis and treatment of aortic valve stenosis. Internist (Berl) 59 (2018): 1279-1290.
- Zakkar M, Bryan AJ, Angelini GD. Aortic stenosis: diagnosis and management. BMJ 355 (2016): i5425.
- Giles TD, Martinez EC, Burch GE. Gallavardin phenomenon in aortic stenosis. A possible mechanism. Arch Intern Med 134 (1974): 747-749.
- Syed K M Gardezi, Saul G Myerson, John Chambers, et al. Cardiac auscultation poorly predicts the presence of valvular heart disease in asymptomatic primary care patients. Heart 104 (2018): 1832-1835.
- Chen RS, Bivens MJ, Grossman SA. Diagnosis and management of valvular heart disease in emergency medicine. Emerg Med Clin North Am 29 (2011): 801-810, vii.
- Gottlieb M, Long B, Koyfman A. Evaluation and Management of Aortic Stenosis for the Emergency Clinician: An Evidence-Based Review of the Literature. J Emerg Med 55 (2018): 34-41.
- Kanwar A, Thaden JJ, Nkomo VT. Management of Patients With Aortic Valve Stenosis. Mayo Clin Proc 93 (2018): 488-508.
- Helmut Baumgartner, Volkmar Falk, Jeroen J Bax, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38 (2017): 2739-2791.
- Alec Vahanian, Friedhelm Beyersdorf, Fabien Praz, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 43 (2022): 561-632.
- Currie PJ, Seward JB, Reeder GS, et al. Continuous-wave Doppler echocardio-graphic assessment of severity of calcific aortic stenosis: a simultaneous Doppler-catheter correlative study in 100 adult patients. Circulation 71 (1985): 1162-1169.
- Helmut Baumgartner Chair, Judy Hung Co-Chair, Javier Bermejo, et al. Recommen-dations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 18 (2017): 254-275.
- Smith MD, Kwan OL, DeMaria AN. Value and limitations of continuous-wave Doppler echocardiography in estimating severity of valvular stenosis. JAMA 255 (1986): 3145-3151.
- Helmut Baumgartner, Judy Hung, Javier Bermejo, et al. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 30 (2017): 372-392.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 343 (2000): 611-617.
- Otto CM. Timing of aortic valve surgery. Heart 84 (2000): 211-218.
- Ralf Koos, Andreas Horst Mahnken, Anil Martin Sinha, et al. Aortic valve calcification as a marker for aortic stenosis severity: assessment on 16-MDCT. AJR Am J Roentgenol 183 (2004): 1813-1818.
- Marwan M, Achenbach S. Role of Cardiac CT Before Transcatheter Aortic Valve Implantation (TAVI). Curr Cardiol Rep 18 (2016): 21.
- Marie-Annick Clavel, Pierre Vladimir Ennezat, Sylvestre Maréchaux, et al. Stress echocardiography to assess stenosis severity and predict outcome in patients with paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. JACC Cardiovasc Imaging 6 (2013): 175-183.
- Patricia A Pellikka, Sherif F Nagueh, Abdou A Elhendy, et al. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 20 (2007): 1021-1041.
- Jean-Luc Monin, Jean-Paul Quéré, Mehran Monchi, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 108 (3): 319-324.
- Stefan Buchner, Kurt Debl, Franz-Xaver Schmid, et al. Cardiovascular magnetic resonance assessment of the aortic valve stenosis: an in vivo and ex vivo study. BMC Med Imaging 15 (2015): 34.
- Czarny MJ, Resar JR. Diagnosis and management of valvular aortic stenosis. Clin Med Insights Cardiol 8 (2014): 15-24.
