Anti-tumour Effect of Mangifera indica Against DMBA Induced Breast Cancer in Rats
Article Information
Jyoti Prakash1, Chandrajeet Kumar1, Aparna Jyoti Kujur2, Sanjiv Kumar3 and Arun Kumar4*
Affiliation:
1Department of Biotechnology, YBN University, Ranchi, Jharkhand
2Patna University, Patna, Bihar, India
3Bihar Veterinary College, Patna, Bihar, India
4Mahavir Cancer Sansthan and Research Centre,
Patna, Bihar, India
*Corresponding author: Dr. Arun Kumar, Senior Scientist, Mahavir Cancer Sansthan & Research Centre, Patna, Bihar- 801505, India.
Received: October 25, 2023; Accepted: November 06, 2023 Published: November 20, 2023
Citation: Jyoti Prakash, Chandrajeet Kumar, Aparna Jyoti Kujur, Sanjiv Kumar and Arun Kumar. Anti-tumour Effect of Mangifera indica Against DMBA Induced Breast Cancer in Rats. Journal of Cancer Science and Clinical Therapeutics 7 (2023): 204-211.
View / Download Pdf Share at FacebookAbstract
In recent times breast cancer incidences have increased manifold globally. It is the most common form of cancer worldwide. It is also the major cause of death in female cancer patients around the world. Despite various drug discoveries and advancements in medical treatment, it remains associated with a high mortality rate. Mangifera indica (L.) has been extensively used in the Indian medicine system Ayurveda, due to its various medicinal properties. It is the most common tree found in the Indian subcontinent and has had religious as well as social connections in life for centuries of years. However, there are very limited reports regarding its anticancer activity. Thus, the present study has been aimed to study the anticancer activity of Mangifera indica leaf extract on 7, 12-dimethylbenz(a)anthracene (DMBA) induced breast cancer in rats. Female Charles Foster rats, 55 – 60 days old weighing around (150±10 g) were used for the study and were induced DMBA (20 mg/mL dissolved in Olive oil) orally. After the development of breast tumors (about 0.8 cm), the rats were treated with Mangifera indica hydroxy-ethanolic leaf extract (200 mg/kg b.w./day) orally for 5 weeks and then the volume of the tumor was measured. Mangifera indica treatment showed significantly reduced mammary tumor volume (p< 0.05), along with significant reduction (p< 0.0001) in the different serum biomarkers such as TNF- α level and serum malondialdehyde (MDA) levels. Significant (p< 0.0001) improvement in both, the kidney and liver serum biomarker parameters were observed after the treatment with Mangifera indica hydroxy-ethanolic leaf extract. From the entire study, taking everything into account it can be interpreted that Mangifera indica hydroxy-ethanolic leaf extract possesses anti-proliferative activity by suppressing the progression of breast tumors in the rat model. The plant extract also possesses a hepato-renal protective effect. Hence, it can be targeted as a novel and safe anti-cancer drug against breast cancer.
Keywords
DMBA-induced breast model, Tumor volume, TNF alpha, Mangifera indica, novel drug discovery.
DMBA-induced breast model articles; Tumor volume articles; TNF alpha articles; Mangifera indica articles; novel drug discovery articles
DMBA-induced breast model articles DMBA-induced breast model Research articles DMBA-induced breast model review articles DMBA-induced breast model PubMed articles DMBA-induced breast model PubMed Central articles DMBA-induced breast model 2023 articles DMBA-induced breast model 2024 articles DMBA-induced breast model Scopus articles DMBA-induced breast model impact factor journals DMBA-induced breast model Scopus journals DMBA-induced breast model PubMed journals DMBA-induced breast model medical journals DMBA-induced breast model free journals DMBA-induced breast model best journals DMBA-induced breast model top journals DMBA-induced breast model free medical journals DMBA-induced breast model famous journals DMBA-induced breast model Google Scholar indexed journals Tumor volume articles Tumor volume Research articles Tumor volume review articles Tumor volume PubMed articles Tumor volume PubMed Central articles Tumor volume 2023 articles Tumor volume 2024 articles Tumor volume Scopus articles Tumor volume impact factor journals Tumor volume Scopus journals Tumor volume PubMed journals Tumor volume medical journals Tumor volume free journals Tumor volume best journals Tumor volume top journals Tumor volume free medical journals Tumor volume famous journals Tumor volume Google Scholar indexed journals TNF alpha articles TNF alpha Research articles TNF alpha review articles TNF alpha PubMed articles TNF alpha PubMed Central articles TNF alpha 2023 articles TNF alpha 2024 articles TNF alpha Scopus articles TNF alpha impact factor journals TNF alpha Scopus journals TNF alpha PubMed journals TNF alpha medical journals TNF alpha free journals TNF alpha best journals TNF alpha top journals TNF alpha free medical journals TNF alpha famous journals TNF alpha Google Scholar indexed journals Mangifera indica articles Mangifera indica Research articles Mangifera indica review articles Mangifera indica PubMed articles Mangifera indica PubMed Central articles Mangifera indica 2023 articles Mangifera indica 2024 articles Mangifera indica Scopus articles Mangifera indica impact factor journals Mangifera indica Scopus journals Mangifera indica PubMed journals Mangifera indica medical journals Mangifera indica free journals Mangifera indica best journals Mangifera indica top journals Mangifera indica free medical journals Mangifera indica famous journals Mangifera indica Google Scholar indexed journals novel drug discovery articles novel drug discovery Research articles novel drug discovery review articles novel drug discovery PubMed articles novel drug discovery PubMed Central articles novel drug discovery 2023 articles novel drug discovery 2024 articles novel drug discovery Scopus articles novel drug discovery impact factor journals novel drug discovery Scopus journals novel drug discovery PubMed journals novel drug discovery medical journals novel drug discovery free journals novel drug discovery best journals novel drug discovery top journals novel drug discovery free medical journals novel drug discovery famous journals novel drug discovery Google Scholar indexed journals Breast cancer articles Breast cancer Research articles Breast cancer review articles Breast cancer PubMed articles Breast cancer PubMed Central articles Breast cancer 2023 articles Breast cancer 2024 articles Breast cancer Scopus articles Breast cancer impact factor journals Breast cancer Scopus journals Breast cancer PubMed journals Breast cancer medical journals Breast cancer free journals Breast cancer best journals Breast cancer top journals Breast cancer free medical journals Breast cancer famous journals Breast cancer Google Scholar indexed journals female malignancy articles female malignancy Research articles female malignancy review articles female malignancy PubMed articles female malignancy PubMed Central articles female malignancy 2023 articles female malignancy 2024 articles female malignancy Scopus articles female malignancy impact factor journals female malignancy Scopus journals female malignancy PubMed journals female malignancy medical journals female malignancy free journals female malignancy best journals female malignancy top journals female malignancy free medical journals female malignancy famous journals female malignancy Google Scholar indexed journals Ayurveda articles Ayurveda Research articles Ayurveda review articles Ayurveda PubMed articles Ayurveda PubMed Central articles Ayurveda 2023 articles Ayurveda 2024 articles Ayurveda Scopus articles Ayurveda impact factor journals Ayurveda Scopus journals Ayurveda PubMed journals Ayurveda medical journals Ayurveda free journals Ayurveda best journals Ayurveda top journals Ayurveda free medical journals Ayurveda famous journals Ayurveda Google Scholar indexed journals breast tumors articles breast tumors Research articles breast tumors review articles breast tumors PubMed articles breast tumors PubMed Central articles breast tumors 2023 articles breast tumors 2024 articles breast tumors Scopus articles breast tumors impact factor journals breast tumors Scopus journals breast tumors PubMed journals breast tumors medical journals breast tumors free journals breast tumors best journals breast tumors top journals breast tumors free medical journals breast tumors famous journals breast tumors Google Scholar indexed journals hydroxy-ethanolic leaf articles hydroxy-ethanolic leaf Research articles hydroxy-ethanolic leaf review articles hydroxy-ethanolic leaf PubMed articles hydroxy-ethanolic leaf PubMed Central articles hydroxy-ethanolic leaf 2023 articles hydroxy-ethanolic leaf 2024 articles hydroxy-ethanolic leaf Scopus articles hydroxy-ethanolic leaf impact factor journals hydroxy-ethanolic leaf Scopus journals hydroxy-ethanolic leaf PubMed journals hydroxy-ethanolic leaf medical journals hydroxy-ethanolic leaf free journals hydroxy-ethanolic leaf best journals hydroxy-ethanolic leaf top journals hydroxy-ethanolic leaf free medical journals hydroxy-ethanolic leaf famous journals hydroxy-ethanolic leaf Google Scholar indexed journals
Article Details
Introduction
In terms of mortality rates, cancer ranks second worldwide. Cancer is responsible for around one in six deaths worldwide. Breast cancer has the highest mortality rate of any female malignancy. Breast cancer accounts for about 11.7% (2,261,419) of all new cancer cases diagnosed among women in 2020 and 6.9% (684,996) of all cancer-related deaths among women in 2020 worldwide; in 2018, that figure is expected to grow to 2.1 million.
Breast cancer accounts for 14% of all new cancer cases in India (1,62,468 cases), according to the latest data from the Global Cancer Report [1-4]. Hereditary and reproductive factors, prolonged exposure to estrogen, not nursing, and other lifestyle variables all have a role in the development of breast cancer. In addition to these, environmental variables are also thought to have a role in the onset of breast cancer. The increase in various illnesses, including breast cancer, may be traced back to improper dietary consumption and poor food quality. Today's farmers increasingly rely on pesticides as a means of increasing agricultural yields. However, xenoestrogens are present in these pesticides and are also present in food preservatives. Increased breast cancer risk is attributable to the interference of these synthetic xenoestrogens with the endocrine system. Polycyclic aromatic hydrocarbons (PAHs) found in grilled, barbecued, and smoked meat are also linked to an increased risk of breast cancer. The PAHs are pro-carcinogens that become active carcinogens after undergoing a sequence of events catalyzed by cytochrome p450 enzymes in the body [5-9]. Women are at increased risk of acquiring breast cancer due to these risk factors and their ever-changing lifestyles. Improvements in cancer treatment methods including chemotherapy, radiation, and the creation of various novel anticancer medications have been made possible by the rapid progress of modern medicine. Although these treatments are quite effective in extending cancer patients' lives, they are not without their share of drawbacks. The liver and kidneys are particularly vulnerable to the toxic effects of chemotherapy medicines. As a result, there is a pressing need for the discovery and development of cheaper, more effective, and less harmful cancer treatments. Experimental animal models show that medicinal herbs and dietary supplements may be extremely successful as chemo-preventive and anticancer medicines with acceptable safety profiles [10]. The South and Southeast Asian fruit Mangifera indica L. is from the Anacardiaceae family. Producing nations that rank high in mangoes include India, China, Thailand, Indonesia, Pakistan, Mexico, Brazil, Bangladesh, Nigeria, and the Philippines. Several phytochemicals in mango leaves have been linked to health benefits, including the antioxidant mangiferin, as well as phenolic acids, benzophenones, flavonoids, ascorbic acid, carotenoids, and tocopherols. The anti-cancer, anti-diabetic, antioxidant, anti-microbial, anti-obesity, lipid-lowering, hepato-protection, and anti-diarrheal properties of mango leaf (ML) extracts have been investigated. Moreover, several types of cancer cell lines (from the liver, breast, prostate, colon, and nasopharynx) have been found to have the cancer- inhibiting effect [11-20]. Very little work has been carried out on the breast cancer models, hence the present study aims to investigate the anti-tumor effect of leaves of Mangifera indica on DMBA-induced breast cancer in rats.
Materials and Methods
Chemicals and reagents
DMBA (7, 12-dimethylbenz(a)anthracene) manufactured by Sigma Aldrich, USA, product number D3254-1G, (CAS Number: 57-97-6), lot# PXLNG2901, p code: 1009330344 was purchased from the Scientific chemical store of Patna, Bihar, India. All the other solvents and chemicals used were of analytical grade 99%.
Preparation of Mangifera indica leaf ethanolic extract:
The leaves of Mangifera indica were collected from the local tree present in the Patna district of Bihar, India. The leaves were identified by a renowned botanist in Patna, Bihar, India. The leaves were shade-dried for 3 days followed by being dried in an incubator at 37°C temperature. Next, the leaves were ground into a powder and soaked in pure ethanol for 48 hours. The resulting ethanolic mixture of leaves was filtered through filter paper to eliminate any remaining solids. Absolute ethanol was used to extract the filtrate after it was transferred to a rota vapor apparatus. After determining the LD50 value (4000 mg/Kg body weight), the dosage of ethanolic extract was determined, and the final dose was titrated to 200 mg/kg body weight.
Animals
The animal house at the Mahavir Cancer Sansthan and Research Centre in Patna, India (CPCSEA Registration number. 1129/PO/ReBi/S/07/CPCSEA) kindly provided 24 female Charles Foster rats for this study. Institutional Animal Ethics Committee (IAEC) NO. 2021/1H-06/10/21 authorized the experiments. All procedures involving animal testing were conducted in compliance with the requirements set out by the Committee for Control and Supervision of Experiments on Animals (CPCSEA), New Delhi. Rats were provided with a free-access diet of food and water. Seven days were used for acclimating the rats before the experiments began. The rats used in the experiments were kept in conventional polypropylene cages, with two rats per cage, and were randomly assigned to either a control or treatment group. Rats were kept in an environment with a constant (24 2°C) temperature and a 12-hour light/dark cycle.
Experimental design
Animals (24 female, Charles Foster strain rats), aged 55 to 60 days, weighing around (150 ± 20 g) were classified into 3 groups of n=6 animals each.
Group I- Control group.
Group II- DMBA group - DMBA induced rats only.
Group III- DMBA + Mangifera indica group – DMBA-induced rats treated with Mangifera indica ethanolic leaf extract (200 mg/kg body weight per day) for 5 weeks after
tumor development (about 0.8 cm). Ketamine was used to anesthetize the rats at the end of the treatment, and were sacrificed in the diestrous part of their estrous cycle. The rat’s blood was obtained by orbital puncture method. Biochemical tests, lipid peroxidation estimates, TNF- estimates were performed on the serum. For the histological analysis, breast tissues were preserved in 10% formalin.
Tumor induction
Female Charles Foster rats (weighting 150 ±10 g) were used to induce tumors in the mammary glands. These rats were 55 days old. Following the protocol of [21] a single dosage of DMBA (7,12-dimethylbenz(a)anthracene) dissolved in olive oil at a concentration of 20 mg/mL was administered intragastrically. Beginning in the fourth week, following DMBA injection, 20 rats were palpated weekly to detect the growth of tumors. By week 20th, all 18 of the rats in the DMBA-treated group had tumors, while the first tumor emerged at week 18.
Evaluation of mammary tumor volume
The volume of breast tumors was determined using a vernier callipers. The tumor's volume (V) was determined using the formula V(cm3) = (L B2)/2, where L and B are the perpendicular tumor diameters in centimetres (cm).
Haematological parameters study
The acquired blood samples were analysed using conventional methods to determine haematological parameters such as complete blood count, white blood cell count, platelet count, and haemoglobin percentage.
Biochemical assays
The UV - Vis spectrophotometer (UV-10, Thermo Scientific, USA) was used for biochemical analysis following the standard kit technique (Coral crest). [22] the method was used to measure alanine transaminase (ALT) and aspartate transaminase (AST), [23] method was used to measure alkaline phosphatase (ALP), [24] method was used to measure total bilirubin [25-29] methods were used to analyze urea, creatinine, and uric acid, respectively, as kidney biomarker measures.
Lipid peroxidation (LPO)
The twofold heating method [30], which is based on the spectrophotometric assessment of colour reproduction during the reaction of thiobarbituric acid (TBA) with malondialdehyde (MDA), was used to assess TBARS, a marker of LPO. In this experiment, 0.5 mL of serum was combined with 2.5 mL of a 10% trichloroacetic acid (TCA) solution in a centrifuge tube, and the whole thing was heated in a water bath at 90°C for 15 minutes. The mixture was centrifuged at 3000 rpm for 10 minutes after cooling at ambient temperature, and the resulting 2 mL supernatant was
combined with 1 mL of 0.675% TBA solution in a test tube before being heated in a water bath at 90°C for 15 minutes. Subsequently, UV-visible spectrophotometer readings were taken at 532 nm (Thermo Scientific UV-10 USA).
Tumor necrosis factor-alpha (TNF- α) assay
Serum TNF- levels were measured with the ELISA technique. Diaclone, a French company, produced a rat TNF- ELISA kit (Cat. No. 872.010.001). According to the manufacturer's instructions and [31], the serum TNF- level was calculated using a Merck ELISA reader.
Histopathology study
Rats' breast tissue was cut into small pieces and fixed in 10% formalin for 24 hours. The tissues were then embedded in paraffin after being dehydrated with ethanol. For histological examination, 5µm sections were cut and stained with haemotoxylin and eosin.
Statistical analysis
The data is shown in a mean and standard error of the mean (SEM) format. Two-way analysis of variance (ANOVA) with time and drug as the two components were used to compare tumor volume between the DMBA group and the DMBA + Mangifera indica group. Biochemical, LPO, and hormonal testing results were compared across groups using one-way analysis of variance (ANOVA) and Tukey's multiple comparison tests for statistical significance. Statistical significance was set at P<0.05, and the analysis was performed with the help of the GraphPad Prism 5 program (GraphPad Software, Inc., San Diego, USA).
Results
Morbidity and mortality
Each of the six rats exposed to DMBA developed a tumor in the area of their respective mammary teats 1, 2, 3, and 7. The DMBA + Mangifera indica group significantly impeded the growth of tumors in the remaining six rats from teats 1, 3, 5, and 6. No deaths were recorded among any of the groups. The DMBA group and the DMBA + Mangifera indica group are shown graphically in (Figure 1).
Changes in tumor volume
Tumor volume increased with time in both the DMBA and DMBA + Mangifera indica groups. Figure 2 shows that when Mangifera indica ethanolic leaf extract was supplemented on DMBA-induced rats the tumor volume was significantly reduced (p< 0.005) compared to when DMBA was used alone. As a result, the Mangifera indica leaf extract led to a 47% decrease in ultimate tumor volume.
Changes in malondialdehyde (MDA) level
There was a statistically significant (p< 0.05) increase in
malondialdehyde (MDA), a measure of lipid peroxidation, between the DMBA group and the control group. However, compared to the DMBA group, the MDA level decreased considerably (p< 0.05) in the DMBA + Mangifera indica group (Table 1.).
Changes in TNF- α levels
The serum TNF- level was significantly higher in the DMBA-treated group compared to the control group (p<0.05). The serum TNF- level was lower in the DMBA + Mangifera indica group compared to the DMBA group (p<0.05) (Table 2).
Haematological parameters Study
According to the results of the haematological analysis, the red blood cell count, white blood cell count, platelet count, and haemoglobin percentage were all significantly decreased in the DMBA-treated rats compared to the control group rats but were significantly normalized in the Mangifera indica leaf extract-treated rats (p<0.05) (Table 3).
Changes in liver and kidney serum biomarker parameters
Serum total bilirubin, ALT, and ALP levels were all substantially (p<0.05) higher in the DMBA group compared to the control group. Serum total bilirubin, ALT, AST, and ALP levels were all considerably lower in the DMBA
Mangifera indica group compared to the DMBA group (p<0.05) (Table 4). Serum creatinine, urea, and uric acid
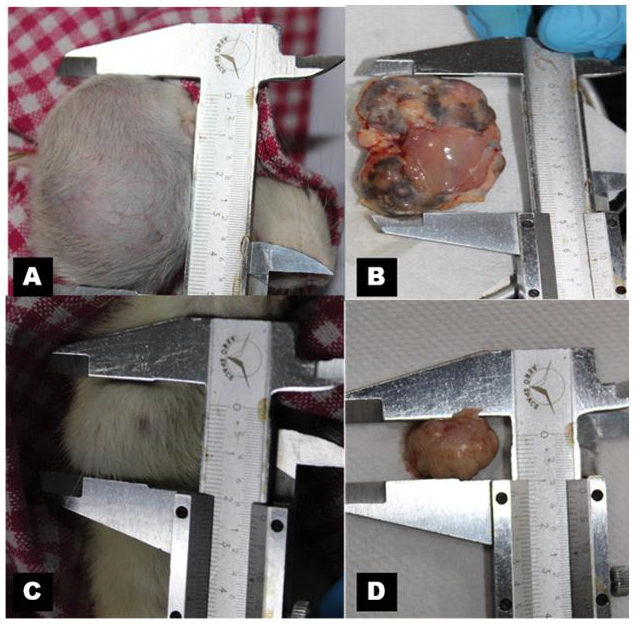
Figure 1: Gross photographs of rat mammary tumor, 1A & 1B of DMBA group (single dose of DMBA at 20 mg/mL in olive oil) and 1C & 1D of DMBA + Mangifera indica group. Mangifera indica was administered at the dose of 200 mg/kg body weight per day for 5 weeks after about 0.8 cm tumor development).
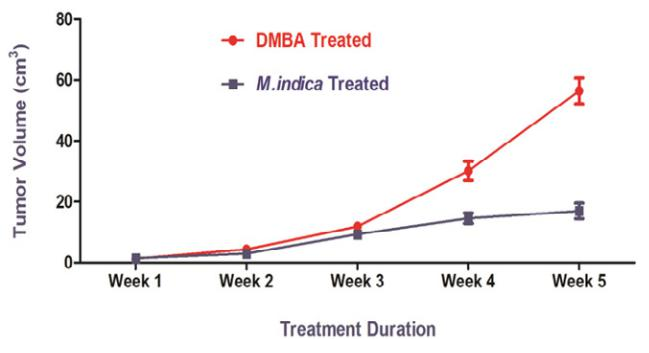
Figure 2: Effect of different treatments on tumor volume in the studied groups. DMBA group (single dose of DMBA at 20 mg/mL in olive oil), DMBA + Mangifera indica group (Mangifera indica at 200 mg/kg body weight per day for 5 weeks after about 0.8 cm tumor development). Values are expressed as mean ± SEM, n=6)
Table 1: Levels of Lipid Peroxidation in different treatment groups
|
Parameters |
Control |
DMBA Treated |
Mangifera indica Treated |
|
Lipid Peroxidation |
2.59 ± 0.94 |
205.53 ± 7.38 |
10.23 ± 2.43 |
Table 2: TNF alpha levels in different treatment groups
|
Parameters |
Control |
DMBA Treated |
Mangifera indica Treated |
|
TNF alpha (pg/mL) |
7.41 ± 0.23 |
98.75 ± 3.22 |
20.5 ± 2.12 |
Table 3: Haematological parameters
|
Parameters |
Control |
DMBA Treated |
Mangifera indica Treated |
|
RBC Count (× 106 mm −3) |
7.4 ± 1.5 |
2.53 ± 0.95 |
7.2 ± 2.83 |
|
WBC Count (mm −3) |
9400 ± 4.7 |
16000 ± 4.3 |
8700 ± 2.55 |
|
Platelets counts (×106 mm−3) |
1.9 ± 0.67 |
0.78 ± 0.83 |
2.5 ± 0.92 |
|
Haemoglobin (g/mL) |
13.7 ± 1.36 |
7.4 ± 2.45 |
13.1 ± 3.76 |
levels were all significantly (p<0.05) higher in the DMBA group compared to the control group, suggesting renal damage. Serum creatinine, urea, and uric acid levels were considerably decreased (p<0.005) in the DMBA + Mangifera indica group compared to the DMBA group (Table 4).
Histopathological findings
In the present histopathological examination Figure 3A, the mammary tissue section of the control rat, shows normal architecture of mammary tissue. The DMBA group rat shows a mammary tumor section in Figure 3B. Both mesenchymal and epithelial cells are involved in this, so it is a mixed mammary gland tumor. Its papillary projections, cystic dilatation, cellular sheet formation, pleomorphic, etc together with patches of embryonic mesenchymal cells confirm the
Tubulo-papillary carcinoma of the breast in rats. The DMBA
Mangifera indica group rat shows a mammary tumor section in Figure 3C. In these sections, there is a regression in the tumour cells.
Table 4: Biochemical Parameters Study
|
Parameters |
Control |
DMBA Treated |
Mangifera indica Treated |
|
SGPT (U/mL) |
30.12 ± 2.2 |
201.57 ± 3.47 |
40.5 ± 3.14 |
|
SGOT (U/mL) |
28.86 ± 1.6 |
230.78 ± 6.34 |
43.2 ± 2.12 |
|
ALP (KA units) |
4.67 ± 1.78 |
50.12 ± 2.46 |
7.56 ± 1.24 |
|
Urea (mg/dL) |
30.55 ± 1.89 |
72.12 ± 4.93 |
37.22 ± 1.78 |
|
Uric acid (mg/dL) |
4.14 ± 1.20 |
14.14 ± 1.45 |
9.24 ± 3.56 |
|
Creatinine (mg/dL) |
0.84 ± 0.49 |
4.17 ± 1.23 |
1.25 ± 1.12 |
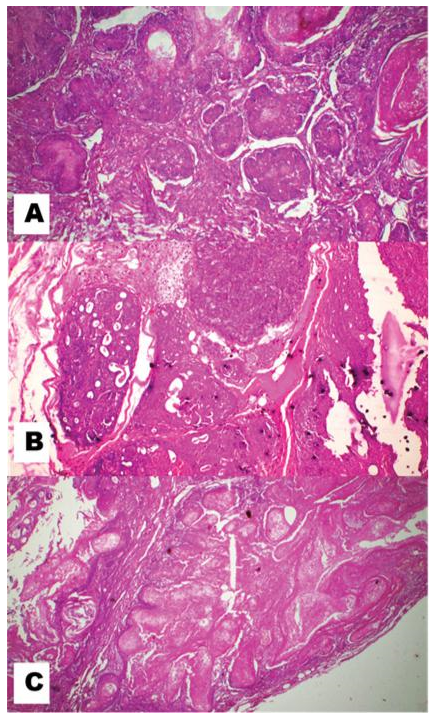
Figure 3: Microphotograph of rat mammary tissue stained with haematoxylin and eosin. (A) Section of control rat mammary tissue showing the normal arrangement of adipocytes and lobules x200.
The mammary tissue section of the DMBA group rat showing papillary projections, cystic dilatation, cellular sheet formation, pleomorphic, etc. together with patches of embryonic mesenchymal cells confirms the Tubulo-papillary carcinoma of breast x500. (C)
The mammary tissue section of DMBA + Mangifera indica leaf-treated group rat showing mature fibrous tissues indicates tumour regression process x500.
Discussion
DMBA is converted into the ultimate carcinogen DMBA-3,4-dihydrodiol-1,2-epoxide (DMBA-DE) by metabolic activation by the cytochrome p450 enzyme. Tissue redox equilibrium is disrupted due to the production of several reactive oxygen species (ROS) during metabolic activity. These reactive species promote malondialdehyde (MDA) production, a byproduct of lipid peroxidation (LPO). In both animal models of cancer and human cancer patients, high levels of MDA have been universally regarded as an indicator of oxidative stress and antioxidant status. Serum MDA levels were found to be significantly higher in the DMBA group compared to the control group in the current investigation. However, compared to the DMBA group, serum MDA levels decreased markedly in the DMBA+ Mangifera indica group. Mangifera indica ethanolic leaf extract's antioxidant capacity may be shown in its ability to lower levels of malondialdehyde (MDA). The primary phytochemical elements of Mangifera indica are responsible for its antioxidant action. As redox imbalance leads to unchecked cellular growth, the antioxidant potential may have helped preserve the redox condition of the cells, which is often disrupted by carcinogen metabolism. Mangiferin is a polyphenolic antioxidant and a glucosyl xanthone with significant antioxidant, anti-lipid peroxidation, immunomodulatory, cardiotonic, hypotensive, wound healing, antidegenerative, and antidiabetic actions, making it an important pharmacological and therapeutic chemical [32-34]. During the carcinogenic process induced by DMBA, the pro-inflammatory cytokine tumor necrosis factor (TNF) is expressed at higher levels, and NF-kB (nuclear factor-kB), a transcription factor involved in the survival and proliferation of neoplastic cells, is translocated into the nucleus via a TNF mediated increase. Elevated levels of TNF play a critical role in driving the development of breast cancer. Serum TNF levels were found to be significantly higher in the DMBA group compared to the control group in the current investigation. However, compared to the DMBA group, the serum TNF level was considerably lower in the DMBA+ Mangifera indica group. Mangifera indica ethanolic leaf extract's anti-inflammatory effects are shown by a lower blood TNF level. Cho et al. similarly noticed a decrease in TNF levels. Mangiferin's anti-proliferative effects on T cells have also been shown to play a vital role in controlling cell proliferation [35-38]. Despite a trend toward a smaller breast tumor volume in the DMBA + Mangifera indica group compared to the DMBA group at the study's end, the difference was not statistically significant. However, a maximum of 47% tumor growth inhibition was also detected in the last week of therapy, and it is highly conceivable that there would have been a large drop in the mammary tumor volume of the medicinal plant-treated group if treatment could have been extended for a longer time. Even though there are several effective anticancer medications now in use, many of them have major side effects that may affect a wide variety of bodily systems, including severely impairing liver and kidney function. It is crucial, therefore, to evaluate the effect of Mangifera indica leaf extract on the functioning of crucial organs including the liver and the kidney. The liver is the principal site where xenobiotic chemicals like DMBA are processed during detoxification. The liver damage and oxidative stress both resulted from the metabolism of the chemical carcinogen. Serum levels of ALT, AST, and ALP were found to be significantly higher in the DMBA group compared to the other two groups. An indicator of liver deterioration is an elevated serum biomarker for hepatitis. But, compared to the DMBA group, the blood total bilirubin, ALT, AST, and ALP levels were considerably lower in the DMBA + Mangifera indica group. Mangifera indica ethanolic leaf extract has hepatoprotective properties, as shown by the decreased serum levels of liver biomarker measures in the DMBA + Mangifera indica group. Similar studies on other models have been well documented [39-42].
The kidney is a crucial organ that not only eliminates harmful byproducts of metabolism but also produces essential chemicals. Consequently, chemotherapeutic drugs may have higher systemic toxicity due to renal impairment, since their excretion and metabolism may be slowed. Serum levels of kidney biomarkers urea, creatinine, and uric acid were all found to be significantly elevated in the DMBA group. The nephrotoxic effects of DMBA are shown by the increased kidney biomarker level. Serum renal biomarkers including urea, creatinine, and uric acid all decreased to a much greater extent in the DMBA + Mangifera indica group than in the DMBA group alone. Mangifera indica leaf extract's beneficial benefits against DMBA-induced renal damage in rats are seen in the rapid restoration of serum kidney biomarker levels as well. Similar studies on other models have been well documented [43-48]. Mangifera indica ethanolic leaf extract has been shown to have anti-proliferative effects in a histopathological examination. Mammary tissue sections from the DMBA group revealed characteristics consistent with tubular-papillary carcinoma of the breast, including papillary projections, cystic dilatation, cellular sheet development, pleomorphic, and patches of embryonic mesenchymal cells, indicating a more rapid tumor growth rate. Most of the fibrous tissues in the DMBA + Mangifera indica group are mature, which is indicative of a slower tumor development rate. The ultimate tumor volume of the two-treatment group was measured, further confirming the antiproliferative characteristics. Similar, studies on other models have been well documented by [49, 50, 51].
Conclusion
All this evidence implies that the ethanolic leaf extract of Mangifera indica has antitumorigenic properties, particularly in its ability to fight free radicals. The plant extract also protects the liver and kidneys. Therefore, it is reasonable to infer that the plant extract has a preventative and curative function in preventing DMBA-induced breast cancer in rats. There is also promising research into using an ethanolic extract of Mangifera indica leaf as a chemotherapeutic agent for the treatment of breast cancer. Furthermore, studies are recommended to get the molecular mechanism and mode of action of leaf extract of Mangifera indica. However, the present study appears to have a promising role in controlling breast cancer in DMBA-induced rat models.
Acknowledgments
The authors are thankful to Mahavir Cancer Sansthan and Research Centre for providing the experimental infrastructure and YBN University for the entire research facilities & Dr. Sanjiv Kumar, Associate Professor cum Scientist, Department of Pathology, Bihar Veterinary College, Patna, Bihar (India) for histopathological confirmation.
Author Contributions:
Design of experiments and preparation of manuscript (Jyoti Prakash, Chandrajeet Kumar, Arun Kumar). Conducted laboratory studies (Jyoti Prakash, Aparna Jyoti Kujur and Arun Kumar). Microphotography and interpretation were done by Sanjiv Kumar. Proofreading of the manuscript (Jyoti Prakash, Chandrajeet Kumar, Sanjiv Kumar, Aparna Jyoti Kujur, Arun Kumar). All authors read and approved the final manuscript.
Funding
The authors declare that no funding was received for this study.
Declaration of conflicting interests
The authors declare that they have no conflict of interest.
References
- GLOBOCAN (2020): 1-2.
- IARC (International Agency for Research on Cancer) (2020).
- WHO cancer Fact sheet (2018).
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144 (2019): 1941-1953.
- Paterni I, Granchi C, Minutolo F. Risks and benefits related to alimentary exposure to xenoestrogens. Crit Rev Food Sci Nutr 57 (2017): 3384-3404.
- Fernandez SV, Russo J. Estrogen and xenoestrogens in breast cancer. Toxicol Pathol 1 (2010): 110-122.
- Kim JH, Lee J, Jung SY, et al. Dietary Factors and Female Breast Cancer Risk: A Prospective Cohort Study. Nutrients 9 (2017): 1331.
- Parada H Jr, Steck SE, Bradshaw PT, et al. Grilled, Barbecued, and Smoked Meat Intake and Survival Following Breast Cancer. J Natl Cancer Inst 109 (2017): 299.
- Rengarajan T, Rajendran P, Nandakumar N, et al. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac J Trop Biomed 5 (2015): 182-189
- Toledo RCL, Brito LF, Caetano MMM, et al. Acute treatment with Mangifera indica leaf extract attenuates liver inflammation in rats fed a cafeteria diet. Food & function 10 (2019): 4861-4867.
- Ganogpichayagrai A, Palanuvej C, Ruangrungsi N. Antidiabetic and anticancer activities of Mangifera indica Okrong leaves. Journal of advanced pharmaceutical technology & research 8 (2017): 19-24.
- Noratto GD, Bertoldi MC, Krenek K, et Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J Agric Food Chem 58 (2010): 4104-4112.
- Kim H, Moon JY, Kim H, et al. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) fllesh and peel. Food Chem 121 (2010): 429-36.
- Parvez GM. Pharmacological activities of mango (Mangifera Indica): A review. J Pharmacogn Phytochem 5 (2016): 1-7.
- Li M, Ma H, Yang L, et al. Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA-182. Oncol Lett 11 (2016): 817-822.
- Wang Y, Guo X, Fan X, et al. The protective effect of mangiferin on osteoarthritis: An in vitro and in vivo study. Physiological Research 71 (2022): 135-145.
- Li H, Huang J, Yang B, et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol Appl Pharmacol 272 (2013): 180-190.
- Li W, Wang K, Liu Y, et al. (2022). A Novel Drug Combination of Mangiferin and Cinnamic Acid Alleviates Rheumatoid Arthritis by Inhibiting TLR4/NFκB/NLRP3 Activation-Induced Pyroptosis. Frontiers in immunology 13 (2022): 912933.
- Laurindo LF, Santos AROD, Carvalho ACA, et al. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 13 (2023): 96.
- Sarfraz M, Khan A, Batiha GE, et al. (2023). Nanotechnology-Based Drug Delivery Approaches of Mangiferin: Promises, Reality and Challenges in Cancer Chemotherapy. Cancers 15 (2023): 4194.
- Liu G, Wang H, Zhang F, et al. The effect of VPA on increasing radiosensitivity in osteosarcoma cells and primary-culture cells from chemical carcinogen-induced breast cancer in rats. Int J Mol Sci 18 (2017): 1027.
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28 (1957): 56-63.
- Kind PRH, King EJ. Determination of Alkaline Phosphatase activity in serum. J Clin Pathol 7 (1954): 322.
- Jendrassik GF, Grofs BM. Quantitative colorimetric determination of bilirubin in serum or plasma. Clin. Chim. Acta 27 (1938): 9.
- Dumas BT, Watson WA, Biggs HG. Quantitative colorimetric determination of albumin in serum or plasma. Clin. Chim. Acta 31 (1971): 87-91.
- Berthelot MPE. Berthelot’s Reaction Mechanism. Report de Chimie Applique (1859): 2884.
- Fawcett JK, Scott J. A rapid and precise method for the determination of urea. J Clin Pathol 13 (1960): 156-159.
- Bones RW, Tausky HH. Colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem 158 (1945): 581-591.
- Fossati P, Prencipe L. Enzymatic colorimetric method of the determination of uric acid in serum. Clin Chem 26 (1980): 227.
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186 (1990): 421-431.
- Beutler B, Cerami A. Cachectin (tumor necrosis factor): a macrophage hormone governing cellular metabolism and inflammatory response. Endo Rev 9 (1988): 57-66.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacognosy reviews 4 (2010): 42-48.
- Lauricella M, Emanuele S, Calvaruso G, et al. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 9 (2017): 525.
- Ediriweera MK, Tennekoon KH, Samarakoon SR. A Review of Ethnopharmacological Applications, Pharmacological Activities, and Bioactive Compounds of Mangifera indica (Mango). Evidence-based complementary and alternative medicine: eCAM (2017): 6949835.
- Imran M, Arshad MS, Butt MS, et al. Mangiferin: a natural miracle bioactive compound against lifestyle-related disorders. Lipids in health and disease 16 (2017): 84.
- Garrido G, Delgado R, Lemus Y, et al. Protection against septic shock and suppression of tumor necrosis factor-alpha and nitric oxide production on macrophages and microglia by a standard aqueous extract of Mangifera indica (VIMANG). Role of mangiferin isolated from the extract. Pharmacological Research 50 (2004): 165-172.
- Saleh S, El-Maraghy N, Reda E, et al. (2014). Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: role of adiponectin and TNF-α. Anais da Academia Brasileira de Ciencias 86 (2014): 1935-1948.
- Naraki K, Rezaee R, Mashayekhi-Sardoo H, et Mangiferin offers protection against deleterious effects of pharmaceuticals, heavy metals, and environmental chemicals. Phytotherapy research: PTR 35 (2021): 810-822.
- Rodeiro I, José Gómez-Lechón M, Perez G, et al. Mangifera indica extract and mangiferin modulate cytochrome P450 and UDP-glucuronosyltransferase enzymes in primary cultures of human hepatocytes. Phytotherapy research: PTR 27 (2013): 745-752.
- Pardo-Andreu GL, Barrios MF, Curti C, et al. Protective effects of Mangifera indica L extract (Vimang), and its major component mangiferin, on iron-induced oxidative damage to rat serum and liver. Pharmacological Research 57 (2008a): 79-86.
- Pardo-Andreu GL, Paim BA, Castilho RF, et al. Mangifera indica extract (Vimang) and its main polyphenol mangiferin prevent mitochondrial oxidative stress in atherosclerosis-prone hypercholesterolemic mice. Pharmacological research 57 (2008b): 332-338.
- Badmus JA, Adedosu TO, atoki JO, et Lipid peroxidation inhibition and antiradical activities of some leaf fractions of Mangifera indica. Acta poloniae pharmaceutica 68 (2011): 23-29.
- Lum PT, Sekar M, Gan SH, et al. Therapeutic potential of mangiferin against kidney disorders and its mechanism of action: A review. Saudi journal of biological sciences 29 (2022): 1530-1542.
- Villas-Boas GR, Paes MM, Gubert P, et al. Evaluation of the toxic potential of the aqueous extract from Mangifera indica (Anacardiaceae) in rats submitted to experimental models of acute and subacute oral toxicity. Journal of Ethnopharmacology 275 (2021): 114100.
- Gondi M, and Prasada Rao UJ. Ethanol extract of mango (Mangifera indica) peel inhibits α-amylase and α-glucosidase activities and ameliorates diabetes-related biochemical parameters in streptozotocin (STZ)-induced diabetic rats. Journal of food science and technology 52 (2015): 7883-7893.
- Parmar HS, and Kar A. Possible amelioration of atherogenic diet-induced dyslipidemia, hypothyroidism, and hyperglycemia by the peel extracts of Mangifera indica, Cucumis melo, and Citrullus vulgaris fruits in rats. BioFactors (Oxford, England) 33 (2008): 13-24.
- Sahu AK, Verma VK, Mutneja E, et al. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of the MAPK pathway. Molecular and cellular biochemistry 452 (2019): 141-152.
- Yang H, Gao L, Niu Y, et al. Mangiferin Inhibits Renal Urate Reabsorption by Modulating Urate Transporters in Experimental Hyperuricemia. Biological & Pharmaceutical Bulletin 38 (2015): 1591-1598.
- Akhouri V, Kumar A, Kumari M. Antitumour Property of Pterocarpus santalinus Seeds Against DMBA-Induced Breast Cancer in Rats. Breast cancer: basic and clinical research, 14 (2020a), 1178223420951193.
- Akhouri V, Kumari M, Kumar A. The therapeutic effect of Aegle marmelos fruit extract against DMBA-induced breast cancer in rats. Scientific reports 10 (2020b): 18016.
- Saunders WB. Tietz Textbook of Clinical Chemistry. 2nd edition. Philadelphia (1994).
