Antioxidant; Coconut water; Drosophila melanogaster; Free radicals; Phenolic compound
Article Information
Oluwarotimi EA*, Odubanjo VO, Adesanmi JI, Alo OJ, Aminu RS, Faturoti IE
Department of Biochemistry, Adekunle Ajasin University, P.M.B 01, Nigeria
*Corresponding Author: Emmanuel Ayo Oluwarotimi. Department of Biochemistry, Adekunle Ajasin University, P.M.B 01, Nigeria.
Received: 16 April 2021; Accepted: 26 April 2021; Published: 28 April 2021
Citation: Oluwarotimi EA, Odubanjo VO, Adesanmi JI, Alo OJ, Aminu RS, Faturoti IE. Antioxidant Capacity and Effect of Coconut Water on Alcl3 Amnesic-Induced Drosophila Melanogaster. Journal of Analytical Techniques and Research 3 (2021): 1-13.
View / Download Pdf Share at FacebookAbstract
Coconut water (CW) has gained wide attraction as a known functional food that offers additional benefits to its basic nutritional value. CW is employed in the management of oxidative stress-associated diseases for its natural antioxidant features. This study sought to investigate CW antioxidant capacity and effect upon its dietary inclusion in an Alcl3-induced amnesic Drosophila melanogaster. CW was extracted from the coconut fruit cotyledon and supplemented in the flies’ diet for five days. The CW extract antioxidant activity was examined in vitro through the total phenol, total flavonoid content, ferric reducing power, iron-chelating ability, ABTS* and DPPH* scavenging ability assays. Likewise, the anti-lipid peroxidation potential of CW extract was also measured in vivo using D. melanogaster. The CW extract has a total phenol content of 1.48 ± 0.43 (mg/g GAE) and a total flavonoid content of 0.53 ± 0.02 (mg/g QE) which could be attributed to its scavenging ability against ABTS* and DPPH* in vitro with an increase in extract concentration. Similarly, a positive trend was detected in the ferric reducing antioxidant power and iron-chelating ability tests. Furthermore, dietary inclusion of CW in groups fed with 0.1% and 1% CW lowers Malondialdehyde (MDA) concentration significantly (p < 0.01 and p < 0.0001 respectively) in Alcl3 induced flies. This correlates with CW’s ability to considerably (p < 0.05) reduced MDA in vitro in a concentration-dependent manner (0.01 – 0.03 mg/mL). These findings provide substantial information that affirms CW's natural antioxidant ability, thus bolstering its therapeutics usage.
Keywords
Antioxidant; Coconut water; Drosophila melanogaster; Free radicals; Phenolic compound
Antioxidant articles; Coconut water articles; Drosophila melanogaster articles; Free radicals articles; Phenolic compound articles
Antioxidant articles Antioxidant Research articles Antioxidant review articles Antioxidant PubMed articles Antioxidant PubMed Central articles Antioxidant 2023 articles Antioxidant 2024 articles Antioxidant Scopus articles Antioxidant impact factor journals Antioxidant Scopus journals Antioxidant PubMed journals Antioxidant medical journals Antioxidant free journals Antioxidant best journals Antioxidant top journals Antioxidant free medical journals Antioxidant famous journals Antioxidant Google Scholar indexed journals Coconut water articles Coconut water Research articles Coconut water review articles Coconut water PubMed articles Coconut water PubMed Central articles Coconut water 2023 articles Coconut water 2024 articles Coconut water Scopus articles Coconut water impact factor journals Coconut water Scopus journals Coconut water PubMed journals Coconut water medical journals Coconut water free journals Coconut water best journals Coconut water top journals Coconut water free medical journals Coconut water famous journals Coconut water Google Scholar indexed journals Drosophila melanogaster articles Drosophila melanogaster Research articles Drosophila melanogaster review articles Drosophila melanogaster PubMed articles Drosophila melanogaster PubMed Central articles Drosophila melanogaster 2023 articles Drosophila melanogaster 2024 articles Drosophila melanogaster Scopus articles Drosophila melanogaster impact factor journals Drosophila melanogaster Scopus journals Drosophila melanogaster PubMed journals Drosophila melanogaster medical journals Drosophila melanogaster free journals Drosophila melanogaster best journals Drosophila melanogaster top journals Drosophila melanogaster free medical journals Drosophila melanogaster famous journals Drosophila melanogaster Google Scholar indexed journals ree radicals articles ree radicals Research articles ree radicals review articles ree radicals PubMed articles ree radicals PubMed Central articles ree radicals 2023 articles ree radicals 2024 articles ree radicals Scopus articles ree radicals impact factor journals ree radicals Scopus journals ree radicals PubMed journals ree radicals medical journals ree radicals free journals ree radicals best journals ree radicals top journals ree radicals free medical journals ree radicals famous journals ree radicals Google Scholar indexed journals Phenolic compound articles Phenolic compound Research articles Phenolic compound review articles Phenolic compound PubMed articles Phenolic compound PubMed Central articles Phenolic compound 2023 articles Phenolic compound 2024 articles Phenolic compound Scopus articles Phenolic compound impact factor journals Phenolic compound Scopus journals Phenolic compound PubMed journals Phenolic compound medical journals Phenolic compound free journals Phenolic compound best journals Phenolic compound top journals Phenolic compound free medical journals Phenolic compound famous journals Phenolic compound Google Scholar indexed journals scientific evidence articles scientific evidence Research articles scientific evidence review articles scientific evidence PubMed articles scientific evidence PubMed Central articles scientific evidence 2023 articles scientific evidence 2024 articles scientific evidence Scopus articles scientific evidence impact factor journals scientific evidence Scopus journals scientific evidence PubMed journals scientific evidence medical journals scientific evidence free journals scientific evidence best journals scientific evidence top journals scientific evidence free medical journals scientific evidence famous journals scientific evidence Google Scholar indexed journals basic nutritional value articles basic nutritional value Research articles basic nutritional value review articles basic nutritional value PubMed articles basic nutritional value PubMed Central articles basic nutritional value 2023 articles basic nutritional value 2024 articles basic nutritional value Scopus articles basic nutritional value impact factor journals basic nutritional value Scopus journals basic nutritional value PubMed journals basic nutritional value medical journals basic nutritional value free journals basic nutritional value best journals basic nutritional value top journals basic nutritional value free medical journals basic nutritional value famous journals basic nutritional value Google Scholar indexed journals oxidative stress-associated diseases articles oxidative stress-associated diseases Research articles oxidative stress-associated diseases review articles oxidative stress-associated diseases PubMed articles oxidative stress-associated diseases PubMed Central articles oxidative stress-associated diseases 2023 articles oxidative stress-associated diseases 2024 articles oxidative stress-associated diseases Scopus articles oxidative stress-associated diseases impact factor journals oxidative stress-associated diseases Scopus journals oxidative stress-associated diseases PubMed journals oxidative stress-associated diseases medical journals oxidative stress-associated diseases free journals oxidative stress-associated diseases best journals oxidative stress-associated diseases top journals oxidative stress-associated diseases free medical journals oxidative stress-associated diseases famous journals oxidative stress-associated diseases Google Scholar indexed journals therapeutics usage articles therapeutics usage Research articles therapeutics usage review articles therapeutics usage PubMed articles therapeutics usage PubMed Central articles therapeutics usage 2023 articles therapeutics usage 2024 articles therapeutics usage Scopus articles therapeutics usage impact factor journals therapeutics usage Scopus journals therapeutics usage PubMed journals therapeutics usage medical journals therapeutics usage free journals therapeutics usage best journals therapeutics usage top journals therapeutics usage free medical journals therapeutics usage famous journals therapeutics usage Google Scholar indexed journals hytohormone Cytokinins articles hytohormone Cytokinins Research articles hytohormone Cytokinins review articles hytohormone Cytokinins PubMed articles hytohormone Cytokinins PubMed Central articles hytohormone Cytokinins 2023 articles hytohormone Cytokinins 2024 articles hytohormone Cytokinins Scopus articles hytohormone Cytokinins impact factor journals hytohormone Cytokinins Scopus journals hytohormone Cytokinins PubMed journals hytohormone Cytokinins medical journals hytohormone Cytokinins free journals hytohormone Cytokinins best journals hytohormone Cytokinins top journals hytohormone Cytokinins free medical journals hytohormone Cytokinins famous journals hytohormone Cytokinins Google Scholar indexed journals
Article Details
1. Introduction
Coconut fruit, botanically called Cocos nucifera, is a member of the Arecaceae family, which provides numerous health benefits beyond its nutritional content [1]. Coconut water has gained attention as a functional food with its application in health and medicine, supported by increasing scientific evidence [2]. This coconut water is the plant's liquid endosperm and an outstanding natural drink with a caloric of 174/100g. Coconut water contains unique beneficial ingredients, namely sugars, vitamins, minerals, amino acids, and plant hormones which justifies its wide applications [3, 4]. The phytohormone Cytokinins' present in coconut water influences the plant cell division and confers its anti-aging effects [5]. According to some experimental research and reviews, coconut water has been revealed to contain minerals and aromatic compounds as its primary composition [3, 6] Young coconut water (about six months) contains estrogen-like compounds that prevent Alzheimer’s disease in menopausal women [7], while the matured coconut water of about 12 months has displayed hypoglycemic effect and also reduced oxidative stress in rats [8].
Reactive oxygen species (ROS) are released as a byproduct during various cellular metabolism. Consequently, continuous exposure to environmental stresses results in oxidative stress and eventually death [9, 10]. Oxidative stress contributes to neurodegeneration and plays an essential role in Alzheimer's disease (AD) and Parkinson’s disease (PD) pathogenesis [11]. Neurodegenerative diseases are often age-associated, usually characterized by cognitive decline, memory loss and behavioral disturbances [12]. The brain antioxidant defensive system is poor and thus more susceptible to free radical’s attack [13]. The cells counteract this attack under normal conditions via homeostatic balance regulation, but the cell loses the ability in a disease condition [14]. This is characterized by accumulated free radicals and antioxidant system dysfunction, ultimately causing oxidative stress [15].
Compounds with high phenolic content easily donate hydroxyl hydrogen due to the resonance stabilization [16], scavenge free radicals, activate the antioxidant system, and chelate metals [17,18]. Natural compounds such as polyphenolic compounds derived from plants act as antioxidants that confer beneficial health functions [19]. These compounds function by reducing or neutralizing the formation of free radicals and hence protect the cell from oxidative damage [20, 21]. Phenolic compounds are important phytochemicals that exhibit several bioactive properties, including antioxidant activity [22]. Many studies have reported the quantification and identification of phenolic compounds in different kinds of fruits and vegetables, but only a few studies have reported coconut water antioxidant activity [23, 24]. However, a study conducted by Chang and Wu [25] reported the identification of phenolic compounds.
Drosophila melanogaster, a fruit fly now commonly used as a multipurpose model organism for biomedical science research due to its economic advantage to culture in the laboratory, rapid generation time, shorter life cycle, high production rate and genetic modifications possibilities [26]. Its lifespan is between 40-120 days, depending on diet and environmental stress conditions. Some diets, such as those rich in free saccharides and cholesterol, can reduce fruit flies life span [27]. This present study was designed to examine the free radical scavenging property of coconut water and the antioxidant effect of its dietary inclusion in an oxidative-induced D. melanogaster.
2. Methods
2.1 Sample preparation
Figure 1a shows a sample of fresh Cocos nucifera fruits (thirty) purchased from a native market in Akungba-Akoko, Ondo State, Nigeria. The plant was identified and authenticated at the Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba, Akoko, Ondo State. The shell of the coconut fruit was removed and washed properly to avoid contamination. The cotyledon was broken (Figure 1b) to extract the water. It was then broken, and the water was collected in labeled vials, kept in a deep freezer until use.
2.2 Experimental design
- melanogaster Harwich strain (both gender, five days old) were divided into four groups containing 40 flies each. Group I was placed on a normal diet, while groups II-IV were placed on a basal diet containing: 10mM Al, 0.1% CW and 1%CW as shown thus:

Figure 1: a. Fresh Coconut fruit b. Cotyledon of C. nucifera (Kannaian et al., 2020).
|
Groups |
Diet |
|
I |
Normal diet (positive control) |
|
II |
Normal diet + 10mM Al3+ (from Alcl3) |
|
III |
Normal diet + 10mM Alcl3 + 0.1% CW |
|
IV |
Normal diet + 10mM Alcl3 + 1% CW |
The flies were exposed to these treatments for five days and sustained at ambient temperature. All experiments were done in triplicates [19].
2.3 Drosophila melanogaster stock culture
Wild type D. melanogaster (Harwich strain) stock culture was obtained from Drosophila research laboratory, Department of Biochemistry, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria. The flies were grown on a normal diet made up of cornmeal medium containing 1% w/v brewer's yeast and 0.08% v/w nipagin at constant temperature and humidity (25 ± 1oC; 60% relative humidity respectively) under 12 h dark/light cycle conditions. All the experiments were executed with equivalent D. melanogaster strain [28].
2.4 Diet preparation
The basal diet was based on the traditional cornmeal medium containing 1% w/v brewer's yeast, 2% w/v sucrose, 1% w/v powdered milk, 1% w/v agar and 0.08% v/w nipagin. The diet was prepared once a week. The coconut water supplemented diet was prepared by adding 0.1mg/g and 1.0 mg/g coconut water, respectively. The media were then mixed and distributed into vials [19].
2.5 Animal transfer for new emergence and treatment
The flies were transferred every five days to prevent overpopulation and contamination and also to breed new flies. The following method was employed for the transfer of the flies from old jars to new jars. A funnel was placed on the new jar, while the old jar was gently tapped on a soft padded surface (towel) so that the flies fall to the bottom of the jar. The jar mouth's cotton plug was quickly removed and then placed on the inverted funnel and slightly banged on the padded surface. Thus, the flies were transferred into a new feed [19].
2.6 Preparation of sample for biochemical assays
Using a Teflon homogenizer, the anesthetized flies were homogenized in 0.1M phosphate buffer, pH 7.4and, the resulting homogenates were centrifuged at 10,000g, 4°C for 10 minutes in a Kenxin refrigerated centrifuge Model KX3400C (KENXIN Intl. Co., Hong Kong). After that, the supernatant was removed from the pellet into an Eppendorf tube used for some bioassays.
2.7 Determination of biochemical parameters
Total phenol content was quantified as described by Singleton et al. [29], total flavonoid content as stated by Meda et al. [30], but with slight modifications. Free radical scavenging activity of coconut water was investigated through the 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonate (ABTS) and 2, 2-diphenyl -1- picrylhydrazyl assays as described by Re et al. [31] and by Brand-Williams et al. [32], respectively. While the Ferric reducing antioxidant power was determined using the method of Oyaizu [33], Iron chelating ability was measured according to Minotti and Aust [34] method, with a slight modification by Puntel et al. [35].
2.8 Anti-lipid peroxidation assay
A modified [36] method was employed for the anti-lipid peroxidation assay. A reaction mixture containing 30 µL of 0.1M pH 7.4 Tris-HCl buffer, coconut water (0-100 µL) and 30 µL of 250 µM freshly prepared FeSO4 was mixed with 100 µL S1 fraction briefly. The volume was top-up to 300 µL with distilled water and incubated at 37? for 1hour. 300 µL 8.1% Sodium dodecyl sulphate (SDS) was added to the reaction mixture for colour reaction development, afterward followed by the addition of 500 µL of acetic acid/HCl (pH 3.4) and 500 µL 0.8% thiobarbituric acid (TBA) mixture. This mixture was incubated at 100? for 1hour. The produced thiobarbituric acid reactive species (TBARS) from the reactions were measured at 532nm using a JENWAY UV-Visible spectrophotometer. The absorbance was compared against the malondialdehyde (MDA) standard curve.
2.9 Statistical analysis
Data were pooled and expressed as mean ± standard deviation (SD). All results were statistically analyzed using the Graph pad PRISM (V.5.0) software. Levels of significance were accepted at p < 0.05, p < 0.01, and p < 0.001 for One-way analysis of variance (ANOVA), while the IC50 (concentration of extract that will cause 50% reducing activity) was determined using linear regression analysis [37].
3. Results
Table 1 presents the total phenol and total flavonoid content of coconut water as 1.48 ± 0.43 (mg/g GAE) and 0.53 ± 0.02 (mg/g QE) respectively.
Figure 1 reveals the 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonate (ABTS) radical scavenging ability of coconut water extract (0 – 400 μg/mL) expressed in mmol. TEAC/g. The extract scavenged ABTS* significantly at p < 0.05 in a concentration-dependent manner. Figure 2, the 2, 2-diphenyl -1- picrylhydrazyl (DPPH) radical scavenging ability of coconut water was presented.
The extract scavenged DPPH radical in a concentration-dependent manner (0-133 μg/mL).
The ferric reducing antioxidant property of coconut water was determined and expressed as ascorbic acid equivalents (Figure 3). The result showed that coconut water reduced Fe3+ to Fe2+ by an observed increase in Fe2+ with extract concentration. Likewise, Figure 4 presents the Fe2+ chelating ability of this coconut water extract. The result revealed that coconut water chelated Fe2+ in a dose-dependent manner.
The effect of coconut water extract against 250mM Fe2SO4 - induced lipid peroxidation in Drosophila melanogaster homogenate flies is presented in Figure 5. Fe2SO4 significantly (p < 0.05) increased malondialdehyde (MDA) level in the fly homogenate (125 ± 2.06). However, coconut water significantly (p < 0.05) decreased the MDA content in Fe2+-stressed homogenate flies in a dose-dependent manner (0.01 – 0.03 mg/mL).
Figure 6 shows the effect of dietary inclusion of coconut water on brain malondialdehyde (MDA) content in Alcl3 induced amnesic flies. The result reveals that the MDA content in flies fed with Alcl3 was significantly (p < 0.05) higher than the control group. However, 0.1% and 1% CW dietary inclusion significantly lowers (p < 0.01 and p < 0.001 respectively) the MDA content against the control without CW.
Table.1: Total phenol and total flavonoid content of Coconut water.
|
CW |
|
|
Total phenol (mg/g GAE) |
1.48 ± 0.43 |
|
Total flavonoid (mg/g QE) |
0.53 ± 0.02 |
|
Values represent means of triplicate readings. |
Key: CW Coconut water.
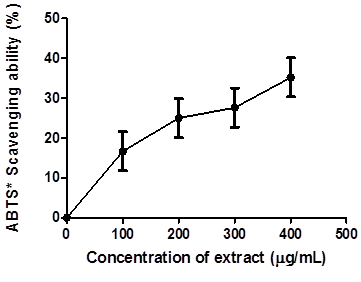
Figure.1: 2, 2-azinobis-3-ethylbenzothiazoline-6-sulfonate (ABTS*) radical scavenging ability of coconut water extract.
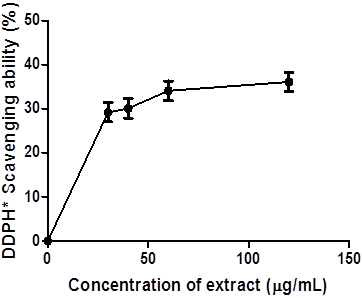
Figure.2: 2, 2-diphenyl -1- picrylhydrazyl (DPPH) radical scavenging ability of coconut water.
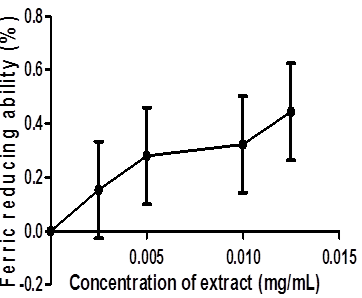
Figure.3: Ferric reducing ability of Coconut water.
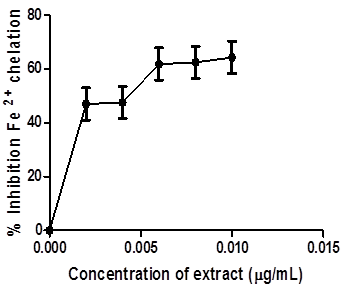
Figure.4: Fe2+ chelating ability of Coconut water.
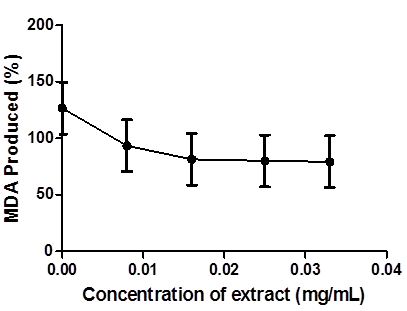
Figure.5: Inhibition of Fe- induced lipid peroxidation in Drosophila melanongaster by Coconut Water
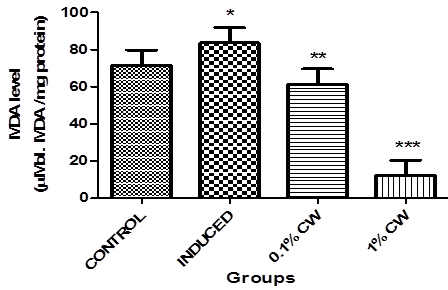
Figure.6: Effect of dietary inclusion of coconut water on brain MDA level in Alcl3 induced amnesic Drosophila melanongaster. Values represent mean ± SD. *Values are significantly different at p < 0.05, **Values are significantly different at p < 0.01, ***Values are significantly different at p < 0.001.
4. Discussion
Over the years, coconut has been extensively explored for its use in different fields. Results from this study revealed high phenol and flavonoid content, consistent with a study on the phytochemical analysis of Cocos nucifera endosperm by Offor et al. [38], conferring its antioxidant ability to significantly lower cellular oxidative stress (Oboh et al. [28]. Flavonoids also function as signaling molecules exerting a positive effect on cognitive function [40]. Phenolic compounds derived mostly from plants possess an antioxidant potential to manage oxidative stress-associated diseases like Alzheimer's and other neurodegenerative diseases [41, 42].
The scavenging ability and the reducing power of coconut water (CW) could be attributed to the total flavonoid and total phenol content. The increase in the scavenging ability and the reducing power of CW in a dose-dependent manner could also be due to the total phenolic concentration [18]. DPPH is a stable nitrogen-centered free radical donor that is stabilized by accepting an electron or hydrogen [43]. The ABTS and DPPH radical scavenging ability of CW results revealed that the extract can prevent radical-induced oxidative damage because phenolic compounds possess hydrogen-donating abilities to function as an antioxidant. Thus, reducing stable DPPH radical [44] and converting the coloured ABTS cation into a colourless form [45].
A similar trend was also observed when the antioxidant activity of the coconut water sample was assessed by the ferric reducing antioxidant power (FRAP) assay, a new antioxidant defence mechanism that is affected by the transfer of electrons and a hydrogen atom [45]. The antioxidants present in the extract can reduce ferric ion (Fe3+) to ferrous ion (Fe2+), which forms a blue complex that is absorbed at 700 nm [46]. Accordingly, several reports have established a correlation between the health benefit of polyphenolic-rich food and its antioxidant effects [47]. Additionally, current findings show that phenolic compounds exhibit health-promoting effects ranging from radicals scavenging to metal chelation responsible for lipid peroxidation [48]. Several animal models have been used to investigate the oxidative stress hypothesis of aging [49].
Specific reports have stated the implication of reactive oxygen species generation and oxidative stress in reducing D. melanogaster's life span [50]. The TBA assay was employed to measure the CW’s scavenging ability of radicals generated in lipid peroxidation. The study revealed that both concentrations (0.1% and 1.0%) dietary inclusions of CW significantly reduce MDA content in the tissue homogenate, which agrees with Das et al. [51], who reported that coconut water significantly reduces free radical generation and has antioxidant activity. The increase in the scavenging activity correlates with a decrease in the MDA level as the induction progresses with the utilization of CW by the brain. Studies have shown that amnestic mild cognitive impaired patient’ exhibit patterns of memory impairment and oxidative stress, similar to those observed in Alcl3-induced amnesic flies' [52, 53]. Therefore, a reduction in the brain MDA (marker of lipid peroxidation) level of flies treated with CW extract indicates a marked improvement in the brain antioxidant status, which could be due to phenolic present in the extract (Table 1). This shows that coconut water extract is capable of inhibiting the formation of lipid peroxidation.
5.Conclusion
The obtained results show that coconut water extract has antioxidant property, inhibited prooxidant-induced TBARS production, scavenged free radicals, chelated Fe2+in vitro and was also able to reduce MDA level in the brain of Alcl3 induced amnesic D. melanogaster fed with coconut water supplemented diet. These abilities could be linked to the action of polyphenolic compounds present in it. Therefore, this study suggests that coconut water could provide a cheap therapeutic means for neurodegenerative disease management/treatment. Further studies should be done to isolate and characterize bioactive compounds present in coconut water.
Acknowledgements
The contributions of all the authors and the supports of the technicians at biochemistry department, Adekunle Ajasin University are immensely appreciated.
References
- Chan E, Elevitch CR. Cocos nucifera (coconut): species profiles for Pacific island agroforestry. Edible Med. Non-Med. Plants (2006).
- Ajibogun OA, Oboma YI. Biochemical Composition of Coconut Water: Nigeria Species. Int J of Med and Bio Sci 1 (2013): 1-4.
- Yong JW, Ge L, Ng YF, et al. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera) Water Molecules 14 (2009): 5144–5164.
- DebMandal M, Mandal S. Coconut (Cocos nucifera: Arecaceae): In health promotion and disease prevention. Asian Pacific Journal of Tropical Medicine 4 (2011): 241-247.
- Huan L, Takamura T, Tanaka, M. Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid. Plant Sci 166 (2004): 1443–1449.
- Prades A, Dornier M, Diop N, et al. Coconut Water Uses, Composition, and Properties: A Review. Fruits 67 (2012): 87–
- Radenahmad N, Saleh F, Sawangjaroen K, et al. Coconut Juice, a Potential Therapeutic Agent That Could Significantly Reduce Some Pathologies Associated with Alzheimer’s Disease: Novel Findings. British Journal of Nutrition 105 (2011): 738–746.
- Preetha PP, Devi VG, Rajamohan T. Hypoglycemic and Antioxidant Potential of Coconut Water in Experimental Diabetes. Food & Function 3 (2012): 753–
- Heyno E, Mary V, Schopfer P, et al. “Oxygen activation at the plasma membrane: relation between superoxide and hydroxyl radical production by isolated membranes,”. Planta 234 (2011): 35–45.
- Blokhina O, Fagerstedt KV. “Reactive oxygen species and nitric oxide in plant mitochondria: origin and redundant regulatory systems,” Physiologia Plantarum 138 (2010): 447–462.
- Uttara B, Singh AV, Zamboni P, et al. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacol 7 (2009): 65-74.
- Chen X, Guo C, Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regeneration Res 7 (2012): 376-385.
- Dröge W. Free radicals in the physiological control of cell function. Physioll Rev 82 (2002): 47-95
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70 (2005): 200-214
- Zuo L, Hemmelgarn BT, Chuang CC, et al. The role of oxidative stress-induced epigenetic alterations in amyloid-B production in Alzheimer’s disease. Oxidative medicine Cellular Longevity (2015).
- Fessenden R, Fessenden J. Organic Chemistry. In: Brooks/Cole Publishing Company. Monterey (1986): pp. 263–4.
- Odubanjo VO, Oboh G, and Ibukun EO. Antioxidant and anticholinesterase activities of aqueous extract of Uraria picta ( Jacq .) DC 7 (2013): 2768–2773.
- Ami D, Davidovi D, Trinajsti N. Structure-Radical Scavenging Activity Relationships of Flavonoids. Croat Chem Acta 76 (2003): 55–61.
- Odubanjo OV, Oluwarotimi AE, Ayeni CO, Akingbola HO, Olabisi PT. Fatty acid composition and antioxidant effect of coconutoil in Drosophila melanogaster. Comp Clin Pathol (2020).
- Pratico D, Delanty N. Oxidative injury in disease of the central nervous system: focus on Alzheimer’s disease. Am J Med 109 (2000): 577–585.
- Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: Antioxidants and beyond. Am J Clin Nutr 81 (2005): 215-217.
- Mahayothee B, Koomyart I, Khuwijitjaru P, et al. Phenolic Compounds, Antioxidant Activity, and Medium Chain Fatty Acids Profiles of Coconut Water and Meat at Different Maturity Stages, International Journal of Food Properties 19: (2016): 2041-2051.
- Mantena SK, Jagadish BSR, Siripurapu KB et al. In Vitro Evaluation of Antioxidant Properties of Cocos nucifera, Linn. Water. Food/Nahrung 47 (2003): 126–
- Leong LP, Shui G. An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food Chemistry 76 (2002): 69–
- Chang CL, Wu RT. Quantification of (+)-Catechin and (-)-Epicatechin in Coconut Water by LC-MS. Food Chemistry 126 (2011): 710–
- Nagarkar-Jaiswal S, DeLuca SZ, Lee PT, et al. A genetic toolkit for tagging intronicMiMIC containing genes. Elife (2015).
- Hirth F. Drosophila melanogaster in the study of human neurodegeneration. CNS & Neurological Disorders - Drug Targets 9 (2010): 504-523.
- Oboh G, Odubanjo VO, Bello F, et al. Aqueous extracts of avocado pear (Persea Americana ) leaves and seeds exhibit anticholinesterases and antioxidant activities in vitro. J Basic Clinical Physiol Pharmacol 27 (2016): 131-140.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth in enzymol 299 (1999): 152-178.
- Meda A, Lamien CE, Romito M, et al. Determination of the total phenolic, flavonoid and proline contents in Burkina Faso honey, as well as their radical scavenging activity. Food Chem 9 (2005): 571-577.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26 (1999): 1231–1237.
- Brand-Williams W, Cuvelier M E, Berset C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensmittel Wissenschaft und Technologie 28 (1995): 25 –30.
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Japanese Journal of Nutrition 44 (1986): 265-267.
- Minotti G, Aust SD. An investigation into the mechanism of citrate-Fe2+-dependent lipid peroxidation. Free Rad Biol Med 3 (1987): 379–387.
- Puntel RL, Nogueira CW, Rocha JB. Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res 30 (2005): 225–235.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95 (1979): 351–358.
- Zar JH. Biostatistical Analysis. Prentice-Hall, Inc., Upper Saddle River, New Jersey, pp. 620 (1984).
- Offor SJ, Mbagwu HO, Orisakwe OE. Lead induced hepato-renal damage in male albino rats and effects of activated charcoal. Front Pharmacol 8 (2017): 107.
- Richetti SK, Blank M, Capiotti KM, et al. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav Brain Res [Internet] 217 (2011): 10–5.
- Ferrari CKB, Torres EAFS. Biochemical pharmacology of functional foods and prevention of chronic diseases of aging. Biomed Pharmaco ther 57 (2003): 251–260.
- Barreira J, Ferreira I, Oliveira M, Pereira J. Antioxidant activity and bioactive compounds of ten Portuguese regional and commercial almond cultivars. Food Chem Toxicol 46 (2008): 2230–2235.
- Dastmalchi K, Dorman D, Laakso I, et al. Chemical composition and antioxidative activity of Moldavian balm (Dracocephalum moldavica L.) extracts. LWT-Food Sci and Tech 40 (2007): 1655-1663.
- Marina AM, Che Man YB, Nazimah SAH, et al. Chemical properties of virgin coconut oil. Journal of the American Oil Chemists’ Society 86 (2009): 301–307.
- Ammar A, Zhang H, Siddeeg A.In Vitro Antioxidant Activity and Total Phenolic and Flavonoid Contents of Alhydwan (Boerhavia elegana Choisy) Seeds. J Food Nutr Res 2 (2014): 215–220.
- Oboh G, Puntel RL, Rocha JB. Hot pepper (Capsicum annuum, Tepin and Capsicum chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain – in vitro. Food Chem 102 (2007): 178–185.
- Prakruthi A, Sunil L, Gopala Krishna AG, et al. Phytochemicals and antioxidant activity of testa extracts of commercial wet dry coconuts and cakes. Int Res. J. Pharm 7 (2016): 9-13.
- Saeed N, Khan RM, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern Med 12 (2012): 221.
- Adefegha SA, Oyeleye SI, Oboh G. Distribution of Phenolic Contents, Antidiabetic Potentials, ANtihypentensive Properties, and Antioxidative Effects of SOursop (Annona muricata L.) Fruit Parts In Vitro; Biochemistry Research International (2015).
- Forbes JM, Coughlan, MT, Cooper ME. Oxidative stressas a major culprit in kidney disease. Diabetes 57 (2008): 1446–1454.
- Lozinsky OV, Lushchak OV, Kryshchuk NI, et al. Snitrosoglutathione-induced toxicity in Drosophila melanogaster: Delayed pupation and induced mild oxidative/nitrosative stress in eclosed flies. Comparative Biochemistry and Physiology A 164 (2013): 162–170.
- Das KK, Das SN, Dasgupta S. The influence of ascorbic acid on nickel-induced hepatic lipid peroxidation in rats.J Basic ClinPhysiolPharmacol 12 (2001): 187–195.
- Odubanjo VO, Ibukun EO, Oboh G, et al. Biomedicine & Pharmacotherapy Aqueous extracts of two tropical ethnobotanicals (Tetrapleura tetraptera and Quassia undulata) improved spatial and non-spatial working memories in scopolamine-induced amnesic rats?: In fl uence of neuronal cholinergic and. Biomed Pharmacother 99 (2018): 198–204.
- Jain A, Rusten TE, Katheder N et al. P62/Sequestosome-1, Autophagy in Drosophila Are regulated by Nuclear Factor Erthroid 2-related Factor 2 (NRF 2), Independent of Transcription Factor TFEB. J. Biol. Chem 290 (2015): 14945-14962.
