Antioxidant, Antimicrobial and Anti-Inflammatory Activities of Edible Rhus Tripartita (Ucria) Hydromethanolic Extracts
Article Information
Zaineb Ben Barka1,3*, Karima Lahbib1, Chedia Aouadhi2, Mohamed Habib Ladjimi1, Hanene Ben Miled1, Khémais Ben Rhouma1, Mohsen Sakly1, Abderrazek Maaroufi2, Yves Jacques Schneider3, Olfa Tebourbi1
1Laboratory of Integrated Physiology, Faculty of Science of Bizerte, University of Carthage, Bizerte, Tunisia
2Laboratory of Epidemiology and Microbiology Veterinary Bacteriology Groups and Biotechnology Development, Pasteur Institute of Tunis, El Manar University, Tunis, Tunisia
3Laboratory of Cellular, Nutritional and Toxicological Biochemistry, Institute of Life Sciences (ISV) UC Louvain, Belgium
*Corresponding Author:Zaineb BEN BARKA, Science Faculty of Bizerte, 7021 Tunisia
Received: 12 June 2019; Accepted: 05 July 2019; Published: 24 September 2019
Citation:
Barka ZB, Lahbib K, Aouadhi C, Ladjimi MH, Miled HB, Rhouma KB, Sakly M, Maaroufi A, Schneider YJ, Tebourbi O. Antioxidant, Antimicrobial and Anti-Inflammatory Activities of Edible Rhus Tripartita (Ucria) Hydromethanolic Extracts. J Pharm Pharmacol Res 3 (2019): 074-087.
View / Download Pdf Share at FacebookAbstract
Background: Rhus tripartita (Anacardiacae) is a plant which is traditionally used for the treatment of ulcer and diarrhea in Tunisia. The core aim of this study is to evaluate the antioxidant, antimicrobial and anti-inflammatory activities of Rhus tripartita (leaf (RLE), stem (RSE) and root (RRE)) extracts.
Results: RLE extract exhibited high total phenolic, flavonoids and condensed tannins. The in vitro antioxidant activity of the tested extracts (RLE, RSE and RRE) showed significant potent total antioxidant capacity (TAC), DPPH and ABTS radical scavenging activity, ferrous ions chelating and ferric reducing ions activities, H2O2 and OH scavenging capacities. The extracts showed great antimicrobial effects with an important inhibition zones (IZs). The minimum inhibitory concentration (MIC) and the lowest minimum bactericidal concentration (MBC) were recorded. The RRE showed the most significant protective anti-inflammatory effects compared to Indomethacin in carrageenan inflammation paw edema test.
Conclusion: This work highlights the importance of Rhus tripartita as dietary source of natural antioxidants and might be appropriate for the development of reliable biotechnologic methods to identify and extract the antimicrobial and anti-inflammatory biomolecules.
Keywords
Rhus tripartita extracts, Antioxidant, Antimicrobial, Anti-inflammatory, Biomolecules
Article Details
Abbreviations:
RRE-Rhus tripartita root extract; RLE-Rhus tripartita leaf extract; RSE-Rhus tripartita stem extract; IZs-diameters of inhibition zones; MIC-minimal inhibitory concentration; MBC-minimal bactericidal concentration
1. Introduction
Oxidation is essential for many living organisms for the production of energy to fuel biological processes. Nevertheless, Oxygen-centered free radicals and other reactive oxygen species (ROS), which are continuously, produced in vivo, result in cell death and tissue damage. The role of oxygen radicals has been implicated in several diseases, including cancer, diabetes, cardiovascular diseases, atherosclerosis, hypertension, ischemia-reperfusion, aging, neurodegenerative diseases and age-related diseases [1, 2]. Antioxidants are vital substances which possess the ability to protect the body from damage caused by free radical-induced oxidative stress [3, 4]. There is an increasing natural antioxidants present in medicine and dietary plants which prevent oxidative damage and play an important role in alleviating chronic diseases by reducing the oxidative damage to cellular components caused by ROS [5].
Naturally occurring flavonoids, tannins, anthocyanins and other phenolic constituents are potential antioxidants [5, 6]. Many synthetic antioxidants such as butylated hydroxyl anisole (BHA) and butylated hydroxyl toluene (BHT) are very effective and are used for industrial processing. However, they possess some side effects and toxic properties to human health, like carcinogenic effects [5, 7, 8, 9]. Therefore, substitution of synthetic antioxidants by natural ones and the screening of the plant species have become critically important in recent years. Polyphenols have an ideal structural chemistry for free radical-scavenging activity.
In fact, they can neutralize free radicals by accepting or donating electrons to eliminate the unpaired condition of the radical [10]. In order to improve human protection against oxidative damage due to environmental pollution or life stress, interest has been paid to explore the antioxidant properties of natural products. Anacardiacea family of plants consists of trees, shrubs and/or woody vines belonging mainly to the genus Rhus (Sumac) with about 250 species which occurs mostly in the tropics and sub-tropics and various temperate zones of the world [11].
Rhus species are widely used in food, and due to their high contents in phenols, flavonoids and other phytochemicals, Rhus species are widely used in both modern and traditional medicine [12, 13]. In Tunisia, Rhus is represented by two species: [Rhus oxyacantha (Shousb).Ex. Cav = R. oxya. canthoides Dum.cours = Rhus tripartita (Ucria) Grande [= R. tripartitum (Ucria) D.C. = Searsia tripartita (Ucria) Moffet] and Rhus pentaphylla [14]. Rhus tripartita known locally as “Jdéri” is a Tunisian plant used in traditional medicine for the treatment of digestive diseases [15, 16]. In Tunisia, the plant root bark is the most important part of the plant due to its high tannins content. Spraying of the root powder on a wound facilitates its healing. In southern Tunisia, powdered root barks are used for dyeing leather to light brown. It’s also used to dye wool and silk providing solid beige and brown colors by washing and rubbing [14, 17].
Rhus tripartita is not growing only in Tunisia but also it’s very abundant in north Africa, especially in the steppes of desert, arid and semi-arid areas [17, 18]. It also exists in Sicily and Western Asia steppes and is used in folkloric and traditional medicine of many countries situated in the cited areas [17, 19, 20]. Recently it has been demonstrated that Rhus tripartita
had anti-diarrheal, anti-ulcer, hepatoprotective and cardio-protective properties [21-24]. Moreover this plant showed a potent activity against inflammatory diseases [20].
We recently demonstrated that the dichloromethanic extract of Rhus tripartita root cortex showed a high anti-inflammatory activity in vitro, inhibiting nitric oxide release by lipopolysaccharide-stimulated RAW 274.7 macrophages [25]. However no in vivo study was performed on anti-inflammatory activities of this plant. Besides, the anti-microbial activities studies performed on Rhus tripartita extracts were limited to diarrhea-related microbes. Accordingly the aim of the present study is to: (i): investigate the antioxidant and radical scavenging activities of three hydromethanolic extracts of Rhus tripartita different organs (Roots, Leafs and stems). (ii) Evaluate the potential protective effects of these extracts against different pathogenic microbes. (iii) Determinate the anti-inflammatory activities of the extracts using carrageenan induced paw edema method in Wistar albino rats.
2. Material and Methods
2.1 Plant material and preparation of extract
2.1.1 Plant material: The Rhus tripartita roots, leaves and stems were collected at the beginning of March 2012 in Djebel Thelja, Gafsa, state of Tunisia. A voucher specimen (R.T.H03-N06) was identified by two taxonomists Dr Boulbeba Ltayef and Pr Mohammed Boussaid.
2.1.2 Preparation of plant extract: The Rhus tripartita roots (RRE), leaves (RLE) and stems (RSE) were dried and grounded. Grounded roots (50 g) were extracted with 300 ml of 50% methanol solution for 24 h at room temperature with magnetic stirring. The extract was centrifuged at 4500 × g for 10 min and
lyophilized. The residues were stored at – 21°C until use.
2.2 Antioxidant study
The following parameters were studied, the total antioxidant capacity (TAC) [26], the DPPH radical scavenging activity [27], the ABTS radical cation scavenging capacity (ABTS) [28], the Ferric reducing power (FRP), Ferrous ion chelating (FIC) activity [29], the hydrogen peroxide scavenging activity [30] and Hydroxyl radical scavenging activity [31].
2.3 Antimicrobial activity
2.3.1 Microbial species: The antimicrobial activities of the plant extracts (RRE, RSE and RLE) were investigated against 8 indicators of microorganisms including five pathogenic bacteria strains (Bacillus cereus ATCC1247, Klebsiella pneumoniae ATCC13883, Listeria monocytogenes ATCC 7644, Pseudomenas aeruginosa ATCC 27853, Aeromonas hydrophila EI, two fungi (Aspergillus flavus and Asergillus Niger) and one yeast (Candida albicans ATCC 2091) species. Bacterial strains were grown in trypto-caseine soja agar (TCSA) and incubated at 37°C for 24h. Fungal species were grown on potato dextrose agar (PDA) plate at 28°C for 72 h and Candida albicans was grown on sabouraud dextrose agar (SDA).
2.3.2 Disk diffusion assay: The antimicrobial activities of the 50% hydromethanolic extracts of Rhus tripartita (RRE, RLE, RSE), were firstly determined by disk diffusion assay. Antibacterial activities were assessed by measuring the diameter of the growth-inhibition zone (IZ) in millimeters (including disk diameter of 6 mm). These measurements of IZs were carried out three times and values were the average of three replicates.
2.3.3 Determination of MIC and MBC: The MIC (minimum inhibitory concentrations) and MBC
(minimum bactericidal concentrations) values of R. tripartita extracts (RRE, RSE and RLE) were determined using agar dilution[32]. The MIC was the lowest concentration which resulted in a significant decrease in inoculum viability (> 90%), while the MBC was the concentration where 99.9% or more of the initial inoculum was killed. Each experiment was repeated at least three times and the modal MIC and MBC values were selected.
2.4 Anti-inflammatory activity
Anti-inflammatory activity was determined by the carrageenan induced paw edema method in Wistar albino rats by using plethysmography following the method of Winter et al. [33]. Diclofenac at an oral dose of 20 mg/kg served as the standard drug for comparison. The test compounds (10 mg/kg) were administered orally 30 min prior to administration of carrageenan (0.1 mL of 1% w/v) in the plantar region of the paw. The paw volumes were measured at 0, 60, 90, 120 and 180 min after carrageenan administration. Animals were cared for in compliance with the code of practice for the Care and Use of Animals for Scientific Purposes. Approval for these experiments was obtained from the Medical Ethical Committee for the Care and Use of Laboratory Animals of Pasteur Institute of Tunis (approval number: LNFP/Pro 152012).
2.5 Phytochemical study
The concentration of the total phenolic contents, flavonoids and proanthocyanidin contents in the RLE were determined [34].
2.6 Statistical analyses
Data obtained are presented as means ± standard error of the mean (S.E.M) for the number of animals in each group (n=3). Data obtained from distilled water-treated control rats were used as baseline values. Statistical analyses were performed using one-way ANOVA followed by a Tukey posthoc test (GraphPad Prism 5, GraphPad soft-ware; Inc; San Diego, CA, USA). Statistical significance required a p<0.05.
3. Results
3.1 Antioxidant study
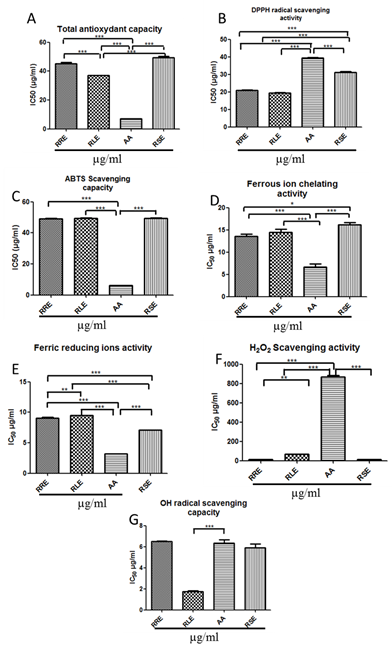
3.1.1 Total antioxidant Activity (TAC): All the extracts had lower TACs when compared to AA TAC value. RLE showed the highest TAC followed by RRE and RSE (36.99 ± 0.20 µg/ml; 45.20 ± 0.98 µg/ml; 49.31 ± 0.89 µg/ml respectively for RLE, RRE and RSE vs 6.95 ± 0.12 µg/ml for AA, p<0.0001.) (Figure 1A).
3.1.2 DPPH radical scavenging activity: All the extracts showed higher DPPH radical scavenging activities than that of the standard molecule which is AA (p<0.0001). IC50 values were 19.33 ± 0.42 µg/ml; 20.81 ± 0.36 µg/ml and 31.18 ± 0.39 µg/ml respectively for RLE, RRE and RSE, whereas AA IC50 was 39.33 ± 0.33 µg/ml (Figure 1B).
3.1.3 ABTS radical cation scavenging capacity (ABTS): High ABTS cation scavenging capacities were recorded with all the extracts. However correspondent IC50 values were significantly higher than that of AA (49.25 ± 0.03 µg/ml; 49.44 ± 0.12 µg/ml and 49.52 ± 0.16 µg/ml respectively for RRE, RLE and RSE vs 6.16 ± 0.07 µg/ml for AA, p<0.0001) (Figure 1C).
3.1.4 Ferric reducing antioxidant power (FRP) and ferrous ion chelating (FIC): The chelating activities of ferrous ions by the plant extracts in this assay were
important (Figure 1D). Results showed that the IC50 values of all tested samples were arranged from 12.21 ± 0.45 µg/ml to 14.50 ± 0.65 µg/ml. But they are significantly higher than that of the AA 6.66 ± 0.66 µg/ml (p<0.0001). Similarly the authentic standard AA exhibited the highest ferric reducing ions activity when compared with the plant extracts (p<0.0001). IC50 values of all tested samples were ranged from 3.18 ± 0.03 µg/ml to 9.52 ± 0.00 µg/ml, and arranged in the following decreasing efficiently order: AA (3.18 ± 0.03 µg/ml) > RSE (7.07 ± 0.05 µg/ml) > RRE (9.06 ± 0.12 µg/ml) > RLE (9.52 ± 0.00 µg/ml).
3.1.5 Hydrogen peroxide and Hydroxyl radical scavenging activity: Striking total H2O2 scavenging activities were observed with the three tested extracts. These activities were significantly higher than that of AA. The IC50 values recorded were 13.12 ± 0.36 µg/ml, 13.47 ± 0.84 µg/ml and 68.55 ± 0.38 µg/ml respectively for RRE, RSE and RLE vs AA IC50 = 866.7 ± 17.65 µg/ml (p<0.0001) (Figure 1F). In contrast, only RLE showed the strongest OH radical scavenging capacity (IC50 RLE = 1.75 ± 0.06 µg/ml vs IC50 AA = 6.33 ± 0.33 µg/ml, p<0.0001) (Figure 1G).
3.2 Antimicrobial activity of Rhus tripartita
The hydromethanolic extract of Rhus tripartita was tested against five undesirable bacteria, two fungi and one yeast strain, in order to estimate their antimicrobial potentials (Table 1). The results of the in vitro antimicrobial activity, assessed by the presence or absence of inhibition zone (IZs), MIC (Minimal inhibitory concentration) and MBC (Minimal Bacteria Concentration) values, showed that regardless bacteria (Gram+ or Gram-), fungi (Aspergilus flavus and Aspergilus niger) or yeast, the extracts act in a non-selective manner. They had a great potential for antimicrobial activity.
The IZs were in the range of 16 ± 1 mm2 and 26 ± 0.5 mm2 for tested bacterial strains. In fact the highest IZ was reached against, Listeria monocytogenes, Bacillus cereus, with IZs of 26 ± 1 mm2 and 26 ± 0.5 mm2 respectively for RSE and RRE. The lowest IZ was recorded for Listeria monocytogenes and was estimated by 16 ±1 mm2 when treated by RLE (Table 1). For the fungi, all the extracts showed great potent antifungal activities and the highest IZ reached was 27 ± 0.5 mm2 for Aspergillus niger when treated with RRE. Whereas the lowest IZ recorded was 19 ± 1.5 mm2, for Aspergillus flavus with RSE (Table 1). The Candida albicans IZs values were 24 ± 1 mm2, 27 ± 1 mm2 and 21 ± 1 mm2 respectively for RRE, RLE and RSE (Table 1). The MIC and the MBC values were determined for all the tested micro-organisms with Rhus tripartita extracts. For Klebsiella pneumoniae the minimum MIC values recorded were 6.25 mg/ml in presence of RRE and the lowest MBC was 12.5 mg/ml with RRE. The highest MIC values were registered with Aeromonas hydrophila, Pseudomonas aeruginosa, Bacillus cereus (50 mg/ml for RLE and RSE). MBC values for these strains were 100 mg/ml for RLE and RSE.
The fungi and the yeast were more sensitive, for all the tested extracts (RRE, RLE and RSE), the MICs were 12.5 mg/ml and the MBCs were 25 mg/ml for the Aspergillus flavus and Aspergillus niger. For Candida albicans the MIC was 12.5 mg/ml and the MBC was 25 mg/ml, for RRE and RLE respectively (Table 1).
3.3 Anti-inflammatory activity
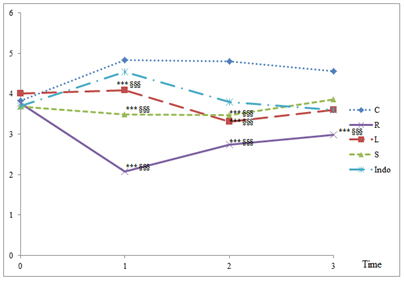
The results showed protective effects of the three extracts (RRE, RLE and RSE) as well as the anti-inflammatory drug Diclofenac (Indomethacin) against acute carrageenan-induced inflammation. In fact, carrageenan induced the appearance of a 4.5 cm height paw edema after 3h of injection. The extracts showed significant protective anti-inflammatory effects compared to Indomethacin from the first hour of carrageenan injection. The highest protective activity was 57%, recorded in the presence of the root extract (2.08 cm vs 4.83 cm for carageenan). After 3h, the plant extracts inhibited the inflammation by 15.26%, 20.95% and 34.67%, respectively for RSE, RLE and RRE. The inflammation inhibiting percentage of Indomethacin was comparable to that of RLE (21.17%) (Figure 2). Data showed that RRE was the most potent extract in inhibiting inflammation (Figure 2).
3.4 Phytochemical study
The phytochemical study of RLE revealed the presence of polyphenols (324.73 ± 7.82 mgGAE/gDW), flavonoids (223.60 ± 2.20 mgCE/gDW) and condensed tannins (proanthocyanidins) (92.62 ± 3.37 mgCE/gDW) (Table 2).
Figure 1: Evaluation of antioxidant activity parameters of Rhus tripartita extracts. (A) Total antioxydant capacity; (B) The DPPH radical scavenging activity; (C) The ABTS radical scavenging capacity; (D) Ferrous ion chelating activity; (E) Ferric Reducing Antioxidant Power; (F) H2O2 Scavenging activity; (G) OH radical scavenging capacity.
RRE-Rhus tripartita root extract; RLE-Rhus tripartita leaf extract; RSE-Rhus tripartita stem extract; AA-Ascorbic acid; DPPH-1,1-diphenyl-2-picrylhydrazyl; ABTS-2,2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid); IC50-half maximal inhibitory concentration. Each value represents the mean ± SEM of three experiments. Values are expressed as means ± SEM (n=3 for each experimental group). *p<0.05, **p<0.01, ***p<0.0001 in Tukey’s multiple comparison post hoc test
|
Micro-organisms |
Inhibition zone diameters (mm2) |
MIC (mg/ml) |
MBC (mg/ml) |
|||||||
|
RRE |
RLE |
RSE |
Gent |
RRE |
RLE |
RSE |
RRE |
RLE |
RSE |
|
|
Gram negative bacteria |
||||||||||
|
Aeromonas hydrophila |
25 ± 2 |
20 ± 2 |
21 ± 2 |
24 |
25 |
50 |
50 |
50 |
100 |
100 |
|
Pseudomonas aeruginosa |
19 ± 1 |
19 ± 1 |
18 ± 1 |
23 |
25 |
50 |
50 |
50 |
100 |
100 |
|
Klebsiella pneumoniae |
22 ± 1.5 |
18 ± 1 |
19 ± 2 |
23 |
6.25 |
12.5 |
12.5 |
12.5 |
25 |
25 |
|
Gram positive bacteria |
||||||||||
|
Listeria monocytogenes |
22 ± 1 |
16 ± 1 |
26 ± 1 |
21 |
25 |
50 |
12.5 |
50 |
100 |
25 |
|
Bacillus cereus |
26 ± 0.5 |
18 ± 1.5 |
17 ± 0.5 |
20 |
25 |
50 |
50 |
50 |
100 |
100 |
|
Fungus |
||||||||||
|
Aspergillus flavus |
26 ± 1 |
26 ± 0.5 |
19 ± 1.5 |
11 |
12.5 |
12.5 |
12.5 |
25 |
25 |
25 |
|
Aspergillus niger |
27 ± 0.5 |
25 ± 1.5 |
20 ± 1 |
12 |
12.5 |
12.5 |
12.5 |
25 |
25 |
25 |
|
Yeast |
||||||||||
|
Candida albicans |
24 ± 1 |
27 ± 1 |
21 ± 1 |
17 |
12.5 |
12.5 |
25 |
25 |
25 |
50 |
IZs-diameters of inhibition zones; MIC-minimal inhibitory concentration; MBC-minimal bactericidal concentrations; RRE-Rhus tripartita root extract; RSE-Rhus tripartita stem extract; RLE-Rhus tripartita Leaf extract.
Table 1: Antimicrobial activity of Rhus tripartita extracts.
C-carrageenan; R-Rhus tripartita root extract; L-Rhus tripartita leaf extract; S-Rhus tripartita stem extract; Indo- Indomethacin. Values are expressed as means ± SEM (n=3 for each experimental group); *** p<0.0001 in Tukey’s multiple comparison post hoc test compared to carrageenan group, §§§ p<0.0001 in Tukey’s multiple comparison post hoc test compared to Indomethacin group
|
RLE |
Total phenolics (mg GAEgDWRLE) |
Flavonoids (mgCE/gDWRLE) |
Proanthocyanidins (mgCE/gDWRLE) |
|
324.73 ± 7.82 |
223.60 ± 2.20 |
92.62 ± 3.37 |
DW-dry weight; CE-catechin equivalent; GAE-gallic acid equivalent; RLE-Rhus tripartita Leaf extract
Table 2: Contents of total phenols, flavonoids and proanthocyanidins in RLE.
4. Discussion
Plants are important sources of potentially useful biomolecules for the development of new chemotherapeutic agents. Plants may help to discover new chemical classes of antibiotics and anti-inflammatory agents that could serve as drugs for the maintenance of animal and human health. The present study is an evaluation of the antioxidant, antimicrobial and anti-inflammatory activities of the different extracts of Rhus tripartita. Our phytochemical investigation showed the presence of high concentrations of polyphenols, flavonoids and tannins (which is the major compound for the leaf) in Rhus tripartita hydromethanolic leaf extract. In previous studies we demonstrated the presence of high amount of these biomolecules in RRE (789 ± 7.82 mgGAE/gDW for Polyphenols, 538.34 ± 18.94 mgCE/gDW for flavonoids and 219.77 ± 50.89 mgCE/gDW for tannins) [21] and RSE (380 ± 11.47 mgGAE/gDW for Polyphenols, 232.58 ± 7.90 mgCE/gDW for flavonoids and 147.65 ± 9.14 mgCE/gDW for tannins) [23].
The secondary metabolites like phenolics, flavonoids and tannins found in all parts of plants such as leaves, seeds, stems, roots and fruits, are known to be potent free radical scavengers and inhibitors of lipid peroxidation [35]. In this context Ben Miled et al. [24] highlighted the richness of a polar extract (water and methanol) of Rhus tripartita roots in phenolic compounds. These extracts have similar and strong antioxidant activities with respectively ORAC values of 8.95 and 8.55 µmol Trolox/ml. Our results showed that the TAA and the radical scavenging activities (DPPH and ABTS) of Rhus tripartita extracts were potent and these activities may be related to their concentrations in phenolics, flavonoids and condensed tannins. Antioxidative properties of these compounds arise from their high reactivity as hydrogen or electron donors [36]. The reducing power could serve as a significant reflection of the antioxidant activity [37]. In fact compounds with reducing power indicate that they are electron donors and can reduce the oxidized intermediates of lipid peroxidation processes, so that they can act as primary and secondary antioxidants. In fact, phenolics compounds can conjugate transition metals, therefore preventing the metal-induced free radical formation. Accordingly, all the extracts tested in our study were capable to reduce the Fe3+ to Fe2+ and to chelate ferrous ions.
Free radicals are very harmful for human health. The hydrogen peroxide (H2O2) itself is not very reactive, but it can be toxic to cells because it may give rise to the very harmful hydroxyl radicals, through the Fenton or the Haber–Weiss reactions [38]. In organism, H2O2 can diffuse through the plasmic, mitochondrial or paroxysmal membranes inducing disruption. Thus, the removing of H2O2 is very important for antioxidant defense in organisms. The hydroxyl radical, which is the most reactive oxygen species is capable to attack and to damage almost every molecule found in living cells. It can hydroxylate purine and pyrimidine bases leading to DNA mutations. It can also initiate the peroxidation of cell membrane lipids and can increase the malondialdehyde (MDA) levels, which is cytotoxic, mutagenic and carcinogenic [39]. The low levels of H2O2 and OH. IC50 showed the strong free radicals scavenging activities of all our tested extracts. Because of resistance to antibiotics, the side effects of many chemical antimicrobial agents such as: toxicity, environmental impact and health risk associated to their use, another alternative must be found to reduce the use of synthetic antibiotics, fungicides and bactericides [40]. Natural compounds will be more efficient, safer and potent. In this context we tried to study the antimicrobial activity of Rhus tripartita extracts.
The in vitro antimicrobial activities of Rhus tripartita extracts against bacteria (Gram-positive and Gram-negative bacteria), fungus and yeast were investigated. The presence or absence of inhibition zones, MIC and MBC values were used for assessing the potency of tested extracts (Table 1). Indeed, the three investigated extracts presented potential for antimicrobial activities against all tested microorganisms. In fact, the maximal inhibition zones for bacteria were observed for the Bacillus cereus and Listeria monocytogenes (Gram positive bacteria). For the fungus the maximum inhibition zone was recorded against Aspergilus niger. In addition, the MIC and the MBC values for different tested microorganisms were ranged from 6.25 mg/ml to 100 mg/ml. Previous recent studies [15, 21] showed that Rhus tripartita extracts have a potent antimicrobial activities against diarrhea-related microbes.
Generally, it is well known that polyphenols are bioactive molecules. According to Zoreky [41], polyphenols played an important role in protein precipitation and enzyme inhibition of microorganisms. Moreover, phenolic are capable to destroy the membrane of the bacteria [42]. These biological activities are related to the molecule structures. In fact by their hydroxyl groups, phenolic compounds have capacity to link with proteins and bacterial membrane to form complexes [31]. The antimicrobial compounds from plants may inhibit bacterial growth by different mechanisms. Previous studies demonstrated that flavonoids have the abilities to inhibit spore germination of plant pathogens. They have been proposed for use against fungal pathogens of man [43], while tannins are able to inhibit the extracellular microbial enzymes, to derivate of the substrates required for microbial growth or have a direct action on microbial metabolism through inhibition of oxidative phosphorylation [44]. The antimicrobial activity observed in our study could be due to the high contents of Rhus tripartita extracts in phenolic compounds as flavonoids and condensed tannins, known to be antibacterial and antifungal agents [45, 46].
The investigation for the anti-inflammatory activities of the extracts, showed great activities when compared with a standard anti-inflammatory agent which is Indomethacin and RRE had the highest anti-inflammatory activity. A recent study performed by Shahat et al. [20], has demonstrated that Rhus tripartita stem extract had a significant in vitro anti-inflammatory activity by inhibiting selectively the cyclo-oxygénase 2, with strong inhibition of acetylcholinesterase. This activity is correlated to the radical-scavenging effects of the extracts. In our knowledge, this study is the first to highlight the anti-inflammatory potential of Rhus tripartita extracts in vivo. In the present study, the antioxidant, antimicrobial and anti-inflammatory activities could be due to presence of specific phenolic compounds known as antimicrobial and anti-inflammatory molecules. In fact in previous studies several biomolecules were identified in Rhus tripartita root cortex such as (+) catechin and (-) epicatechine-3-O-gallate [47], the masazinoflavanone [48], as well as the catechol, gallic acid, and syringic acid [25]. All those compounds are known for their great and potent anti-microbial and anti-inflammatory activities [48-51].
5. Conclusion
The obtained results clearly showed that Rhus tripartita extracts exhibited interesting antioxidant, antimicrobial and anti-inflammatory activities due to their high polyphenols contents. These biomolecules act as antioxidants due to their redox properties. They act as reducing agents, hydrogen donors, free radical scavenging and metal chelators. This supported their use in traditional medicine in the treatment of infections and encouraged their potential use as alternative safe antioxidant antimicrobial and anti-inflammatory agents.
Conflict of Interest
None.
Acknowledgements
This research was funded by the Tunisian Ministry of Higher Education, Scientific Research and Technology, Carthage University and Faculty of Sciences of Bizerte. The authors gratefully acknowledge the technical assistance of Béchir AZIB and Yessine ELMEGDICHE.
References
- Bjørklund G, Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 33 (2017): 311-321.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity 2 (2009): 270-278.
- Falleh H, Oueslati S, Guyot S, et al. LC/ESI-MS/MS characterisation of procyanidins and propelargonidins responsible for the strong antioxidant activity of the edible halophyte Mesembryanthemum edule L. Food Chemistry 127 (2011): 1732-1738.
- Silva EM, Souza JNS, Rogez H, et al. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chemistry 101 (2007): 1012-1018.
- Elzaawely AA, Xuan TD, Tawata S. Antioxidant and antibacterial activities of Rumex japonicus HOUTT. Aerial parts. Biological and Pharmaceutical Bulletin 28 (2005): 2225-2230.
- Banerjee A, Dasgupta N, De B. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chemistry 90 (2005): 727-733.
- Kahl R, Kappus H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Zeitschrift Fur Lebensmittel-Untersuchung Und -Forschung 196 (1993): 329-338.
- Anagnostopoulou MA, Kefalas P, Papageorgiou VP, et al. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chemistry 94 (2006): 19-25.
- Ito N, Hirose M, Fukushima S, et al. Studies on antioxidants: Their carcinogenic and modifying effects on chemical carcinogenesis. Food and Chemical Toxicology 24 (1986): 1071-1082.
- Lu JM, Lin PH, Yao Q, et al. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. Journal of Cellular and Molecular Medicine 14 (2010): 840-860.
- Kossah R, Nsabimana C, Zhao J, et al. Comparative Study on the Chemical Composition of Syrian Sumac (Rhus coriaria L.) and Chinese Sumac (Rhus typhina L.) Fruits. Pakistan Journal of Nutrition (2009): 1570-1574.
- Lee SK, Jung HS, Eo WK, et al. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: report of two cases. Annals of Oncology 21 (2010): 1383-1385.
- Wu T, McCallum JL, Wang S, et al. Evaluation of antioxidant activities and chemical characterisation of staghorn sumac fruit (Rhus hirta L.). Food Chemistry 138 (2013): 1333-1340.
- Floc'h E, Boulos L, Vela E. Flora of Tunisia: Contribution to an ethnobotanical study of Tunisian flora. Ministry of Higher Education and Scientific Research (2nd part). The Tunisian Republic.
- Abbassi F, Hani K. In vitro antibacterial and antifungal activities of Rhus tripartitum used as antidiarrhoeal in Tunisian folk medicine. Natural Product Research 26 (2012): 2215-2218.
- Alimi H, Mbarki S, Barka ZB, et al. Phytochemical, antioxidant and protective effect of Rhus tripartitum root bark extract against ethanol-induced ulcer in rats. General Physiology and Biophysics 32 (2013): 115-127.
- Mahmoud SB, Saad H, Charrier B, et al. Characterization of sumac (Rhus tripartitum) root barks tannin for a potential use in wood adhesives formulation. Wood Science and Technology 49 (2015): 205-221.
- Muhaisen HMH, Ab-Mous MM, Ddeeb FA, et al. Antimicrobial agents from selected medicinal plants in Libya. Chinese Journal of Integrative Medicine 22 (2016): 177-184.
- Qasem JR. Ephedra alte (joint pine): an invasive, problematic weedy species in forestry and fruit tree orchards in Jordan. The ScientificWorld Journal (2012).
- Shahat AA, Ibrahim AY, Al-Ghamdi AAM, et al. Phytochemical investigation of Rhus tripartita and its activity against cyclooxygenases and acetylcholinesterase. Tropical Journal of Pharmaceutical Research 15 (2016): 1697.
- Ben Barka Z, Aouadhi C, Tlili M, et al. Evaluation of the anti-diarrheal activity of the hydromethanolic root extract of Rhus tripartita (Ucria) (Anacardiacae). Biomedicine and Pharmacotherapy 83 (2016): 827-834.
- Shahat AA, Alsaid MS, Rafatullah S, et al. Treatment with Rhus tripartita extract curtails isoproterenol-elicited cardiotoxicity and oxidative stress in rats. BMC Complementary and Alternative Medicine 16 (2016): 351.
- Ben Barka Z, Tlili M, Alimi H, et al. Protective effects of edible Rhus tripartita (Ucria) stem extract against ethanol-induced gastric ulcer in rats. Journal of Functional Foods 30 (2017): 260-269.
- Ben Miled H, Barka ZB, Hallègue D, et al. Hepatoprotective activity of Rhus oxyacantha root cortex extract against DDT-induced liver injury in rats. Biomedicine and Pharmacotherapy 90 (2017): 203-215.
- Ben Miled H, Saada M, Jallali I, et al. Variability of antioxidant and biological activities of Rhus tripartitum related to phenolic compounds. EXCLI Journal 16 (2017): 439-447.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Analytical Biochemistry 269 (1999): 337-341.
- Braca A, Sortino C, Politi M, et al. Antioxidant activity of flavonoids from Licania licaniaeflora. Journal of Ethnopharmacology 79 (2002): 379-381.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 26 (1999): 1231-1237.
- Chew YL, Chan EWL, Tan PL, et al. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complementary and Alternative Medicine 11 (2011): 12.
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry 43 (1995): 27-32.
- Sánchez-Moreno C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Revista de Agaroquimica Y Tecnologia de Alimentos 8 (2002): 121-137.
- Riahi L, Elferchichi M, Ghazghazi H, et al. Phytochemistry, antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L. in Tunisia. Industrial Crops and Products 49 (2013): 883-889.
- Winter CA, Risley EA, Nuss GW. Anti-Inflammatory and Antipyretic Activities of Indo-Methacin, 1-(p-Chlorobenzoyl)-5-Methoxy-2-Methyl-Indole-3-Acetic Acid. Journal of Pharmacology and Experimental Therapeutics 141 (1963): 369-376.
- Falleh H, Ksouri R, Medini F, et al. Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Industrial Crops and Products 34 (2011): 1066-1071.
- Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food and Chemical Toxicology 44 (2006): 198-206.
- Chanda S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. African Journal of Microbiology Research 3 (2009): 981-996.
- Oktay M, Gulçin ?, Küfrevio?lu Ö?. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Science and Technology 36 (2003): 263-271.
- Evans P, Halliwell B. Free radicals and hearing: cause, consequence, and criteria. Annals of the New York Academy of Sciences 884 (1999): 19-40.
- Favier A. The oxidative stress?: interest of its monitoring in clinical chemistry and problems of the choice of an appropriate parameter. Annales de Biologie Clinique 55 (1997): 9-16.
- Cobos R, Mateos RM, Álvarez-Pérez JM, et al. Effectiveness of Natural Antifungal Compounds in Controlling Infection by Grapevine Trunk Disease Pathogens through Pruning Wounds. Applied and Environmental Microbiology 81 (2015): 6474-6483.
- Al-Zoreky NS. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International Journal of Food Microbiology 134 (2009): 244-248.
- Bisignano G, Tomaino A, Lo Cascio R, et al. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. The Journal of Pharmacy and Pharmacology 51 (1999): 971-974.
- Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents 26 (2005): 343-356.
- Hamburger M, Hostettmann K. Bioactivity in plants: the link between phytochemistry and medicine. Phytochemistry 30 (1991): 3864-3874.
- Olchowik-Grabarek E, Swiecicka I, Andreeva-Kovaleskaya Z, et al. Role of Structural Changes Induced in Biological Membranes by Hydrolysable Tannins from Sumac Leaves (Rhus typhina L.) in their Antihemolytic and Antibacterial Effects. The Journal of Membrane Biology 247 (2014): 533-540.
- Rauha JP, Remes S, Heinonen M, et al. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology 56 (2000): 3-12.
- Tebourbi O, Trabelsi C, Ben Nasr C, et al. Antioxidant activity of extract of Rhus oxyacantha root cortex. Indian Journal of Experimental Biology 44 (2006): 246-249.
- Mahjoub MA, Ammar S, Edziri H, et al. Anti-inflammatory and antioxidant activities of some extracts and pure natural products isolated from Rhus tripartitum (Ucria). Medicinal Chemistry Research 19 (2010): 271-282.
- Zheng LT, Ryu GM, Kwon BM, et al. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: Inhibition of microglial neurotoxicity. European Journal of Pharmacology 588 (2008): 106-113.
- Kürbitz C, Heise D, Redmer T, et al. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells. Cancer Science 102 (2011): 728-734.
- Kroes BH, van den Berg AJ, Quarles van Ufford HC, et al. Anti-Inflammatory Activity of Gallic Acid. Planta Medica 58 (1992): 499-