Anterior Ankle Full Thickness Skin Necrosis Treated with Copper Oxide Dressings without Debridement and Skin Grafting – A Case Report
Article Information
Eyal Melamed1*, Alexei Rovitsky1, Tohar Roth2, Gadi Borkow2
1Foot and Ankle Service, Department of Orthopedics, Rambam Health Care Campus, Haifa, Israel
2MedCu Technologies Ltd., Herzliya, Israel
*Corresponding Author: Eyal Melamed, Foot and Ankle Service, Department of Orthopedics, Rambam Health Care Campus, Haifa, Israel
Received: 07 June 2022; Accepted: 17 June 2022; Published: 30 June 2022
Citation:
Eyal Melamed, Alexei Rovitsky, Tohar Roth, Gadi Borkow. Anterior Ankle Full Thickness Skin Necrosis Treated with Copper Oxide Dressings without Debridement and Skin Graft – A Case Report. Archives of Clinical and Medical Case Reports 6 (2022): 501-510.
View / Download Pdf Share at FacebookAbstract
Full thickness skin necrosis can result from number of causes, including trash emboli, external pressure, burns and severe skin infections and necrotizing fasciitis. Treatment is often dependent on the size and location of the necrotic skin, involvement of adjacent structures, the presence of active infection and the general condition of the patient. The anterior aspect of the ankle is unique in the sense that the structures there (skin, subcutaneous fat, fascia and tendons) are tight without muscles or padding structures to allow healing of skin necrosis without involvement of the deep structures. Therefore, in cases of skin and subcutaneous tissue necrosis the tendons have a very scarce blood underlying facia (e.g. the extensor retinaculum) is often involved, jeopardizing the extensor foot tendons. In such conditions, the treatment normally includes debridement of the necrotic and infected tissues, which often involves resecting the superior extensor retinaculum and the underlying extensors tendons, if they are involved. Since the tendons have a very scarce blood supply, their fate is often doomed either in the first debridement surgery or in the following period. Eventually, the goal of treatment is to fill the area with abundant granulation tissue to afford skin grafting in follow-up surgery. We describe a case in which treatment with Copper Oxide Dressings (COD) prevented infection by its antibacterial activity while at the same time induced debridement of the necrotic fascia and granulation tissue formation. Subsequently rapid epithelization occurred allowing the wound to heal with normal skin and little scar. Thus, the copper dressing enabled to replace debridement and skin graft surgeries and achieve complete healing within 5.5 months with low cost, very little discomfort and full tendon function. We discuss the mechanisms of COD action that enabled this achievement.
Keywords
Copper; Wounds; Dressings; Necrosis; Tendons; Ankle
Copper articles; Wounds articles; Dressings articles; Necrosis articles; Tendons articles; Ankle articles
Copper articles Copper Research articles Copper review articles Copper PubMed articles Copper PubMed Central articles Copper 2023 articles Copper 2024 articles Copper Scopus articles Copper impact factor journals Copper Scopus journals Copper PubMed journals Copper medical journals Copper free journals Copper best journals Copper top journals Copper free medical journals Copper famous journals Copper Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Necrosis articles Necrosis Research articles Necrosis review articles Necrosis PubMed articles Necrosis PubMed Central articles Necrosis 2023 articles Necrosis 2024 articles Necrosis Scopus articles Necrosis impact factor journals Necrosis Scopus journals Necrosis PubMed journals Necrosis medical journals Necrosis free journals Necrosis best journals Necrosis top journals Necrosis free medical journals Necrosis famous journals Necrosis Google Scholar indexed journals Tendons articles Tendons Research articles Tendons review articles Tendons PubMed articles Tendons PubMed Central articles Tendons 2023 articles Tendons 2024 articles Tendons Scopus articles Tendons impact factor journals Tendons Scopus journals Tendons PubMed journals Tendons medical journals Tendons free journals Tendons best journals Tendons top journals Tendons free medical journals Tendons famous journals Tendons Google Scholar indexed journals Ankle articles Ankle Research articles Ankle review articles Ankle PubMed articles Ankle PubMed Central articles Ankle 2023 articles Ankle 2024 articles Ankle Scopus articles Ankle impact factor journals Ankle Scopus journals Ankle PubMed journals Ankle medical journals Ankle free journals Ankle best journals Ankle top journals Ankle free medical journals Ankle famous journals Ankle Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals atherosclerosis articles atherosclerosis Research articles atherosclerosis review articles atherosclerosis PubMed articles atherosclerosis PubMed Central articles atherosclerosis 2023 articles atherosclerosis 2024 articles atherosclerosis Scopus articles atherosclerosis impact factor journals atherosclerosis Scopus journals atherosclerosis PubMed journals atherosclerosis medical journals atherosclerosis free journals atherosclerosis best journals atherosclerosis top journals atherosclerosis free medical journals atherosclerosis famous journals atherosclerosis Google Scholar indexed journals Debridement articles Debridement Research articles Debridement review articles Debridement PubMed articles Debridement PubMed Central articles Debridement 2023 articles Debridement 2024 articles Debridement Scopus articles Debridement impact factor journals Debridement Scopus journals Debridement PubMed journals Debridement medical journals Debridement free journals Debridement best journals Debridement top journals Debridement free medical journals Debridement famous journals Debridement Google Scholar indexed journals SARS-CoV2 articles SARS-CoV2 Research articles SARS-CoV2 review articles SARS-CoV2 PubMed articles SARS-CoV2 PubMed Central articles SARS-CoV2 2023 articles SARS-CoV2 2024 articles SARS-CoV2 Scopus articles SARS-CoV2 impact factor journals SARS-CoV2 Scopus journals SARS-CoV2 PubMed journals SARS-CoV2 medical journals SARS-CoV2 free journals SARS-CoV2 best journals SARS-CoV2 top journals SARS-CoV2 free medical journals SARS-CoV2 famous journals SARS-CoV2 Google Scholar indexed journals
Article Details
Abbreviations:
COD: Copper Oxide Dressings; AI: Artificial Intelligence
1. Introduction
Full thickness skin necrosis can result from a number of causes, including trash emboli, external pressure, burns and severe skin infections and necrotizing fasciitis [1-3]. Treatment of skin necrosis is often dependent on the size and location of the necrotic skin, involvement of adjacent structures, the presence of active infection and the general condition of the patient [1]. An important aspect of taking care of a wound or any injury is to retain the function of the affected tissue, limb or any other organ and to protect it from becoming infected until full skin coverage is achieved. Treatment of skin necrosis often includes debridement of the necrotic skin and infected tissues [1]. However, skin necrosis of the anterior ankle necessitates special attention, as the anterior aspect of the ankle is unique in the sense that the structures there (skin, subcutaneous fat, fascia and tendons) are tight without muscles or padding structures. Thus, in cases of skin necrosis of the anterior ankle, the underlying facia (extensor retinaculum) is often involved, jeopardizing the extensor foot tendons (tibialis anterior, extensor hallucis longus and extensor digitorum longus). Since tendons have poor blood supply, once they are exposed, they are prone to microbial infection and granulation tissue rarely and scarcely grow over the tendons. Therefore, in the case of exposed tendons, the race competition between granulation tissue growth and infection of the dysvascular tendon often ends with tendon loss. Hence, the common clinical practice in such cases is debridement of the necrotic tissue and often resecting the extensor tendons if they are infected or exposed. Tendon’s function is most likely lost in cases of anterior ankle necrosis.
2. Case Description
Sixty-years-old man was admitted to the hospital and operated on due to infected olecranon bursitis in the right elbow (Figure 1). Past medical history included non- insulin-dependent diabetes mellitus, ischemic heart disease and chronic obstructive pulmonary disease secondary to heavy smoking in the past. On examination, the patient was febrile (39.0ºC), and redness plus fluctuation were evident on the right elbow. Laboratory tests were compatible with acute bacterial infection with 12,700 leucocytes and CRP of 30 (normal < 0.5). Aspiration of the abscess revealed pus and methicillin sensitive staphylococcus aureus (MSSA) grew on culture. Incision and drainage were performed in the operating room on two occasions and intravenous (IV) antibiotic treatment was instituted (Cefamizine 2.0 gr x 3 times per day). The patient responded favorably to treatment. The surgical wound at the elbow looked clean and delayed primary suture was carried 10 days following the first surgery.
2.1 Infected foot description
The patient did not have prior foot infection or episode of diabetic foot. He did not have neuropathy. Pulses in the foot were normal. Due to poor venous access in the arm, an IV catheter was installed in the left ankle (Figure 2a). The 12th post admission day, while the patient seemed to heel completely from the elbow infection, he developed local erythema, warm and bulla formation in the anterior ankle region, at the area of the venous catheter portal (Figure 2b). Clinical and imaging findings, were consistent with local infection involving the skin, subcutaneous tissue and fascia deep to the extensor tendons. Sagittal view with soft tissue window of computerized tomography demonstrated swelling and opacity of the sub-dermal tissues down to the extensor tendons and air bubbles, compatible with cellulitis and necrotizing fasciitis spreading deep to the peri-tendon area (Figure 3).
The patient responded to antibiotic treatment (Augmentin and Ciprofloxacin) and the infection remained contained at the anterior aspect of the ankle. The patient was discharged home with oral antibiotic treatment. The patient returned to the clinic for a follow visit. At that time there was full thickness necrosis at the anterior aspect of the ankle (Figure 2c). The wound was covered with thick dry eschar which seemed to be contracted below the level of the adjacent skin surface (Figure 2c). There was no redness surrounding the wound and it seemed that there was no active infection. On direct examination, the wound width (from medial to lateral) was 70 mm and on the long axis of the leg (from proximal to distal) it measured 40-45 mm. Total wound and necrotic tissue area was 20.5 cm2, as measured by an artificial intelligence (AI) program (Tissue Analytics software (https://www.tissue- analytics.com/), used for assessment and management of wounds [4, 5]. The patient was admitted to the ward. The treatment plan was to debride all the necrotic tissue, excise the tendons if they would seem to be compromised or infected, and allow granulation tissue formation growth with subsequent split thickness skin grafting (STSG). An alternative plan, with application of Copper Oxide Dressings (COD), was presented to the patient. The patient was aware of lack of experience with this approach, but elected the non-operative mode of treatment. The patient was discharged home with 10 cm x 12 cm COD dressing to be changed twice weekly without antibiotic treatment. The patient was instructed that if infection will flare up, surgical debridement will need to be conducted and he should come immediately for a re-evaluation. Normal follow up was carried out in the clinic at first by weekly intervals and later by bi-weekly and tri-weekly visits.
Hereafter, the time count described is from the beginning of COD treatment onward (Figure 4a). At one week the wound was dry with very little discharge on the dressing (Figure 4b). Gradually the eschar was contracted, and white amorphous tissue of fibrous-necrotic fascia was seen in the interval between the healthy skin and the eschar (Figure 4c). This interval area was covered by an additional layer of COD, to assure that all the wound area and the adjacent skin are protected by COD (Figures 4d and 4e). The white fascial-necrotic tissue underwent autolysis and red granulation tissue proliferated beneath the necrotic tissue and began replacing it, as was seen on Day 35 (Figure 4f). The red granulation tissue became dense at the periphery of the wound and the epithelial tissue began crawling into the wound (Figure 4g). During all this period until final healing, no signs of infection were seen, and antibiotic was not prescribed. At 10 weeks, the eschar was contracted and partially detached (Figure 4g). It was trimmed in the clinic (Figure 4h) to reveal the same whitish fibrous-necrotic tissue throughout the wound bed with granulation tissue forming underneath (Figure 4i). No tendons were seen (Figures 4h and 4i). Area of thick white tissue was bed side debrided but most of it was left for autolysis by the granulation tissue enzymes, as was seen at 14 and 19 weeks of COD treatment (Figures 4j and 4k). The percent of granulation tissue coverage of the wound constantly increased reaching almost 100% of the area at 14 weeks (Figure 5), as determined by the artificial intelligence program. During this stage, epithelization was rapid and accompanied by deposition of thick corneal layer. At the next visit, at 25 weeks from the beginning of COD treatment, there was no discharge on the dressing (Figure 4) and the wound was closed (Figures 4 and 5). Despite having the wound closed, the patient was asked to continue applying the COD on the closed wound area to assist in better skin regeneration and cosmetic appearance [6]. Nine months after the beginning of COD treatment, there was full function of the ankle including the extensor tendons (Figure 6). The patient full tendon activity at the anterior aspect of the ankle was noted (Figure 6). The scar was minimal and hardly discernible at one year following the COD treatment (Figure 7).
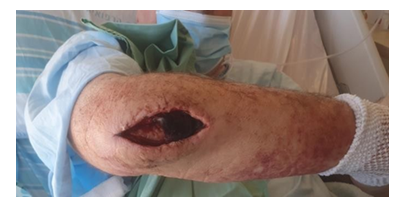
Figure 1: The patient was operated due to infected olecranon bursitis in the right elbow.
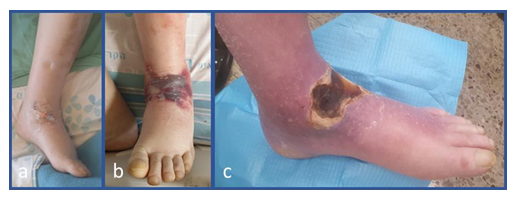
Figure 2: a) IV catheter was installed in the left ankle; b) infection developed with local erythema, warm and bulla formation was formed in the area of the venous catheter portal; c) full thickness necrosis developed at the anterior aspect of the ankle.
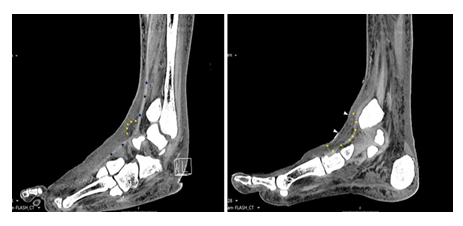
Figure 3: Computerized tomography of the infected area at the anterior aspect of the ankle. The yellow arrows show swelling, air bubbles, and opacity of the sub-dermal tissues down to the extensor tendons revealing the depth of the infection. The white arrows show depression of the skin which became necrotic, dry and shrinked.
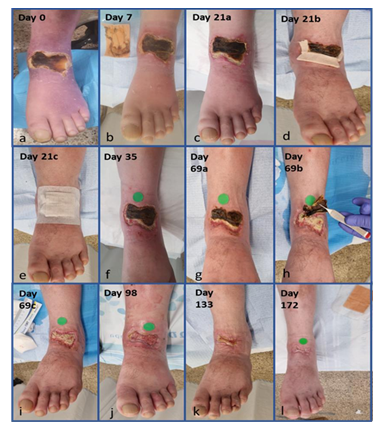
Figure 4: Pictures taken of the wound from the beginning of the COD treatment of the wound until the wound was closed. The Days from the beginning of treatment are depicted.
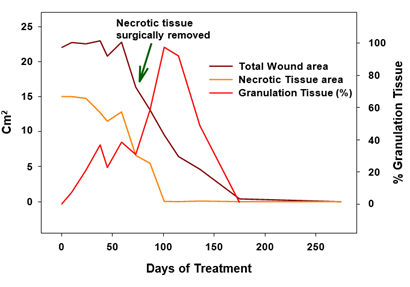
Figure 5: Necrotic tissue area, total wound area and % granulation tissue over time as determined by the AI program.
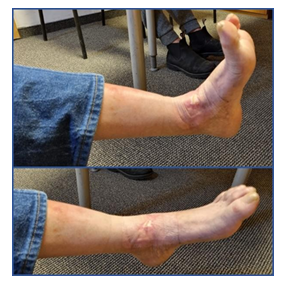
Figure 6: Wound healed with full tendon activity.
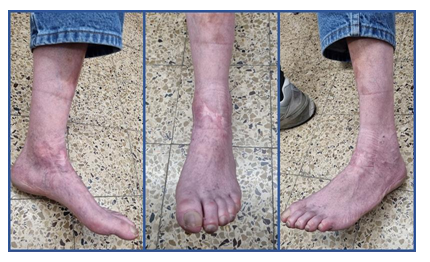
Figure 7: Wound is solidly closed and scar is hardly discernible.
3. Discussion
An important aspect of taking care of a wound or any injury, is to retain the function of the affected tissue, limb or area. The illustrated case report shows a case of full thickness skin necrosis anterior to the ankle joint in a diabetic patient, which was resolved by the sole management of the wound with COD. Skin necrosis at this site is usually full thickness due to lack of padding and no muscles between the deep layers and the fascia. The extensors tendons are just below the fascia, which at this site is the superior extensor retinaculum. The computerized tomography done during the infectious episode illustrated the depth of the infection down to and surrounding the extensor tendons. Blurring and air in the deep tissues at that area (Figure 3) were compatible with severe infection that led to the necrosis of the integuments and facia. The patient responded to antibiotic treatment and the infection did not spread further but the whole epicenter of the infection became necrotic. Although the infection was resolved with eschar formation, the adjacent tendons were prone to infection and necrosis due to their poor blood supply. The granulation tissue was not expected to grow over the bare tendons. Regular treatment of debridement, most probably, would result in loss of the tendon function and loss of active extension of the foot and ankle. Nevertheless, conducting tissue surgical debridement of the dead eschar is usually the safe clinical intervention to avoid colonization of the dead tissue with bacteria and infection. The eschar itself, composed of dry coagulated proteins [7], is relatively resistant to infection, and may rightly be referred to as a temporary biological dressing. The infection though usually begins or penetrates through the interface between the eschar tissue (the dry gangrene) and the intact skin. It makes sense therefore to dress this interface with an antibacterial dressing such as dressings containing copper or silver. While dressings containing silver may have good antibacterial properties, there are several reports indicating that silver may impede the wound healing processes (e.g.; [8-10]).
In the current case we used antimicrobial dressings containing copper oxide microparticles to cover the wound [5, 6, 11, 12]. No development of infection occurred even when the interval between the intact skin and the “biological dressing” e.g. the eschar, was interrupted due to contracture and desiccation of the eschar (Figures 4c-e). Throughout the 5 months to wound closure there was no episode of redness of any other signs of suspected infection and antibiotic was never prescribed. Remarkably, in parallel, intense granulation promoting activity took place at the wound, both at the interface between the eschar and the skin (Figures 4d & 4e), but also underneath the eschar. This was clearly seen when the eschar was trimmed at 10 weeks of treatment (Figure 4f). The necrotic fascial-fibrous tissue was already seeded with large nests of granulation tissue, which emerged at first as islands or pockets or red stains (Figure 4f) to become a uniform layer 4 weeks later (Figure 4g). The induction of granulation tissue is in accordance with experimental studies conducted in full thickness non-infected wounds in diabetic mice in which induction of angiogenesis was induced by the COD but not by silver dressings or control dressings without an active ingredient [13]. This observation was substantiated in a clinical study in which diabetic foot ulcers that were not healing despite standard of care were enrolled in the trial and treated with COD. Already after one week of using the COD, there was a statistically significant increase in granulation tissue formed [5].
One of the most striking observations of the current case was the apparent induction of autolytic debridement by the COD. This can be attributed to autolytic enzymes, such as matrix metalloproteinases, released by the granulation tissue being formed. It has been found that copper stimulates the expression and secretion of matrix metalloproteinase-1 by dermal fibroblasts, a collagenase that degrades interstitial collagen [14]. Direct effect of copper in promoting collagenase release from leukocytes in the cornea was described [15]. We found that copper induced the upregulation of fibroblast growth factors secretion in full thickness wounds [13]. Thus, the increased number of dermal fibroblasts and blood cells in the newly formed granulation tissue, together with the increased secretion of collagenases by the fibroblasts and possibly by the white blood cells, as was seen in the cornea, following their exposure to copper that reached them in situ beneath the necrotic tissue, may explain the autolytic debridement process we saw in the current case. In any case, the debriding effect of COD on the wound was unequivocally presented in this case.
The effect of copper as a potent regenerative agent was also demonstrated in the increased epithelization. While during the first stages of wound healing, epithelization was not observed and the total wound surface did not significantly change (Figure 5), once granulation tissue prevailed and the necrotic tissue was debrided, the epithelization was very rapid, as can be seen in the transition from 10 to 19th weeks. The positive effect of copper on the epidermal layer expressed itself also in abundant formation of corneal layer (Figure 4g-j). The effect of copper in inducing epithelization was reported [13, 16]. We thus can see that the copper and the COD acted in accordance with the known orchestrated wound healing mechanism. This case thus demonstrates the complimentary concentrated effect of copper oxide, which, on one hand, prevented infection during the healing period due to its antibacterial activity; and on the other hand, at the same time, it stimulated autolytic debridement, saved the surgical procedure, and promoted granulation tissue formation. In addition, once granulation tissue covered the wound surface, rapid epithelization took over, sparing the need for skin graft surgery. The positive effect of COD continued after wound closure allowing elastin formation and more natural skin to be formed with better quality and cosmetic appearance. This is in accordance with previous observations [6], in which the scar formed was minimal and hardly discernible at one year following COD treatment (Figure 7). This is in line with the role that copper plays at the maturation stages of wound healing, with the capacity of copper to penetrate the epidermal layers of the skin [17, 18], with its stimulation of collagen and elastin secretion by dermal fibroblast [18, 19] and with copper's involvement with the efficient production and stabilization of the dermal extracellular matrix [20, 21]. Taken together, the combined effects of COD enabled healing without damage to the tendons, allowing complete recovery of the tendons function, and return of the foot and leg to normal function. This not only saved pain from the patient, was comfortable and easy to use both by the patients and care givers, but it also saved significant cost to the healthcare system and to the patient.
Conflict of Interests
G.B. and T.R. are employees of MedCu; E.M. is a medical consultant of MedCu; MedCu developed the COD. A.R. has no conflict of interests.
References
- Karimi K, Odhav A, Kollipara R, et al. Acute Cutaneous Necrosis: A Guide to Early Diagnosis and Treatment. J Cutan Med Surg 21 (2017): 425-437.
- Kiat HJ, En Natalie YH, Fatimah L. Necrotizing Fasciitis: How Reliable are the Cutaneous Signs?. J Emerg Trauma Shock 10 (2017): 205-210.
- Marques SA, Abbade LPF. Severe bacterial skin infections. An Bras Dermatol 95 (2020): 407-417.
- Barakat-Johnson M, Jones A, Burger M, et al. Reshaping wound care: Evaluation of an artificial intelligence app to improve wound assessment and management amid the COVID-19 pandemic. Int Wound J (2022).
- Melamed E, Rovitsky A, Roth T, et al. Stimulation of Healing of Non-Infected Stagnated Diabetic Wounds by Copper Oxide-Impregnated Wound Dressings. Medicina (Kaunas ) 57 (2021).
- Borkow G, Melamed E. Copper, an abandoned player returning to the wound healing battle. In: Shahin A, editor. Scars and Kelloids. 1st ed. London: IntechOpen (2021).
- Thomas AM, Harding KG, Moore K. The structure and composition of chronic wound eschar. J Wound Care 8 (1999): 285-287.
- Lee AR, Moon HK. Effect of topically applied silver sulfadiazine on fibroblast cell proliferation and biomechanical properties of the wound. Arch Pharm Res 26 (2003): 855-860.
- Szmyd R, Goralczyk AG, Skalniak L, et al. Effect of silver nanoparticles on human primary keratinocytes. Biol Chem 394 (2013): 113-123.
- Burd A, Kwok CH, Hung SC, et al. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen 15 (2007): 94-104.
- Borkow G, Okon-Levy N, Gabbay J. Copper oxide impregnated wound dressings: biocidal and safety studies. Wounds 22 (2010): 310-316.
- Melamed E, Kiambi P, Okoth D, et al. Healing of Chronic Wounds by Copper Oxide-Impregnated Wound Dressings-Case Series. Medicina (Kaunas ) 57 (2021).
- Borkow G, Gabbay J, Dardik R, et al. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen 18 (2010): 266-275.
- Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res 51 (2010): 224-229.
- Lin MT, Chen YL. Effect of copper ion on collagenase release. Its implication in corneal vascularization. Invest Ophthalmol Vis Sci 33 (1992): 558-563.
- Ogen-Shtern N, Chumin K, Silberstein E, et al. Copper Ions Ameliorated Thermal Burn-Induced Damage in ex vivo Human Skin Organ Culture. Skin Pharmacol Physiol 34 (2021): 317-327.
- Hostynek JJ, Dreher F, Maibach HI. Human stratum corneum penetration by copper: in vivo study after occlusive and semi-occlusive application of the metal as powder. Food Chem Toxicol 44 (2006): 1539-1543.
- Ogen-Shtern N, Chumin K, Cohen G, et al. Increased pro-collagen 1, elastin, and TGF-beta1 expression by copper ions in an ex-vivo human skin model. J Cosmet Dermatol 19 (2020): 1522-1527.
- Philips N, Samuel P, Parakandi H, et al. Beneficial regulation of fibrillar collagens, heat shock protein-47, elastin fiber components, transforming growth factor-beta1, vascular endothelial growth factor and oxidative stress effects by copper in dermal fibroblasts. Connect Tissue Res 53 (2012): 373-378.
- Dahl SL, Rucker RB, Niklason LE. Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant 14 (2005): 861-868.
- Philips N, Samuel P, Siomyk H, et al. Improved cell metabolism and strengthening of the extracellular matrix by nicotinamide, and copper for anti-skin aging. In: Ekiguchi A, Imada M, editors. Skin Aging: New Research. , Siomyk H, Parakandi H, Jia H, Gopal S, Shahin H. ed. ebook: Nova Science (2013): 43-58.
