Alterations of Plasma Glutamine and Surgical Stress in Gastric Cancer Surgery
Article Information
Tsutomu Hayashi1*, Hiroshi Yamamoto2, Takanobu Yamada3, Tsutomu Sato4, Toru Aoyama4, Yasushi Rino4, Munetaka Masuda4, Haruhiko Cho5, Akira Tsuburaya4, Takashi Ogata3, Takaki Yoshikawa1
1Department of Gastric Surgery, National Cancer Hospital, Tokyo, Japan
2Research Institute for Bioscience Products & Fine Chemicals, Ajinomoto Co., Inc.
3Department of Gastrointestinal Surgery, Kanagawa Cancer Center, Yokohama, Japan
4Department of Surgery, Yokohama City University, Yokohama, Japan
5Department of Surgery, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Tokyo, Japan
*Corresponding Author: Tsutomu Hayashi, Department of Gastric Surgery, National Cancer Hospital, Tokyo, Japan
Received: 14 July 2022; Accepted: 19 July 2022; Published: 29 July 2022
Citation: Tsutomu Hayashi, Hiroshi Yamamoto, Takanobu Yamada, Tsutomu Sato, Toru Aoyama, Yasushi Rino, Munetaka Masuda, Haruhiko Cho, Akira Tsuburaya, Takashi Ogata, Takaki Yoshikawa. Alterations of Plasma Glutamine and Surgical Stress in Gastric Cancer Surgery. Journal of Surgery and Research 5 (2022): 462-467.
View / Download Pdf Share at FacebookAbstract
Background: The plasma glutamine levels are known to decrease after major surgery, which is related to a negative clinical outcome. However, the influence of surgical stress on the decrease in the plasma glutamine concentration is unclear. The aim of this study was to evaluate the change in glutamine in relation to the types of gastrectomy and approaches as well as the amount of surgical stress evaluated by serum interleukin-6(IL-6) as an objective indicator.
Methods: This was a prospective observational study. The eligibility criteria were (1) gastric adenocarcinoma of the stomach confirmed by pathology and (2) patients scheduled to receive gastrectomy with lymphadenectomy for gastric cancer. Blood samples were taken at 7 AM on the day of surgery and at 12 h after surgery to measure the plasma glutamine and interleukin-6 (IL-6) levels.
Results: Between May 2011 and December 2011, 81 consecutive patients were enrolled in this study. The plasma glutamine level was significantly decreased in all patients, regardless of distal or total gastrectomy and laparoscopic or open surgery. No significant differences were observed in the IL-6 level between total and distal gastrectomy patients or between patients treated via a laparoscopic or open approach. Decreases in the plasma glutamine level were positively correlated with the logarithmically transformed-plasma IL-6 (logIL6) (r =0.471, p<0.001) overall.
Conclusions: Decreases in the glutamine concentration depended on the amount of surgical stress. When conducting a clinical trial to evaluate glutamine administration, personalized adjustment may be key to avoiding glutamine depletion in response to surgical stress.
Keywords
Gastric cancer, Surgical stress, Glutamine
Gastric cancer articles; Surgical stress articles; Glutamine articles
Gastric cancer articles Gastric cancer Research articles Gastric cancer review articles Gastric cancer PubMed articles Gastric cancer PubMed Central articles Gastric cancer 2023 articles Gastric cancer 2024 articles Gastric cancer Scopus articles Gastric cancer impact factor journals Gastric cancer Scopus journals Gastric cancer PubMed journals Gastric cancer medical journals Gastric cancer free journals Gastric cancer best journals Gastric cancer top journals Gastric cancer free medical journals Gastric cancer famous journals Gastric cancer Google Scholar indexed journals Surgical stress articles Surgical stress Research articles Surgical stress review articles Surgical stress PubMed articles Surgical stress PubMed Central articles Surgical stress 2023 articles Surgical stress 2024 articles Surgical stress Scopus articles Surgical stress impact factor journals Surgical stress Scopus journals Surgical stress PubMed journals Surgical stress medical journals Surgical stress free journals Surgical stress best journals Surgical stress top journals Surgical stress free medical journals Surgical stress famous journals Surgical stress Google Scholar indexed journals Glutamine articles Glutamine Research articles Glutamine review articles Glutamine PubMed articles Glutamine PubMed Central articles Glutamine 2023 articles Glutamine 2024 articles Glutamine Scopus articles Glutamine impact factor journals Glutamine Scopus journals Glutamine PubMed journals Glutamine medical journals Glutamine free journals Glutamine best journals Glutamine top journals Glutamine free medical journals Glutamine famous journals Glutamine Google Scholar indexed journals gastric adenocarcinoma articles gastric adenocarcinoma Research articles gastric adenocarcinoma review articles gastric adenocarcinoma PubMed articles gastric adenocarcinoma PubMed Central articles gastric adenocarcinoma 2023 articles gastric adenocarcinoma 2024 articles gastric adenocarcinoma Scopus articles gastric adenocarcinoma impact factor journals gastric adenocarcinoma Scopus journals gastric adenocarcinoma PubMed journals gastric adenocarcinoma medical journals gastric adenocarcinoma free journals gastric adenocarcinoma best journals gastric adenocarcinoma top journals gastric adenocarcinoma free medical journals gastric adenocarcinoma famous journals gastric adenocarcinoma Google Scholar indexed journals Gastrectomy articles Gastrectomy Research articles Gastrectomy review articles Gastrectomy PubMed articles Gastrectomy PubMed Central articles Gastrectomy 2023 articles Gastrectomy 2024 articles Gastrectomy Scopus articles Gastrectomy impact factor journals Gastrectomy Scopus journals Gastrectomy PubMed journals Gastrectomy medical journals Gastrectomy free journals Gastrectomy best journals Gastrectomy top journals Gastrectomy free medical journals Gastrectomy famous journals Gastrectomy Google Scholar indexed journals lymphadenectomy articles lymphadenectomy Research articles lymphadenectomy review articles lymphadenectomy PubMed articles lymphadenectomy PubMed Central articles lymphadenectomy 2023 articles lymphadenectomy 2024 articles lymphadenectomy Scopus articles lymphadenectomy impact factor journals lymphadenectomy Scopus journals lymphadenectomy PubMed journals lymphadenectomy medical journals lymphadenectomy free journals lymphadenectomy best journals lymphadenectomy top journals lymphadenectomy free medical journals lymphadenectomy famous journals lymphadenectomy Google Scholar indexed journals Gastric cancer surgery articles Gastric cancer surgery Research articles Gastric cancer surgery review articles Gastric cancer surgery PubMed articles Gastric cancer surgery PubMed Central articles Gastric cancer surgery 2023 articles Gastric cancer surgery 2024 articles Gastric cancer surgery Scopus articles Gastric cancer surgery impact factor journals Gastric cancer surgery Scopus journals Gastric cancer surgery PubMed journals Gastric cancer surgery medical journals Gastric cancer surgery free journals Gastric cancer surgery best journals Gastric cancer surgery top journals Gastric cancer surgery free medical journals Gastric cancer surgery famous journals Gastric cancer surgery Google Scholar indexed journals serum interleukin-6 articles serum interleukin-6 Research articles serum interleukin-6 review articles serum interleukin-6 PubMed articles serum interleukin-6 PubMed Central articles serum interleukin-6 2023 articles serum interleukin-6 2024 articles serum interleukin-6 Scopus articles serum interleukin-6 impact factor journals serum interleukin-6 Scopus journals serum interleukin-6 PubMed journals serum interleukin-6 medical journals serum interleukin-6 free journals serum interleukin-6 best journals serum interleukin-6 top journals serum interleukin-6 free medical journals serum interleukin-6 famous journals serum interleukin-6 Google Scholar indexed journals
Article Details
Background
Complete tumor removal is essential for the cure of gastric cancer. Gastrectomy with D2 lymphadenectomy is accepted as a standard procedure worldwide [1]. Although D2 surgery is a safe procedure, morbidity and mortality are unavoidable. Surgical complications impair patients’ quality of life and prolong the hospital stay. Recently, infectious complications have been reported to be associated with a poor prognosis [2,3]. Peri-operative glutamine administration may be an attractive approach to reducing the rate of infectious complications, as plasma glutamine levels are decreased following various critical states and because low plasma glutamine levels are associated with a negative clinical outcome [4]. A shortage of glutamine may lead to dysfunction of the immune system, which can cause infectious complications related to invasion [5]. Based on these previous findings, several randomized controlled trials were conducted to evaluate the efficacy of peri-operative glutamine administration in critically ill patients. However, findings were conflicting, with some trials noting positive results [6-8] and others [9-11] negative results. These contradictory results may be explained by the hypothesis that the reduction or demand of glutamine depends on the severity of the surgical stress. Two previous studies [12,13] demonstrated that plasma glutamine concentrations were reduced following major surgery but not after minor surgery, suggesting that severity of the surgical stress determines the reduction of the plasma glutamine concentration. However, the relationship between surgical stress and the reduction in the plasma glutamine remains unclear. Gastric cancer surgery may be a good candidate for such analyses, because surgical stress would be various depending on the approach or extent of gastrectomy. The present study therefore examined the change in the glutamine in relation to the type of gastrectomy and approach, as well as the amount of surgical stress evaluated by serum interleukin-6 (IL-6) as an objective indicator.
Methods
Patients
This was a prospective observational study. The eligibility criteria were (1) gastric adenocarcinoma of the stomach confirmed by pathology and (2) patients scheduled to receive gastrectomy with D1 or D2 lymphadenectomy for gastric cancer. The extent of lymph node dissection was determined by the JGCA guidelines [1]. Distal gastrectomy was selected when the tumors were located at the lower to middle third of the stomach, while total gastrectomy was chosen when the tumors were located at the upper third of the stomach. Laparoscopic distal gastrectomy was only performed when eligible patients with clinical stage I were enrolled in the phase III trial comparing open and laparoscopic surgery and had been randomly allocated to the laparoscopic approach. Laparoscopic total gastrectomy was only selected when eligible patients with clinical T1 disease were enrolled in the in-house phase II trial to evaluate feasibility. All other patients received open surgery. All clinical information was obtained prospectively.
Study schedule and measurements
The patients were admitted to the hospital by two days before surgery and allowed to eat until midnight the day before surgery. On the day of surgery, patients were allowed to drink Oral Rehydration Solution (OS-1®) until 3 h before surgery but did not receive any preoperative intravenous infusion. Blood samples were taken at 7 AM on the day of surgery and at 12 h after surgery to measure the IL-6 and glutamine levels. Glutamine concentrations were measured by high-performance liquid chromatography (HPLC)–electrospray ionization (ESI)–mass spectrometry (MS) by precolumn derivatization. These analytical methods have been described elsewhere [14]. Plasma IL-6 levels were measured using a chemiluminescent enzyme immunoassay (CLEIA).
Statistical analyses
The SPSS software program, version 19.0 (Statistical Package for Social Sciences, Chicago, IL, USA), was used for statistical calculations. To compare the IL-6 levels between surgical procedures, the Mann-Whitney U test was used. The differences in the plasma glutamine concentration before and 12 h after surgery were examined using a paired t-test. Differences were considered significant at p<0.05 in both analyses. The change in the plasma concentration of glutamine was calculated by subtracting the value at 12 h after surgery from the preoperative value. The values of IL-6 were converted to log scale (log10) referring previous studies [15-18]. This prospective observational study was approved by the research ethics committee of Kanagawa Cancer Center, Yokohama.
Results
Between May and December 2011, 81 consecutive patients were prospectively enrolled in this study. No patient refused to enter the study during this period.
Clinicopathological characteristics
The clinicopathological characteristics are shown in table 1. Fourteen patients (17%) received pre-operative chemotherapy. Laparoscopic surgery was performed in 17 patients (21%), laparoscopy-assisted total gastrectomy (LATG) in 4 and laparoscopy-assisted distal gastrectomy (LADG) in 13. Forty-four patients underwent D2 lymphadenectomy, whereas 37 underwent D1. All patients who underwent total gastrectomy with D2 lymphadenectomy received splenectomy. The median intraoperative blood loss was 270 ml (range: 5-2045 ml), and 4 patients received blood transfusion during the operation.
The clinicopathological characteristics are compared between the open and laparoscopic approaches in table 2. The patients who received pre-operative chemotherapy underwent open gastrectomy. In addition, 9 patients (53%) had an ASA score 1 in laparoscopic surgery, while 16 (25%) had ASA 1 in open surgery. There were significant differences in the surgical details and disease stage between the open and laparoscopic surgery patients.
|
Overall N=81 |
Total gastrectomy N=32 |
Distal gastrectomy N=49 |
|
|
Age |
|||
|
Median (range) |
67 (36-84) |
68 (45-84) |
65 (36-83) |
|
Gender |
|||
|
Male/Female |
55/26 |
25/7 |
30/19 |
|
ASA score* |
|||
|
1/2/3 |
25/55/1 |
10/22/0 |
15/33/1 |
|
Pre-operative chemotherapy |
|||
|
No/Yes |
14/67 |
22/10 |
45/4 |
|
Albumin (g/dl) |
|||
|
Mean (SD) |
4.43 (0.42) |
4.49 (0.62) |
4.20 (0.48) |
|
Hemoglobin (g/dl) |
|||
|
Mean (SD) |
12.9 (1.9) |
12.7 (2.1) |
13 (1.8) |
|
Tumor location** |
|||
|
U/M/L |
23/26/32 |
23-06-2003 |
0/20/29 |
|
Tumor depth*** |
|||
|
T1/T2/T3/T4 |
39/10/13/19 |
9/4/9/10 |
30/6/4/9 |
|
Nodal involvement*** |
|||
|
N0/N1/N2/N3 |
49/12/7/13 |
15/5/2/10 |
34/7/5/3 |
|
Stage (TNM 7th) |
|||
|
I/II/III/IV |
46/14/18/3 |
13/5/11/3 |
33/9/7/0 |
|
Surgical procedure |
|||
|
Laparotomy/Laparoscopic surgery |
64/17 |
28/4 |
36/13 |
|
Extent of lymphadenectomy |
|||
|
D1/D2 |
37/44 |
13/19 |
24/25 |
|
Splenectomy |
|||
|
No/Yes |
62/19 |
13/19 |
49/0 |
|
Pancreatectomy |
|||
|
No/Yes |
78/3 |
29/3 |
49/0 |
|
Intraoperative blood loss (ml) |
|||
|
Median (range) |
270 (5-2045) |
380 (30-2045) |
190 (5-1660) |
|
Blood transfusion |
|||
|
No/Yes |
4/77 |
30/2 |
47/2 |
*: The American Society of Anesthesiologists score
**: U, upper third; M, Middle third; L, Lower third
***: Tumor depth and nodal involvement were based on the TNM 7th edition
Table 1: Clinicopathologic characteristics
|
Open N=64 |
Lap N=17 |
P value |
|
|
Age |
|||
|
Median (range) |
62.0 (38-79) |
67.5 (36-84) |
0.234 |
|
Gender |
|||
|
Male/Female |
45/19 |
10/7 |
0.392 |
|
ASA score* |
|||
|
1/2/3 |
16/48/0 |
09-07-2001 |
0.008 |
|
Pre-operative chemotherapy |
|||
|
No/Yes |
50/14 |
17/0 |
0.034 |
|
Albumin (g/dl) |
|||
|
Mean (SD) |
4.40 (0.47) |
4.40 (0.21) |
0.772 |
|
Hemoglibin (g/dl) |
|||
|
Mean (SD) |
12.9 (1.99) |
12.7 (1.65) |
0.807 |
|
Tumor location** |
|||
|
U/M/L |
20/20/24 |
03-06-2008 |
0.564 |
|
Stage (TNM 7th) |
|||
|
I/II/III/IV |
30/13/18/3 |
16/1/0/0 |
0.004 |
|
Extent of lymphadenectomy |
|||
|
D1/D2 |
24/40 |
13/4 |
0.006 |
|
Sprenectomy |
|||
|
No/Yes |
45/19 |
17/0 |
0.009 |
|
Pancreanectomy |
|||
|
No/Yes |
3/61 |
0/17 |
1 |
|
Intraoperative blood loss (ml) |
|||
|
Median (range) |
340 (50-2045) |
45 (5-270) |
<0.001 |
|
Blood transfusion |
|||
|
No/Yes |
45/4 |
0/17 |
0.574 |
*: The American Society of Anesthesiologists score
**: U, upper third; M, Middle third; L, Lower third
Table 2: Comparison between open and laparoscopic approach
Plasma concentration of glutamine
The plasma concentrations of glutamine before and at 12 h after surgery are shown in table 3. The plasma glutamine level was significantly decreased in all patients after surgery, regardless of distal or total gastrectomy or laparoscopic or open surgery (table 2, p<0.001 by the paired t-test). The decreases in the plasma concentrations of glutamine before and at 12 h after surgery are shown in figure 1. There were no significant differences between the total gastrectomy and distal gastrectomy patients (p=0.117) or between the patients treated with the laparoscopic and open approach (p=0.927).
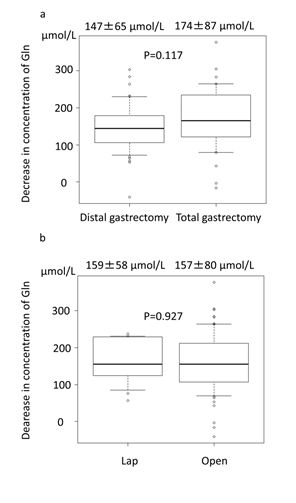
Figure 1: Decreases in the plasma glutamine levels before and at 12 h after surgery compared between procedure (a) and approach (b).
Plasma levels of IL-6
The median plasma level of IL-6 at 12 h after surgery was 55 pg/dl (range: 10-858 pg/dl) overall, 68.0 pg/dl (range: 10-858pg/dl) in the total gastrectomy patients, 53.0 pg/dl (range: 14-405 pg/dl) in the distal gastrectomy patients, 58 pg/dl (range: 24-217 pg/dl) in the laparoscopic surgery patients and 55 pg/dl (range: 10-858 pg/dl) in the laparotomy patients. There were no significant differences between the total and distal gastrectomy groups (p=0.398 by Mann-Whitney U test) or between the laparoscopic and open approach groups (p=0.894 by Mann-Whitney U test).
Analyses of the correlations between decreases in plasma glutamine and IL-6
Overall, a decrease in the plasma glutamine level was positively correlated with the logarithmically transformed-plasma IL-6 (log IL6); r=0.471 p<0.001 in overall, r=0.500 p<0.001 in open approach, and r=0.241 p=0.351 in laparoscopic approach (Figure 2a). When stratified by each procedure, strong correlations between the log IL-6 and a decrease in the plasma glutamine levels were observed in both the open and laparoscopic total gastrectomy patients; r=0.677 p<0.001 in overall, r=0.677 p<0.001 in open approach, and r=0.775, p=0.2251 in laparoscopic approach (Figure 2b). In contrast, a significant but weak correlation was found only in the overall patients who received distal gastrectomy; r=0.286 p=0.046 in overall. No significant correlation was observed when stratified by open and laparoscopic surgery; r=0.313 p=0.063 in open approach, and r=0.098 p=0.751 in laparoscopic approach (Figure 2b).
|
Pre-surgery |
Post-surgery* |
P value** |
|
|
Overall (mean, SD) |
569, 62 |
412, 60 |
< 0.001 |
|
Total gastrectomy (mean, SD) |
573, 51 |
399, 66 |
< 0.001 |
|
Open (mean, SD) |
574, 51 |
400, 68 |
< 0.001 |
|
Lap (mean, SD) |
571, 56 |
393, 54 |
0.012 |
|
Distal gastrectomy (mean, SD) |
566, 69 |
419, 55 |
< 0.001 |
|
Open (mean, SD) |
567, 73 |
423, 57 |
< 0.001 |
|
Lap (mean, SD) |
565, 57 |
411, 51 |
< 0.001 |
*: 12hours after surgery
**: paired t test
Table 3: The plasma concentrations of glutamine before and 12hours after surgery
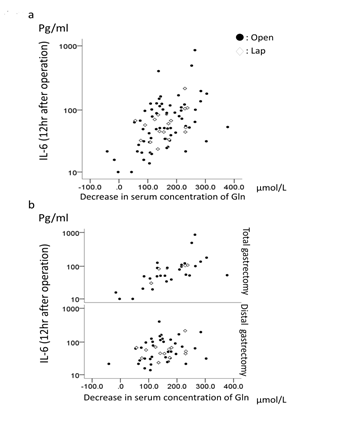
Figure 2: Logarithmically transformed-plasma IL-6 and decreases in the plasma glutamine levels (a) in all patients and (b) stratified by procedure.
Discussion
This is the first study to examine the correlation between decreases in the plasma glutamine levels and the amount of surgical stress. As previously reported [12,13,19], the plasma glutamine concentration was decreased after gastrectomy, which is a major abdominal surgery, regardless of total or distal gastrectomy and laparoscopic or open surgery. Furthermore, the present study clearly demonstrated that decreases in the plasma glutamine level were positively correlated with the surgical stress estimated by logarithmically transformed plasma IL-6. We therefore confirmed our hypothesis of this study. When conducting clinical trials to evaluate glutamine administration, personalized adjustment may be key to avoiding glutamine depletion in response to surgical stress. Interestingly, the plasma IL-6 values were similar between the open and laparoscopic surgery groups. Furthermore, a reduction in the plasma glutamine was similarly found, regardless of open or laparoscopic surgery. Laparoscopic surgery has been considered less invasive than an open approach. In the present study, patients who received laparoscopic surgery had a higher proportion of early-stage disease, less-extensive nodal dissection, no splenectomy, and less blood loss than those in the open surgery group. The surgical invasiveness may therefore have been greater in the open surgery group than in those treated via a laparoscopic approach. Nevertheless, the IL-6 and decrease in the plasma glutamine levels were similar between the open and laparoscopic surgery groups in the present study. Previously, a few investigators reported the difference of IL-6 level between the open and the laparoscopic gastrectomy. One randomized study showed that increase of IL-6 was greater in the open surgery than the laparoscopic surgery [20]. Conversely, another randomized study targeting only clinical stage I demonstrated that increase of IL-6 was not different between open surgery and laparoscopic approach [21]. This disagreement had been also reported in colorectal surgery [21-23]. Thus, it remains controversial whether the laparoscopic approach has less surgical stress as compared with open surgery. Resection of the stomach itself might be a major determinant of surgical stress in gastric cancer surgery than difference of open or laparoscopic approach. This idea can also be applied in cases of total or distal gastrectomy. Decreases in the plasma glutamine levels were positively correlated with surgical stress estimated by logarithmically transformed plasma IL-6. When stratified by approach, this correlation was weakened, especially in the laparoscopic approach. As a result, there were no significant correlations in laparoscopic total and distal gastrectomy. These results may be explained by less variation in the IL-6 values in laparoscopic surgery than in the open approach. Looking at the patients’ background factors, the ASA scores, pre-operative chemotherapy, and stage were significantly different between the two approaches. Similarly, with regard to surgical factors, the extent of lymphadenectomy, rate of splenectomy, and intraoperative blood loss were significantly different. These findings may suggest that homogeneity of the patients and surgery was higher in laparoscopic surgery than in open surgery, resulting in narrowed variations in surgical stress and decreases in plasma glutamine in laparoscopic surgery. The present study has several limitations. At first, the study only showed just an association of glutamine and IL-6, which did not mean direct causal relationship. Second, IL-6 level was only measured 12 hours after surgery. This might not represent the highest IL-6 caused by surgery. Finally, the sample size may be not enough to strictly interpret variation in the IL-6 value effected by various factors involved in surgical stress including surgical factors and patient’s backgrounds.
Conclusions
In conclusion, the plasma glutamine concentration was decreased after gastrectomy, regardless of the approach and type of gastrectomy. Decreased glutamine concentrations depended on the amount of surgical stress. This may account for the contradictory results of phase III trials of glutamine administration. Thus, personalized adjustment may be necessary when conducting clinical trials to evaluate glutamine administration.
Abbreviations
IL-6: interleunkin-6
Highlights
Plasma glutamine was decreased depending on the amount of surgical stress.
Declarations
Funding
This work being reported was partially funded by Ajinomoto Co., Ltd. All authors declare no commercial associations that might represent conflicts of interest with this article and have nothing to disclose.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Kanagawa Cancer Center Hospital, and written informed consent was obtained before surgery from all patients.
Competing interests
The authors declare that they have no competing interests in association with the present study.
References
- Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20 (2017): 1-19.
- Tokunaga M, Tanizawa Y, Bando E, et al. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol 20 (2013): 1575-1583.
- Hayashi T, Yoshikawa T, Aoyama T, et al. Impact of infectious complications on gastric cancer recurrence. Gastric Cancer 18 (2015): 368-374.
- Oudemans-van Straaten HM, Bosman RJ, Treskes M, et al. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 27 (2001): 84-90.
- Parry-Billings M, Evans J, Calder PC, et al. Does glutamine contribute to immunosuppression after major burns? Lancet 336 (1990): 523-525.
- Jacobi CA, Ordemann J, Zuckermann H, et al. The influence of alanyl-glutamine on immunologic functions and morbidity in postoperative total parenteral nutrition. Preliminary results of a prospective randomized trial]. Zentralbl Chir 124 (1999): 199-205.
- Klek S, Kulig J, Szczepanik AM, et al. The clinical value of parenteral immunonutrition in surgical patients. Acta Chir Belg 105 (2005): 175-179.
- Oguz M, Kerem M, Bedirli A, et al. L-alanin-L-glutamine supplementation improves the outcome after colorectal surgery for cancer. Colorectal Dis 9 (2007): 515-520.
- Jo S, Choi SH, Heo JS, et al. Missing effect of glutamine supplementation on the surgical outcome after pancreaticoduodenectomy for periampullary tumors: a prospective, randomized, double-blind, controlled clinical trial. World J Surg 30 (2006): 1974-1982.
- Gianotti L, Braga M, Biffi R, et al. Perioperative intravenous glutamine supplemetation in major abdominal surgery for cancer: a randomized multicenter trial. Ann Surg 250 (2009): 684-690.
- Heyland D, Muscedere J, Wischmeyer PE, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 368 (2013): 1489-1497.
- Parry-Billings M, Baigrie RJ, Lamont PM, et al. Effects of major and minor surgery on plasma glutamine and cytokine levels. Arch Surg 127 (1992): 1237-1240.
- Viggiano E, Passavanti MB, Pace MC, et al. Plasma glutamine decreases immediately after surgery and is related to incisiveness. J Cell Physiol 227 (2012): 1988-1991.
- Yoshida H, Kondo K, Yamamoto H, et al. Validation of an analytical method for human plasma free amino acids by high-performance liquid chromatography ionization mass spectrometry using automated precolumn derivatization. J Chromatogr B Analyt Technol Biomed Life Sci 12 (2015): 993-996.
- Sanders J, Hawe E, Brull DJ, et al. Higher IL-6 levels but not IL6 -174G>C or -572G>C genotype are associated with post-operative complication following coronary artery bypass graft (CABG) surgery. Atherosclerosis 204 (2009): 196-201.
- Miwa K, Tanaka M, Okazaki S, et al. Association between interleukin-6 levels and first-ever cerebrovascular events in patients with vascular risk factors. Arterioscler Thromb Vasc Biol 33 (2013): 400-405.
- Shah T, Zabaneh D, Gaunt T, et al. Gene-centric analysis identifies variants associated with interleukin-6 levels and shared pathways with other inflammation markers. Circ Cardiovasc Genet 6 (2013): 163-170.
- Park SY, Kim J, Kim OJ, et al. Predictive value of circulating interleukin-6 and heart-type fatty acid binding protein for three months clinical outcome in acute cerebral infarction: multiple blood markers profiling study. Crit Care 17 (2013): R45.
- Blomqvist BI, Hammarqvist F, Von der Decken A, et al. Glutamine and alpha-ketoglutarate prevent the decrease in muscle free glutamine concentration and influence protein synthesis after total hip replacement. Metabolism 44 (1995): 1215-1222.
- Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 19 (2005): 1172-1176.
- Aoyama T, Yoshikawa T, Hayashi T, et al. Randomized comparison of surgical stress and the nutritional status between laparoscopy-assisted and open distal gastrectomy for gastric cancer. Ann Surg Oncol 21 (2014): 1983-1990.
- Leung KL, Lai PB, Ho RL, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: A prospective randomized trial. Ann Surg 231 (2000): 506-511.
- Tang CL, Eu KW, Tai BC, et al. Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg 88 (2001): 801-807.
- Dunker MS, Ten Hove T, Bemelman WA, et al. Interleukin-6, C-reactive protein, and expression of human leukocyte antigen-DR on peripheral blood mononuclear cells in patients after laparoscopic vs. conventional bowel resection: a randomized study. Dis Colon Rectum 46 (2003): 1238-1244.
