Aging Adjusted Serum Levels of Parathyroid Hormone and 25-Hydroxyvitamin D in Outpatients of a Tertiary Care Center using Generalized Additive Models for Location, Scale, and Shape
Article Information
Tatiana Munhoz da Rocha Lemos Costa1, Salomão Cury Riechi2, Ricardo Rasmussen Petterle3, Kátia Cristina Boritza2, Carolina Aguiar Moreira1, Cesar Luiz Boguszewski1, Victoria Zeghbi Cochenski Borba1,*
1Serviço de Endocrinologia e Metabologia do Hospital de Clínicas da Universidade Federal do Paraná (SEMPR), Curitiba, Brazil
2Laboratório de Análises Clínicas do Hospital de Clínicas da Universidade Federal do Paraná, Curitiba, Brazil
3Departamento de Medicina Integrada, Universidade Federal do Paraná, Curitiba, Brazil
*Corresponding author: Victoria Zeghbi Cochenski Borba, SEMPR, Serviço de Endocrinologia e Metabologia do Hospital de Clínicas da UFPR, Avenida Agostinho Leão Junior, 285, Curitiba, Brazil
Received: 13 November 2020; Accepted: 20 November 2020; Published: 27 November 2020
Citation: Tatiana Munhoz da Rocha Lemos Costa, Salomão Cury Riechi, Ricardo Rasmussen Petterle, Kátia Cristina Boritza, Carolina Aguiar Moreira, Cesar Luiz Boguszewski, Victoria Zeghbi Cochenski Borba. Aging adjusted serum levels of parathyroid hormone and 25-hydroxyvitamin D in outpatients of a tertiary care center using generalized additive models for location, scale, and shape. Archives of Clinical and Biomedical Research 4 (2020): 738-748.
View / Download Pdf Share at FacebookAbstract
Purpose: Evaluate serum concentrations of 25 hydroxyvitamin D (25OHD), calcium and parathyroid hormone (PTH) in samples obtained from a tertiary hospital, and establish the relationship between them, adjusted for age.
Methods: Cross-sectional study, samples by convenience from all 25OHD ordered for outpatients in one year, excluding clinical trials, research protocols, chronic kidney or liver diseases. Calcium corrected for albumin, PTH and 25OHD were measured. 25OHD levels were classified as deficiency (< 20 ng/ml), insufficiency (20-30 ng/ml), or sufficiency (> 30 ng/ml). The relationship between PTH and 25OHD adjusted by age was studied using the semi-parametric GAMLSS regression model (generalized additive models for location, scale, and shape).
Results: Samples from 1,031 subjects (82% female, age 53.1 ± 18.4 years) were included, mean 25OHD level was 26.6 ± 13.4 ng/mL, 321 (31.1%) patients were vitamin D deficient, and 409 (39.7%) insufficient. 25OHD had a seasonal variation, with lower levels in winter and spring and higher in summer and fall. PTH was elevated in 393 (38.6%) patients, from these, 2.5% were hypercalcemic and classified as primary hyperparathyroidism (HPT), 19.1% as normocalcemic primary HPT, and 78.4% as secondary HPT. The association of PTH and 25OHD adjusted by age showed that PTH decreased up to the 25OHD level close to 40 ng/mL and remained constant after 45 ng/mL.
Conclusion: Outpatients from a tertiary hospital had a high prevalence of vitamin D deficiency, which was influenced by seasonality, the PTH plateau was at a 25OHD level of 40 ng/mL when corrected for age.
Keywords
Parathyroid hormone; Calcium; Vitamin D; Outpatient; Tertiary care; GAMLSS regression
Parathyroid hormone articles; Calcium articles; Vitamin D articles; Outpatient articles; Tertiary care articles; GAMLSS regression articles
Article Details
Abbreviations
SEMPR=Serviço de Endocrinologia e Metabologia do Hospital de Clínicas da Universidade Federal do Paraná
25OHD =25 hydroxyvitamin D
PTH =Parathyroid hormone
GAMLSS=Generalized additive models for location, scale, and shape regression model
HPT =Hyperparathyroidism
SD =Standard deviation
CaSR= Calcium sensor receptor
1. Introduction
It is believed that one billion people worldwide have hypovitaminosis D, defined by serum levels of 25-hydroxyvitamin D (25OHD) below 75 nmol/L (30 ng/mL) [1]. The recommended levels of vitamin D intake and optimal levels of circulating 25OHD are a matter of long and controversial debate. The levels vary somewhat from country to country, and what is optimal for one group might be suboptimal for another group and age [2]. However, it is recognized that there is an inverse relationship between serum parathyroid hormone (PTH) and 25OHD levels, but it is not well known how aging could affect this relationship. PTH levels usually start to plateau at approximately 30 ng/mL, which has been observed in two different populations using linear regression not adjusted for age [3, 4].
Secondary hyperparathyroidism is a consequence of extra-glandular stimuli in response to serum mineral changes, leading to an imbalance of calcium and phosphorus in the blood. It is usually caused by kidney failure and vitamin D deficiency. Other less common causes of secondary hyperparathyroidism are long-term lithium therapy, gastrointestinal malabsorption syndromes, malnutrition, vitamin D-resistant rickets, or hypermagnesemia. The insufficient amount of calcium ions in the blood acts as a stimulus for proliferation of parathyroid cells, increasing parathyroid secretion [5].
Vitamin D deficiency is well known to be prevalent in hospitalized patients and with specific diseases [6, 7]. Around 70 to 80% of inpatients might have hypovitaminosis D [8, 9], with higher prevalence observed in elderly individuals, even in sunny countries such as Brazil. In our institution, located in Curitiba, in the south of Brazil, we found a high prevalence of deficiency around 30% or insufficiency up to 90% in specific populations, such as postmenopausal women, patients with systemic lupus erythematosus, chronic obstructive pulmonary disease, and inflammatory bowel disease [10-12].
In contrast, much less is known about 25OHD levels in outpatients, which motivated us to study serum concentrations of 25OHD, calcium, and PTH in a consecutive cohort of outpatients with different ages, visiting a tertiary care center in a city located at 25° 25’S 49° 15’W, for diverse medical reasons, and to establish the association of PTH and 25OHD adjusted for age.
2. Patients and Methods
This was a cross-sectional, observational study, with samples obtained by convenience from all 25OHD measurements ordered for outpatients visiting various clinics of our university hospital within one year. Dosages required as part of clinical trials or research protocols and from patients with known chronic renal or liver diseases were excluded.
Serum levels of 25OHD were measured by chemiluminescence in an automated analyzer (LIAISON®, DiaSorin, inter-assay variation = 20%; detection limit = 4 – 150 ng/mL). 25OHD was classified according to Endocrine Society Guidelines as normal (25OHD ≥ 30 ng/mL), insufficient (25OHD = 20 - <30 ng/mL), or deficient (25OHD < 20 ng/mL) [13]. In the same blood sample used for 25OHD determination, total calcium was analyzed by colorimetry (inter-assay variation = 5%; reference range = 8.4-10.2 mg/ml) and corrected for albumin (bromocresol purple; reference range= 3.4 to 5 g/dL); hypo- and hypercalcemia were defined as levels below 8.4 or above 10.2 mg/ml, respectively. PTH levels were analyzed by chemiluminescence (inter-assay variation = 2.51%; reference range = 15 - 68.3 pg/mL). Hyperparathyroidism was classified as primary when serum levels of PTH and calcium were elevated with normal 25OHD; as normocalcemic primary hyperparathyroidism when PTH was elevated with serum calcium and 25OHD normal; and as hyperparathyroidism secondary to vitamin D deficiency when PTH was elevated, calcium level was normal or low, and 25OHD was below 20 ng/mL.
Samples were included throughout the year, and only the first sample measured during the study period for the same individual was included. All measurements were taken in the routine work of the laboratory, soon after collection, with prior centrifugation of 4.200 rpm for 10 min. No personal contact existed between patients and investigators.
The relationship between PTH and 25OHD adjusted by age and calcium was studied using the semi-parametric GAMLSS regression models [14]. This study was approved by the Ethics Committee of our institution (CEP- 2655.262/2011-11).
2.1 Statistical analysis
R statistical software (R Core Team; 2019) [15] was used. Data are presented as mean ± standard deviation (SD), median, and range, or frequencies and percentages. Data distribution was tested using the Kolmogorov-Smirnov and Shapiro-Wilk tests, which verified the assumption of their symmetrical (normal) distribution. Symmetrical distribution of the variables is presented as mean ± SD, while the asymmetric variables are presented as median, minimum, and maximum values. Differences for categorical variables were analyzed using the chi-square test and multiple comparisons using the Bonferroni test. To analyze the nonlinear relationship between PTH values and vitamin D adjusted by age, we used the GAMLSS framework. This regression technique makes assumptions on a distribution form and provides a variety of different distribution families for the response variable; additionally, it has a platform to fit, compare, and check many different models. GAMLSS models the distribution parameters: μ (a location parameter, i.e., mean, median), σ (a scale, i.e., standard deviation, dispersion), ν (a shape, i.e., modeling skewness), and τ (a shape, i.e., modeling kurtosis). It is possible to fit with GAMLSS additive or multiplicative models for μ using identity or log links, respectively [14]. PTH was assumed as the dependent variable, age, calcium, and 25OHD as explanatory variables. We adopted the Box-Cox t distribution for modeling PTH [14]. For all analyses, two-tailed tests with a p-value below 0.05 were considered statistically significant.
3. Results
For one year, 1,265 samples were sent to our Clinical Chemistry Laboratory for the determination of serum 25OHD levels. A total of 234 were excluded from our analysis due to reasons mentioned before (samples from inpatients or patients with chronic renal or liver disease, duplicate samples of the same patient, or dosages required as part of clinical trials or research protocols). The final analysis was carried out on 1,031 individual samples from outpatients (81.8% women; mean age 53.1 ± 18.4 years) visiting various clinics of the hospital. The mean serum 25OHD levels were 26.6 ± 13.4 ng/mL, 321 (31.1%) patients had 25OHD deficiency, and 409 (39.7%) had insufficiency. PTH levels were higher in patients with vitamin D deficiency compared with insufficient or sufficient patients (p < 0.001). Serum calcium levels did not differ among patients sufficient, insufficient, and deficient in vitamin D, with hypocalcemia observed in 69 (6.68%) patients and hypercalcemia in 23 (2.22%) (Table 1).
There was a difference between 25OHD classification according to age; the median age was 59 years (1 - 86) for patients with sufficiency, 54 (3 - 86) years for insufficiency, and 56 (1 - 86) years for sufficiency, p<0.01 for all. Patients older than 65 years had higher levels of 25OHD (n = 726; 29.5 ± 17.3 ng/mL) compared to the younger ones (n = 294; 25.3 ± 11.1 ng/mL; p < 0.001). The prevalence of low 25OHD (deficiency and insufficiency) of 25OHD was 75.3% in patients older than 65 years and 61.9% in those younger than 65 years.
|
Variables |
All Samples |
25OHD Deficiency |
25OHD Insufficiency |
25OHD Sufficiency |
P |
|
Number (%) |
1,031 (100) |
321 (31.1) |
409 (39.7) |
301 (29.2) |
|
|
Age (Years) |
53.1 ± 18.4 |
53.9 ± 17.8 |
50.7 ± 18.1 |
55.4 ± 19.0 |
< 0.001 |
|
Female N (%) Male N (%) |
843 (81.8) 188 (18.2) |
259 (80.7) 62 (19.3) |
347 (84.8) 62 (15.2) |
237 (78.7) 64 (21.2) |
*< 0.001 |
|
25OHD (ng/mL) |
26.60 ± 13.40 |
14.3 ± 4.1 |
24.80 ± 2.8 |
42.1 ± 13.3 |
< 0.001 |
|
Calcium (mg/dL) |
9.26 ± 0.64 |
9.28 ± 0.72 |
9.26 ± 0.56 |
9.25 ± 0.66 |
0.629 |
|
PTH (pg/dL) median (min - max) |
60.8 (5.0 - 841.4) |
95.25 (68.0 - 841.4) |
56.20 (5.0 - 792.9) |
53.35 (5.0 - 209.7) |
< 0.001 |
25OHD = 25-hydroxyvitamin D; % = percentage; min = minimum; max = maximum; N = number; PTH = parathyroid hormone; *= difference between sex
Table 1: Clinical and laboratory data of outpatients visiting various clinics of a tertiary care center within one year.
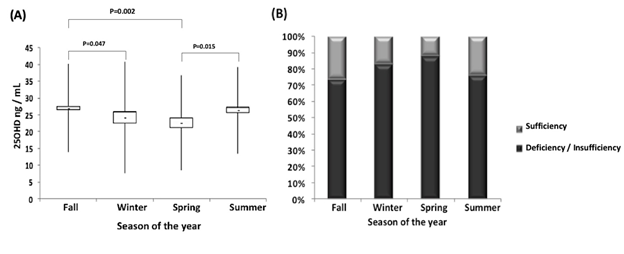
Serum 25OHD levels were different according to the season of the year: lower in the spring (21.59 ± 8.44 ng/mL) and winter (24.05 ± 7.50 ng/mL) and higher in the fall (29.34 ± 9.19 ng/mL) and summer (27.49 ± 9.63 ng/mL). Accordingly, the prevalence of deficiency/insufficiency was higher in the winter (83%) and spring (87.9%) than in the other two seasons (75.9% in fall and 73.4% in summer) (p < 0.001) (Figures 1A and 1B).
25OHD: 25-hydroxyvitamin D. (A) levels of 25OHD in ng/mL each season of the year; (B) Percentage of patients classified according to the Endocrine Society in each season of the year [12]. Statistical significance p < 0.05.
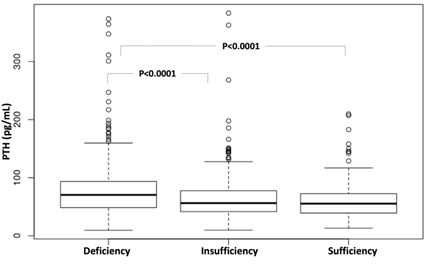
The 25OHD levels were lower in patients with elevated PTH levels (23.57 ± 8.62 ng/mL) compared to patients with normal PTH (28.52 ± 9.72 ng/mL) (p < 0.001). Furthermore, PTH levels were higher in patients with deficiency compared to patients with insufficiency and sufficiency (p < 0.0001 for both) (Table 1, Figure 2).
Parathyroid hormone. Percentage of patients in each age group classified according to the Endocrine Society [12]. Statistical significance p < 0.05.
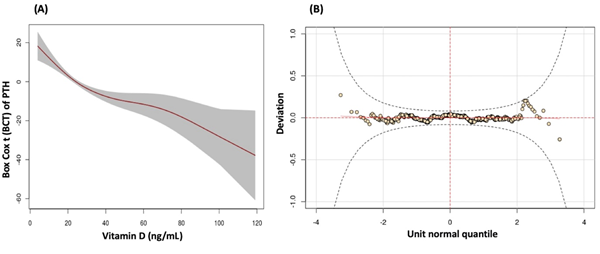
Nonlinear regression was conducted using the GAMLSS framework of serum PTH levels as a function of serum 25OHD levels, which were adjusted for calcium and age; however, calcium was not significant, and only age remained in the final model. The equation µi = β01 + pb (vitamin D) + pb (age) was used to adjust the model, with the parameters being σi = β02; vi = β03; τi = β04. In this equation, the mean PTH was modeled as a function of explanatory variables (25OHD and age), and the other variables were kept fixed, Table 2. The worm plot based on the residuals showed the adequacy of the model, with all points inside the 95% confidence band, Figure 3 (B). PTH decreased up to a 25OHD level close to 40 ng/mL, remained constant after 45 ng/mL, and declined again after 80 ng/mL, Figures 3 (A) and 3 (B).
|
Parameter |
Estimate |
Standard error |
p-value |
|
Intercept |
44.1891 |
2.575 |
< 0.001 |
|
pb (25OHD) |
-0.46647 |
0.0565 |
< 0.001 |
|
pb (Age) |
0.54260 |
0.04183 |
< 0.001 |
Legend: pb = penalized B-splines; 25OHD= 25hydroxyvitamin D; PTH= parathyroid hormone
Table 2: Estimates, approximate standard errors and p-values from the Box-Cox t distribution for modeling PTH.
4. Discussion
The high prevalence of insufficiency and deficiency of vitamin D found in this study is in agreement with the results presented in the literature. Of the population studied, 70.8% had hypovitaminosis D, similar to the data from Amsterdam, with 86% of patients having hypovitaminosis, 79% having insufficiency, and 7% having deficiency [16]. In Brazil, the prevalence of vitamin D deficiency was 55.8% and 40%, respectively, in the elderlies from the community in São Paulo [3] and in a group of Brazilian postmenopausal women [17]. In the same study, data from Curitiba showed an average 25OHD level of 26.2 ± 12.5 ng/ml, with 78% of patients categorized as having deficiency/insufficiency, similar to this study [17]. Low vitamin D levels were also present in 63% of outpatients from a rheumatology clinic [18].
The study sample consisted of middle-aged individuals, mostly women, with higher 25OHD levels in patients older than 65 years. The high prevalence of hypovitaminosis D was independent of age, and although we found higher levels in the group older than 65 years, both groups were vitamin D insufficient. It has been described that the decrease in the concentrations of 7-dehydrocholesterol in the skin, the vitamin D precursor, related to aging, negatively interferes with vitamin D levels. By the age of 70, there is approximately a 75% reduction in the ability to synthesize vitamin D3 in the skin [2]. This study, however, showed higher vitamin D levels with aging, similar to the data from Australia [19]. We believe that this fact, was not due to metabolic reasons but because of possible vitamin D replacement therapy in this population; unfortunately, we did not have information about vitamin D supplementation.
Sex in this study did not affect vitamin D deficiency, which differs from other studies that observed a higher prevalence of vitamin deficiency in women [6, 20]. The seasonality of vitamin D levels were maintained and were shown to be lower in the spring and winter and higher in the fall and summer, similar to what was reported in a study conducted in São Paulo with 250 elderly individuals, with a 25OHD peak in the fall and a dip in the spring, reflecting the higher and lower incidence of sun irradiation [21].
Although consistent with the literature, the prevalence of hypovitaminosis D in this study was higher, which can be explained by multiple factors that interfere with the individual production of vitamin D that were not evaluated here. Moreover, other factors such as the diversity of studied population regarding age, ethnic origin, and inclusion of chronic patients followed up in a tertiary hospital, might have influenced the high rate of vitamin D deficiency, which has been observed in other studies [22-25].
Among patients with elevated PTH, the prevalence of primary hyperparathyroidism, normocalcemic primary hyperparathyroidism, and secondary hyperparathyroidism was 2.5%, 19.1%, and 78.4%, respectively, different of what is reported in the literature, which shows 0.5-1%, 8.5%, and 18% prevalence, respectively, probably because this study was conducted with outpatients of a tertiary hospital with multiple comorbidities and other possible secondary causes possibly underdiagnosed [26], and a longitudinal evaluation was not possible by the design of the study.
There is a discussion in the literature about the optimal level of 25OHD. When taking into account calcium homeostasis and bone health, levels range from 20 to 32 ng/ml (50 to 80 nmol/L). To correct secondary hyperparathyroidism, reducing the risk of falls and fractures and maximum absorption of calcium, the best cutoff point of 25OHD has not yet been defined. Some studies have shown that a 25OHD value around 30 ng/mL (75 nmol/L) could be the best to suppress PTH [3, 4, 27]. Our study used a GAMLSS model, which is a better model to evaluate a nonlinear association such as PTH and vitamin D. Furthermore, it was adjusted for age, a known interfering factor. A better cutoff point according to this model would be 40 ng/ml (99 nmol/L), which is in agreement with the study of Ginde et al. [28].
Several studies have shown the importance of PTH being within the normal range, especially in patients with chronic diseases and elderly populations [29]. In a cohort of older men and women, it was shown that higher PTH concentrations were associated with a 93% higher risk of all-cause mortality [30]. There are potential mechanisms through which PTH excess could affect mortality risk. Serum PTH might influence intracellular signaling, which might increase intracellular calcium concentrations and ultimately result in cell death [31]; still, it can activate the renin-angiotensin system with subsequent hypertension and cardiac remodeling. Furthermore, PTH stimulates vascular inflammation and arterial stiffness, augmenting atherogenesis [31, 32]. Also, another study found higher concentrations of PTH in frequent fallers, suggesting a possible role of PTH in falling [33].
Although this study did not assess calcium intake, which could influence the correlation of PTH and 25OHD, we interestingly observed that a group of patients with low 25OHD did not exhibit any change in calcium and PTH levels. One explanation is that there are possible significant individual differences in mean plasma calcium level, reflecting small individual differences in parathyroid secretory set point due to polymorphism of the calcium sensor receptor (CaSR) gene, which also accounts for significant differences between families [34].
We conclude that in the samples analyzed, the prevalence of vitamin D deficiency was high, and for this population of outpatients from a tertiary hospital, when age was taking into account, a 25OHD level of 40 ng/mL could better suppress PTH. These data suggest that even patients being treated in a tertiary care hospital do not receive enough supplementation to maintain adequate levels of vitamin D.
Declarations of Interest
None.
References
- Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 18 (2017): 153-165.
- Christakos S, Li S, De La Cruz J et al. New developments in our understanding of vitamin metabolism, action and treatment. Metabolism S98 (2019): 112-120.
- Maeda SS, Saraiva GL, Kunii IS, et al. Factors affecting vitamin D status in different populations in the city of São Paulo, Brazil: the São PAulo vitamin D Evaluation Study (SPADES). BMC Endocr Disord 13 (2013): 14
- Silva BC, Camargos BM, Fujii JB, et al. Prevalence of vitamin D deficiency and its correlation with PTH, biochemical bone turnover markers and bone mineral density, among patients from ambulatories. Arq Bras Endocrinol Metabol 52 (2008): 482-488.
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet 13 (2018): 168-178.
- Muschitz C, Kocijan R, Stütz V, et al. Vitamin D levels and comorbidities in ambulatory and hospitalized patients in Austria.; Wien Klin Wochenschr 127 (2015): 675-684.
- Borges RC, Barbeiro HV, Barbeiro DF, et al. Muscle degradation, vitamin D and systemic inflammation in hospitalized septic patients. J Crit Care 56 (2020): 125-131.
- Boettger SF, Angersbach B, Klimek CN, et al. Prevalence and predictors of vitamin D-deficiency in frail older hospitalized patients. BMC Geriatr 18 (2018): 219.
- Tanabe S, Yano S, Mishima S, et al. Physical inactivity and vitamin D deficiency in hospitalized elderlies. J Bone Miner Metab 37 (2019): 928-934.
- Borba VZ, Vieira JG, Kasamatsu T, et al. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int 20 (2009): 427-433.
- Franco CB, Paz-Filho G, Gomes PE, et al. Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of vitamin D. Osteoporos Int 20(2009): 1881-1887.
- Souza HN, Lora FL, Kulak CA, et al. Low levels of 25-hydroxyvitamin D (25OHD) in patients with inflammatory bowel disease and its correlation with bone mineral density. Arq Bras Endocrinol Metabol 52 (2008): 684-691.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96 (2011): 1911-1930.
- Stasinopoulos MD, Rigby RA, De Bastiani F. GAMLSS: A distributional regression approach. Statistical Modelling 18 (2018): 248-273.
- R Core Team. R: A Language and Environment for Statistical Com - puting, R Foundation for Statistical Computing, Vienna, Austria (2019).
- Deckers MM, de Jongh RT, Lips PT, et al. Prevalence of vitamin D deficiency and consequences for PTH reference values. Clin Chim Acta 15 (2013): 41-45.
- Arantes HP, Kulak CA, Fernandes CE, et al. Correlation between 25-hydroxyvitamin D levels and latitude in Brazilian postmenopausal women: from the Arzoxifene Generations Trial. Osteoporos Int 24 (2013): 2707-2712.
- Sabbagh Z, Markland J, Vatanparast H. Vitamin D status is associated with disease activity among rheumatology outpatients. Nutrients 5 (2013): 2268-2275.
- Voo VTF, Stankovich J, O'Brien TJ, et al. Vitamin D status in an Australian patient population: a large retrospective case series focusing on factors associated with variations in serum 25(OH)D. BMJ Open 10 (2020): e032567.
- AlQuaiz AM, Mujammami M, Kazi A, et al. Vitamin D cutoff point in relation to parathyroid hormone: a population based study in Riyadh city, Saudi Arabia. Arch Osteoporos 14 (2019): 22.
- Maeda SS, Saraiva GL, Hayashi LF, et al. Seasonal variation in the serum 25-hydroxyvitamin D levels of young and elderly active and inactive adults in São Paulo, Brazil: The São PAulo Vitamin D Evaluation Study (SPADES). Dermatoendocrinol 5 (2013): 211-217.
- Caccamo D, Ricca S, Currò M, et al. Health Risks of Hypovitaminosis D: A Review of New Molecular Insights. Int J Mol Sci 19 (2018): E892.
- Jiang W, Wu DB, Xiao GB, et al. An epidemiology survey of vitamin D deficiency and its influencing factors. Med Clin (Barc) 154 (2020): 7-12.
- Herrmann M, Farrell CL, Pusceddu I, et al. Assessment of vitamin D status - a changing landscape. Clin Chem Lab Med 55 (2017): 3-26.
- Huang CH, Huang YA, Lai YC, et al. Prevalence and predictors of hypovitaminosis D among the elderly in subtropical region. PLoS One 12 (2017): e0181063.
- Majid H, Khan AH, Riaz M, et al. Identifying parathyroid hormone disorders and their phenotypes through a bone health screening panel: it's not simple vitamin D deficiency. Endocr Pract 22 (2016): 814-821.
- Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7 (1997): 439-443.
- Ginde AA, Wolfe P, Camargo CA Jr, et al. Defining vitamin D status by secondary hyperparathyroidism in the U.S. population. J Endocrinol Invest 35 (2012): 42-48.
- Toraman A, Aras F, Hekimsoy Z, et al. Is there a relationship between parathyroid hormone and neutrophil lymphocyte ratio or platelet lymphocyte ratio? Acta Endocrinol (Buchar) 5 (2019): 96-101.
- van Ballegooijen AJ, Reinders I, Visser M, et al. Serum parathyroid hormone in relation to all-cause and cardiovascular mortality: the Hoorn study. J Clin Endocrinol Metab 98 (2013): E638-E645.
- Jablonski KL, Chonchol M, Pierce GL, et al. 25 Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57 (2011): 63-69.
- Pirro M, Manfredelli MR, Helou RS, et al. Association of parathyroid hormone and 25-OH-vitamin D levels with arterial stiffness in postmenopausal women with vitamin D insufficiency. J Atheroscler Thromb 19 (2012): 924-931.
- de França NAG, Murthy LS, Phu S, et al. High parathyroid hormone levels are associated with poor balance in older persons: A cross-sectional study. Maturitas 121 (2019): 57-62.
- Parfitt AM. Plasma calcium homeostasis. J Musculoskelet Neuronal Interact 4 (2004): 109-110.