(-)-Agelastatin A is a Potent Modulator of Osteopontin Expression in Normal and Dystrophic Canine Myoblasts
Article Information
Madison Feng, Sara Mata López, and Peter P. Nghiem*
The Department of Veterinary Integrative Sciences, School of Veterinary Medicine and Biomedical Sciences, Texas A & M University, College Station, Texas, TX 77843-4458, USA
*Corresponding author:
Peter P. Nghiem, DVM, Ph.D., The Department of Veterinary Integrative Biosciences, School of Veterinary Medicine and Biomedical Sciences, Texas A & M University, College Station, Texas, TX 77843-4458, USA.
Received: 04 October 2023; Accepted: 17 October 2023; Published: 01 November 2023
Citation: Madison Feng, Sara Mata López, and Peter P. Nghiem. (-)-Agelastatin A is a Potent Modulator of Osteopontin Expression in Normal and Dystrophic Canine Myoblasts. Fortune Journal of Health Sciences. 6 (2023): 422-426.
View / Download Pdf Share at FacebookAbstract
(-)-Agelastatin A [(-)-AA] is a naturally occurring alkaloid of marine sponge origin that is a potent modulator of osteopontin. The latter is a multifunctional cytokine expressed in many types of cells and is involved with several important cellular processes, including adhesion, proliferation, immune response stimulation, and so on. As such, modulating OPN could be of therapeutic benefit for various diseases. On that note, (-)-AA has been shown to reduce OPN in xenografted human tumors in nude mice. Additionally, an OPN genotype was associated loss of ambulation in Duchenne muscular dystrophy (DMD) boys and its expression associated with pathology. In this study, we analyzed OPN expression in normal and canine DMD stem cells (myoblasts) after AA treatment. When suspended in 1,2-propanediol (3-deoxy-DL-glycerol), (-)-AA elicited a dose-dependent decrease in OPN mRNA expression in canine DMD myoblasts at doses ranging from 0.01nM – 30nM. There was also a dose-dependent cell detachment after 24 hours of AA treatment with concentrations of 10nM and greater. Further studies are warranted to determine if (-)-AAis safe and can provide therapeutic benefit in animal models of DMD.
Keywords
Osteopontin, (-)-agelastatin A, Duchenne muscular dystrophy (DMD), canine, myoblasts
Article Details
1. Introduction
Duchenne muscular dystrophy (DMD) is a devastating, monogenic, muscle-wasting disease caused by a loss of dystrophin protein [1]. Dystrophin deficiency disrupts the normal structural integrity and proper functioning of the costameric dystrophin-glycoprotein complexes that link the sarcolemma to the myofibrils [2, 3]. The cytoskeleton is then unable to support the normal repeated muscle stretching and contracting that occurs during exercise-induced damage [4]. These events then bring about the chronic degeneration and regeneration within the affected muscle. The necrotic injury that ensues then promotes widespread muscle inflammation and fibrosis; a situation that causes the regenerating muscle cells to transform into fibroadipocytes [5], which then promote fat infiltration into the skeletal muscle and allied fibrosis. Together, these actions cause severe muscle wasting and weakness; circumstances which typically induce loss of ambulation by 12 years of age, and death by their 30-40s, frequently from attendant cardiac or respiratory arrest.
There is remarkable variability in the extent to which many individuals are affected by DMD, with the first debilitating signs of the disease typically manifesting themselves between the ages of 2 to 5. Most of the phenotypic variability is associated with the type of DMD gene mutation that has arisen. Either in-frame or out-of-frame, with the former leading to the much milder Becker MD syndrome, in which some truncated functional dystrophins can still be found; and the latter leading to the more severe DMD disease, where dystrophin is significantly reduced to absent [6]. That said, there are several genetic modifiers that may dramatically accelerate DMD progression [7]. One of these is osteopontin (OPN); also known as secreted phosphoprotein 1 (SPP1) [8]. It can markedly exacerbate the amount of muscle damage, inflammation, and fibrosis that can occur [9-13], particularly when it is chronically overexpressed within DMD muscle, as happens, when there is repeated mechanically-induced muscle injury.
While it is possible to delay the progressive muscle weakness, attendant loss of ambulation, and generally declining motor function with the aid of corticosteroid drugs (e.g. prednisolone or deflazacort) [14], these benefits are only temporary. Steroidal drugs also often elicit many undesirable side-effects such as immunosuppression, neuropsychiatric deficits, osteoporosis, slow wound healing, metabolic and endocrine derangements, growth retardation in children, amongst others. While dystrophin restoration therapies hold quite considerable promise in restoring dystrophin expression, other treatments are needed to stop and even reverse the secondary sequela, including inflammation and fibrosis. (-)-Agelastatin-A [(-)-AA] is a naturally occurring oroidin alkaloid of marine sponge origin that exerts strong antitumor effects against a wide range of xenografted human tumors in nude mice [15-17]. Its mechanism of action includes downregulation of OPN. In this study, we sought to determine if (-)-AA could downregulate OPN in muscle stem cells (myoblasts) from normal and dystrophic canine origin.
2. Methods
Materials. The (-)-AA used here was a kind gift from the laboratory of Dr. Karl Hale and was synthesized according to experimental procedures detailed previously [18-20].
(-)-AA Dilution. (-)-AA was diluted in 1,2-propanediol (3- deoxy-DL-glycerol; 1,2-propylene-glycol) to make concentrations of 0.01, 0.1, 1, 5, 10, 30, 100, and 1,000 nanomolar (nM).
Cell Culture. Myoblast isolation was performed as previously described [21, 22]. Briefly, myoblasts were isolated with a pre-plate technique and cryopreserved at -80°C until analysis. Myoblasts were plated in flasks and then transferred to collagen-coated 6, 12, and 24 well plates with 1-5 x105 cells per well in complete growth/proliferation medium. Myoblasts were allowed to attach to the collagen plate and proliferate until approximately 70-90% confluency.
Cell Treatments. Complete proliferation medium was changed to DMEM (Dulbecco’s Modified Eagle’s Medium). Myoblasts were then treated for 72 h with either (-)-AA (diluted in 1,2-propanediol) or placebo (equal volume of 1,2-propanediol).
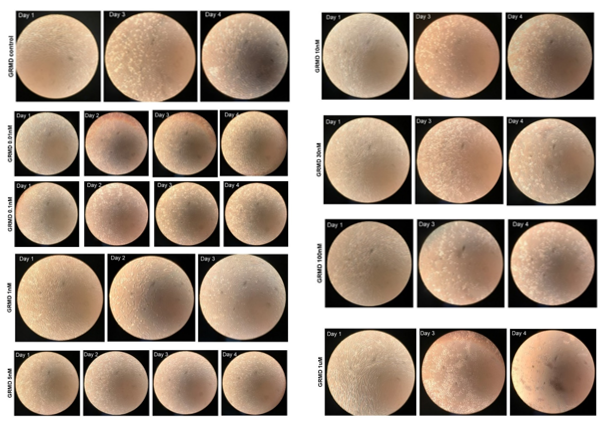
Microscopic Analysis and Necrotic Fiber Characterization. A light microscope and cellular camera were used to take images before treatment (day 1) and every 24 h (day 2 [24 hr of tx], 3 [48 hr of tx], and 4 [72 hr of tx]).
RNA Analysis. The cells were first washed with 1XPBS. Total RNA was then extracted from cell culture with Tripure, and its quality and quantity were determined with a Bioanalyzer, as previously described [22]. Complementary DNA was generated by reverse transcription. (-)-AA-treated cultures were analyzed for OPN mRNA expression using SYBR green assays. Primers for OPN and the internal control, HPRT1, as well as quantitative, real-time (qRT-) PCR parameters can be found in a previously published study [22].
Statistical Analysis. We analyzed CT values from the qRT-PCR of mRNA for OPN in normal and GRMD myoblasts following treatment with various doses of (-)-AA using a pairwise fixed reallocation randomization test to determine fold-change and standard error of the mean (SEM). Bar graphs were plotted as the mean of each treated group and the SEM.
3. Results
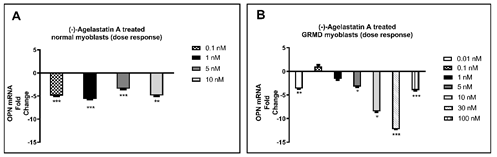
We treated for 72 hours normal and dystrophic myoblasts with (-)-AA dissolved in 1,2-propanediol over a range of drug concentrations from 0.01-1,000 nM, and then analyzed mRNA for OPN expression. Although we did see statistically significant changes in OPN mRNA in normal dog myoblasts, the (-)-AA responses were not dose-dependent (Fig. 1A). Nonetheless, after observing positive results in the normal cells, we chose to focus our remaining efforts on dystrophic myoblasts. A clear dose-dependent decrease in OPN mRNA was observed following (-)-AA treatment at concentrations of 0.01 nM-30 nM in canine DMD myoblasts (Fig. 1B).
Figure 1: (-)-AA reduced OPN mRNA expression after 72 hrs in A) normal and B) dystrophic myoblasts, the latter was dose-dependent. Quantitative real-time PCR was performed to quantify OPN mRNA. Expression of OPN was determined as a fold change of 1xPBS control-treated cells. HPRT1 was used as an internal control for 1,2-propanediol (placebo) and (-)-AA-treated cells. A pair-wise fixed reallocation randomization test was performed to determine the fold change. *p ≤0.05; **p ≤0.01; ***p ≤0.001.
In control (placebo)-treated, canine DMD cells, there was a small amount of cell detachment evident by days 3 [48 h of tx] and 4 [72 h of tx] (Figure 2, left panel). In AA-treated cells, on day 4, increased cell detachment was observed at 10nM and greater (Figure 2, right panel), which was dose-dependent. At the 1,000 nm (1 uM) concentration, cell detachment was almost 100% by day 4 (Figure 2, right panel).
4. Discussion
Although we did see changes in OPN mRNA in normal dog myoblasts, the (-)-AA responses were not dose-dependent, making these results rather difficult to interpret. Nonetheless, in canine DMD myoblasts, a dose-dependent decrease in OPN mRNA was observed following (-)-AA treatment. This suggested that the OPN gene is somehow primed in dystrophic muscle cells, presumably because of the well-known upregulation of OPN in dystrophic muscles [12]. Whatever OPN downregulatory mechanism is operational, the most salient observation made here is that canine DMD myoblasts are much more susceptible to a dose-dependent decrease in OPN expression when they are treated with (-)-AA than are normal myoblasts.
We observed a small amount of cell detachment after 24 hours of treatment with placebo. This was not surprising, as primary myoblasts are susceptible to the removal of proliferation media and to various treatments, despite the innocuous nature of the conditions. In (-)-AA treated cells, however, we did observe a dose-dependent cell detachment in normal (not shown) and dystrophic myoblasts at doses of 10nM or greater. Since OPN regulates a multitude of cellular processes, including cell adhesion, it was no surprise that the potent (-)-AA significantly inhibited OPN expression and its adhesion function.
In this study we have documented the downstream effects of treating dystrophin deficient myoblasts with (-)-AA. The ability of (-)-AA to pharmacologically manipulate the expression of OPN, in a way where its injurious pro-inflammatory/fibrotic effects can be diminished, and its beneficial, pro-regenerative, properties [23] can be maintained, is of substantive interest. Care should be taken to dampen the immune response, but not to negatively effect other important cellular functions.
There are some drawbacks of our study. Our multi-year quest to identify an antibody to detect OPN protein in canine cells and tissue has been met with failure. Also, some of the (-)-AA treatments were missing imaging days due to logistical difficulties. We selected myoblasts due to their availability in our cryopreserved biobank; that said, important knowledge of OPN manipulation can be gleamed from our myoblast study. In the future, immune cells, myofibroblasts, and fibroadipogenic progenitor cells from normal and dystrophic individuals should also be assessed with (-)-AA treatment, as well as in vivo pre-clinical studies.
Acknowledgements
We would like to acknowledge Dr. Karl Hale and his team, Soraya Manaviazar and Hamish A. Watson, for their guidance and for synthesizing and providing the (-)-Agelastatin A. This study was funded by startup funds from Texas A&M University.
References
- Hoffman EP, Brown RH, and Kunkel LM. Dys- trophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51 (1987): 919-928.
- Ervasti JM, and Campbell KP. Membrane organiza- tion of the dytrophin-glycoprotein complex. Cell 66 (1991): 1121-1131.
- Michele DE, and Campbell KP. Dystrophin- Glycoprotein Complex: post translational processing and dystro- glycan function. Biol. Chem 278 (2003): 15457-15460.
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, and Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Natl. Acad. Sci.USA 90 (1993): 3710-3714.
- Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. Cell Sci 124 (2011): 3654-3664.
- Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, and Kunkel LM. An explanation for the phenotypic differ- ences between patients bearing partial deletions of the DMD locus. Genomics 2 (1988): 90-95.
- Vo AH, and McNally EM. Modifier genes and their effect on Duchenne muscular dystrophy. Opin. Neurol. 28 (2015): 528-534.
- Denhardt DT, and Guo X. Osteopontin: a protein with diverse FASEB J. 7 (1993): 1475-1482.
- Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, et al. Osteopontin promotes fibrosis in dystrophic mouse mus- cle by modulating immune cell subsets and intramuscular TGFbeta. J Clin. Invest. 119 (2009): 1583-1594.
- Capote J, Kramerova I, Martinez L, Vetrone S, Barton ER, Sweeney HL, et al. Osteo- pontin ablation ameliorates muscular dystrophy by shifting macro- phages to a pro-regenerative phenotype. Cell Biol 213 (2016): 275-288.
- Uaesoontrachoon K, Wasgewatte Wijesinghe DK, Mackie EJ, and Pagel CN. Osteopontin deficiency delays inflam- matory infiltration and the onset of muscle regeneration in a mouse model of muscle injury. Disease Models and Mechanisms 6 (2013): 197-205.
- Many GM, Yokosaki Y, Uaesoontrachoon K, Nghiem PP, Bello L, Dadgar S, et al. OPN-? induces muscle inflammation by in- creasing recruitment and activation of pro-inflammatory macro- phages. Physiol 101 (2016): 1285-1300.
- Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, et al. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology 76 (2011): 219-226.
- McDonald CM, Sajeev G, Yao Z, McDonnell E, Elfring G, Souza M, et al. Deflazacort vs prednisone treatment for Duchenne muscular dystrophy: A meta-analysis of dis- ease progression rates in recent multicenter clinical trials. Muscle & Nerve 61 (2019): 26-35.
- D’Ambrosio M, Guerriero A, Debitus C, Ribes O, Pusset J, Leroy S, and Pietra F. Agelastatin A, a new skele- ton cytotoxic alkaloid of the oroidin family. Isolation from the axinel- lid sponge Agelas dendromorpha of the coral sea. Chem. Soc. Chem. Commun (1993): 1305-1306.
- D’Ambrosio M, Guerri- ero A, Pietra F, Ripamonti M, Debitus C, and Waikedre J. The active centres of agelastatin A, a strongly cytotoxic alka- loid of the coral sea axinellid sponge Agelas dendromorpha, as de- termined by comparative bioassays with semi-synthetic derivatives. Chim. Acta 79 (1996): 727-735.
- Mason CK, McFarlane S, Johnston PG, Crowe P, Er-win PJ, et al. Agelastatin A: A novel inhibitor of os- teopontin-mediated adhesion, invasion, and colony formation. Cancer Ther 7 (2008): 548-558.
- Hale KJ, Domostoj MM, Tocher DA, Irving E, and Scheinmann F. Enantiospecific formal total synthesis of the tumor and GSK-3?-inhibiting alkaloid (-)-agelastatin A. Lett. 5 (2003): 2927-2930.
- Domostoj MM, Irving E, Scheinmann F and Hale KJ. New total synthesis of the marine antitumor alkaloid (-)-agelastatin A. Lett. 6 (2004): 2615-2618.
- Hale KJ, Domostoj MM, El-Tanani M, Campbell FC, and Mason CK. Total synthesis and mechanism of action studies on the antitumor alkaloid, (-)-agelastatin A. Strategies and Tactics in Organic Synthesis 6 (2005): 352-394.
- Mata López S, Balog-Alvarez C, Vitha S, Bettis AK, Canessa EH, Kornegay JN, et al. Challenges associated with homologous directed repair using CRISPR-Cas9 and TALEN to edit the DMD genetic mutation in canine Duchenne muscular dystrophy. PLOS One 15 (2020):
- López SM, Balog-Alvarez C, Canessa EH, Hathout Y, Brown KJ, Vitha S, et al. Creation and characterization of an immortalized canine myoblast cell line: Myok9. Mamm Genome 31 (2020): 95-109.
- Nghiem PP, Kornegay JN, Uaesoontrachoon K, Bello L, Yin Y, Kesari A, et al. Osteopontin is linked with AKT, FoxO1, and myostatin in skeletal muscle cells. Muscle Nerve 56 (2017): 1119-1127.