African Americans Possessed High Prevalence of Comorbidities and Frequent Abdominal Symptoms, and Comprised A Disproportionate Share of Covid-19 Mortality among 9,873 Us-Hospitalized Patients Early in the Pandemic
Article Information
Hassan Ashktorab1, Antonio Pizuorno1, Lakshmi Gayathri Chirumamilla1, Folake Adeleye1, Maryam Mehdipour Dalivand1, Zaki A. Sherif2, Gholamreza Oskrochi3, Suryanarayana Reddy Challa1, Boubini Jones-Wonni1, Sheldon Rankine1, Chiamaka Ekwunazu1, Abigail Banson1, Rachel Kim1, Chandler Gilliard1, Elizabeth Ekpe1, Nader Shayegh1, Constance Nyaunu1, Chidi Martins1, Ashley Slack1, Princess Okwesili1, Malachi Abebe1, Yashvardhan Batta1, Do Ly1, Valarie Ogwo1, Tori Smith1, Kyra Watson1, Oluwapelumi Kolawole1, Sarine Tahmazian1, Sofiat Atoba1, Myra Khushbakht1, Gregory Riley1, Warren Gavin4, Areeba Kara4, Manuel Hache-Marliere5, Leonidas Palaiodimos5, Vishnu R Mani6, Aleksandr Kalabin7, Vijay Reddy Gayam8, Pavani Reddy Garlapati8, Joseph Miller9, Fatimah Jackson2, John M. Carethers10, Vinod Rustgi11, Hassan Brim2
1Department of Medicine, GI Division, Cancer Center, Howard University Hospital, Washington DC, USA.
2Department of Pathology and Cancer Center, Department of Biochemistry & Molecular
Biology, Howard University College of Medicine, Washington DC, USA
3College of Engineering and Technology, American University of the Middle East, Kuwait
4Division of General Internal Medicine and Geriatrics, Indiana University School of Medicine,
IN, USA
5Department of Medicine, Albert Einstein College of Medicine, Bronx, NY, USA
6Department of Trauma, Acute and Critical Care Surgery, Duke University Medical Center, NC,
USA.
7Department of Surgery, Columbia University College of Physicians and Surgeons at Harlem Hospital, NY, USA
8Department of Medicine, Interfaith Medical Center, NY, USA.
9Departments of Emergency Medicine and Internal Medicine, Henry Ford Hospital, Detroit,
MI, USA.
10Division of Gastroenterology and Hepatology, Department of Medicine; Moores Cancer Center; and the Herbert Wertheim School of Public Health and Longevity Science, University of California San Diego, San Diego, CA, USA
11Division of Gastroenterology and Hepatology, Robert Wood Johnson University Hospital - New Brunswick, NJ
*Corresponding author: Hassan Ashktorab, Department of Medicine, GI Division, Cancer Center, Howard University Hospital, Washington DC, USA.
Received: 30 April 2023; Accepted: 11 May 2023; Published: 16 February 2024
Citation: Hassan Ashktorab, Antonio Pizuorno, Lakshmi Gayathri Chirumamilla, Folake Adeleye, Maryam Mehdipour Dalivand, Zaki A. Sherif, Gholamreza Oskrochi, Suryanarayana Reddy Challa, Boubini Jones-Wonni, Sheldon Rankine, Chiamaka Ekwunazu, Abigail Banson, Rachel Kim, Chandler Gilliard, Elizabeth Ekpe, Nader Shayegh, Constance Nyaunu, Chidi Martins, Ashley Slack, Princess Okwesili, Malachi Abebe, Yashvardhan Batta, Do, Ly, Ogwo Valarie, Tori Smith, Kyra Watson, Oluwapelumi Kolawole, Sarine Tahmazian, Sofiat Atoba, Myra Khushbakht, Gregory Riley, Warren Gavin, Areeba Kara, Manuel Hache-Marliere, Leonidas Palaiodimos, Vishnu R Mani, Aleksandr Kalabin, Vijay Reddy Gayam, Pavani Reddy Garlapati, Joseph Miller, Fatimah Jackson, John M. Carethers, Vinod Rustgi, Hassan Brim. African Americans possessed high prevalence of comorbidities and frequent abdominal symptoms, and comprised a disproportionate share of COVID-19 mortality among 9,873 UShospitalized patients early in the pandemic. Archives of Internal Medicine Research. 7 (2024): 27-41.
View / Download Pdf Share at FacebookAbstract
Background and aim:
Identifying clinical characteristics and outcomes of different ethnicities in the US may inform treatment for hospitalized COVID-19 patients. Aim of this study is to identify predictors of mortality among US races/ethnicities.
Design, Setting and participants:
We retrospectively analyzed de-identified data from 9,873 COVID-19 patients who were hospitalized at 15 US hospital centers in 11 states (March 2020-November 2020). Main Outcomes and Measures: The primary outcome was to identify predictors of mortality in hospitalized COVID-19 patients.
Results:
Among the 9,873 patients, there were 64.1% African Americans (AA), 19.8% Caucasians, 10.4% Hispanics, and 5.7% Asians, with 50.7% female. Males showed higher in-hospital mortality (20.9% vs. 15.3%, p=0.001). Non-survivors were significantly older (67 vs. 61 years) than survivors. Patients in New York had the highest in-hospital mortality (OR=3.54 (3.03 - 4.14)). AA patients possessed higher prevalence of comorbidities, had longer hospital stay, higher ICU admission rates, increased requirement for mechanical ventilation and higher in-hospital mortality compared to other races/ethnicities. Gastrointestinal symptoms (GI), particularly diarrhea, were more common among minority patients. Among GI symptoms and laboratory findings, abdominal pain (5.3%, p=0.03), elevated AST (n=2653, 50.2%, p=<0.001, OR=2.18), bilirubin (n=577, 12.9%, p=0.01) and low albumin levels (n=361, 19.1%, p=0.03) were associated with mortality. Multivariate analysis (adjusted for age, sex, race, geographic location) indicates that patients with asthma, COPD, cardiac disease, hypertension, diabetes mellitus, immunocompromised status, shortness of breath and cough possess higher odds of in-hospital mortality. Among laboratory parameters, patients with lymphocytopenia (OR2=2.50), lymphocytosis (OR2=1.41), and elevations of serum CRP (OR2=4.19), CPK (OR2=1.43), LDH (OR2=2.10), troponin (OR2=2.91), ferritin (OR2=1.88), AST (OR2=2.18), D-dimer (OR2=2.75) are more prone to death. Patients on glucocorticoids (OR2=1.49) and mechanical ventilation (OR2=9.78) have higher in-hospital mortality.
Conclusion:
These findings suggest that older age, male sex, AA race, and hospitalization in New York were associated with higher in-hospital mortality rates from COVID-19 in early pandemic stages. Other predictors of mortality included the presence of comorbidities, shortness of breath, cough elevated serum inflammatory markers, altered lymphocyte count, elevated AST, and low serum albumin. AA patients comprised a disproportionate share of COVID-19 death in the US during 2020 relative to other races/ethnicities.
Keywords
COVID-19; United States; African Americans; Mortality
COVID-19 articles; United States articles; African Americans articles; Mortality articles
Article Details
Abbreviation list:
C-Reactive Protein (CRP); Lactose dehydrogenase (LDH); Creatine Phosphokinase (CPK); Intensive Care Unit (ICU); Aspartate Transaminase (AST); Odds Ratio (OD).
1. Introduction
Coronavirus disease-2019 (COVID-19) was first recognized in December 2019 in China [1]. As of April 28th 2022, SARS CoV-2, the virus has infected more than 509 million people, including 6.2 million deaths [2]. More than 70 million cases have been confirmed in the US, with over 900,000 reported deaths [3]. Patients who acquire COVID-19 can experience a range of clinical manifestations, from no symptoms to severe critical illness to death. Available data suggest that older age groups, those with preexisting comorbid conditions and male sex are common predisposing risk factors for COVID-19 infection [ 4-7,8]. By March 2021, for each 100,000 Americans based on race/ethnicity, 180 African Americans (AA), 150 Caucasians, and 147 Hispanics had died from COVID-19. Nationwide, AA have died at 1.4 times the rate of White Americans [9,10]. To date where race is known, 15% of COVID-19 deaths in the US were African Americans [11]. People from racial/ethnic minority groups in Western countries experience undue socioeconomic and structural determinants of health burdens. These conditions resulted in inequalities and inequities in healthcare access and unsatisfactory health outcomes [8]. The present study aimed to examine hospitalized SARS-CoV-2-positive patients' racial/ethnic background, clinical manifestations including gastrointestinal symptoms, clinical inflammatory biomarkers, comorbidities, and disease severity to ascertain predictors of mortality from COVID-19 across 15 hospital centers in 11 states in US.
2. Patients and Methods
Patient selection: In this retrospective cross-sectional study we collected de-identified information and reviewed demographics, clinical manifestations, laboratory tests and outcomes of COVID-19 patients hospitalized in 15 US hospital representing 11 states between March 2020 and November 2020. We received approval from the Howard University Institutional Review Board (IRB) and individual participating hospitals. As this study includes the de-identified patient data, we were granted an exemption of informed consent from the IRB. An excel file template was shared with our collaborators to standardize the process of data collection and database construction. The total number of patients included in this study was 9,873; however, the total number for each variable in the overall analysis varies due to some missing values. Inclusion and exclusion criteria: The following inclusion criteria were used: confirmed SARS-CoV-2 RT-PCR and hospitalization for COVID-19, without distinction on sex, age, treatment regimen, clinical manifestations, comorbidities, or outcomes. Individuals involved in studies that did not include AA or Hispanic patients, studies from outside of the US, cases that were not confirmed by RT-PCR, cases with incomplete symptoms and comorbidities, and cases with overlapping data were
Statistical analysis: Patient demographics, symptoms, underlying comorbidities, treatment, and outcomes were compared among AA, Caucasian, Hispanic, and other ethnic groups. Predictors of hospital mortality were evaluated by using logistic and/or multiple logistic regression employing four models to assess the effect of each risk factor: OR1: no adjustment; OR2: adjusted for gender, age, ethnicity, and center; OR3: the OR2 model further adjusted for comorbidities; and OR4: the OR3 model further adjusted for disease severity. In each analysis, odds ratios (ORs) and associated 95% confidence intervals were calculated. The 95% confidence interval was investigated to see if it contained unity. Confidence intervals that included one were considered not statistically significant.
3. Results
3.1 COVID-19 hospitalized patient characteristics: There are 9,873 patients from 15 US hospitals across 11 states in the United States (Table 1). The mean age was 60.2 years. Males (n=4511, 49.3%) and females (n=4640, 50.7) distribution was balanced. Of the total cohort there were, 5591 (64.1%) AA, 1729 (19.8%) Caucasian, 903 (10.4%) Hispanic, and 498 (5.7%) Asian. The average hospital stay was 8.9 days, with length of stay significantly higher in non-survivors than in survivors (15.3 vs 6.07 days, p=0.03). A majority of patients were obese (n=3262, 46.5%). Hypertension (n=5101, 60.2%), diabetes mellitus (n=3046, 35.3%), and chronic kidney disease (n=1156, 18.1%) were the most common comorbidities (Table 2). The overall mortality was 18.10% (n=1654).
DC: District of Columbia, GA: Georgia, IN: Indiana, KY: Kentucky, LA: Louisiana, MD: Maryland, MI: Michigan, NJ: New Jersey, NY: New York, RI: Rhode Island, TX: Texas. LOS: Length of Hospital Stay, AA: African Americans, BMI: Body Mass Index, ICU: Intensive care unit.
Table 1: Demographics and Disease severity of COVID-19 across the 11 states in the United States.
|
All patients |
Survivor |
Non-survivor |
p-Values |
|
|
n/N (%) |
n/N (%) |
n/N (%) |
||
|
Characteristics (N: Total no of patients) |
||||
|
Age (N=9873) |
60.2 ± 17.6 |
61 ± 14 |
67 ± 12 |
<0.001 |
|
Length of stay in hospital (days) |
8.9 ± 6.9 |
6.07 ± 8.5 |
15.3 ± 7.5 |
0.02 |
|
Sex (N=9151) |
||||
|
Male |
4511(49.3) |
3569 (79.1) |
942 (20.9) |
<0.001 |
|
Female |
4640 (50.7) |
3931 (84.7) |
709 (15.3) |
|
|
Race (N=8721) |
||||
|
African American |
5591(64.1) |
4524(80.9) |
1069(19.1) |
< 0.009 |
|
Caucasian |
1729(19.8) |
1425(82.4) |
304(17.6) |
|
|
Hispanic |
903(10.4) |
757(83.8) |
146(16.2) |
|
|
Asian |
498(5.7) |
428(85.9) |
70(14.1) |
|
|
BMI (N=7016) |
||||
|
Normal |
1746(24.9) |
1356(77.7) |
210 (22.3) |
<0.001 |
|
Overweight |
2008(28.6) |
1607(801) |
390(20) |
|
|
Obese |
3262(46.5) |
2748(84.2) |
67.5(15.8) |
|
|
Comorbidities |
||||
|
COPD |
715/ 7449(9.6) |
507(70.9) |
208(29.1) |
<0.001 |
|
Chronic Kidney Disease |
1156/6370(18.1) |
852(73.7) |
304(26.3) |
<0.001 |
|
Hypertension |
5101/8472(60.2) |
4041(79.2) |
1060(20.8) |
<0.001 |
|
Diabetes Mellitus |
3046/8616(35.3) |
2345(77) |
701(23) |
<0.001 |
|
Malignancy |
596/6498(9.2) |
438(73.5) |
158(26.5) |
<0.001 |
|
Cardiac Disease |
1218/7732(15.7) |
880(72.2) |
338(27.8) |
<0.001 |
|
Chronic Liver Disease |
622/3812(16.3) |
429(69) |
193(31) |
<0.001 |
|
Asthma |
1250/7742(16.1) |
921(73.7) |
329(26.3) |
<0.001 |
|
Immunocompromised |
288/4481(6.4) |
200(69.4) |
88(30.6) |
0.01 |
|
Smoking |
1291/7725(16.7) |
1014(78.5) |
277(21.5) |
<0.001 |
|
Symptoms |
||||
|
Fever |
3487/8441 (41.3) |
2862(82.1) |
625(17.9) |
<0.001 |
|
Shortness of Breath |
3127/7115 (43.9) |
2524 (80.7) |
603 (19.3) |
<0.001 |
|
Headache |
639/ 6903(9.21) |
480 (75.1) |
159(24.9) |
<0.001 |
|
Cough |
3648/8853 (41.2) |
2911(79.8) |
737(20.2) |
<0.001 |
|
Chest Pain |
552/6555(8.4) |
485(87.9) |
67(12.8) |
0.103 |
|
Fatigue |
976/6433(15.2) |
814(83.4) |
162(16.6) |
0.03 |
|
Altered Mental Status |
201/1105(18.2) |
139(69.1) |
62(30.9) |
<0.001 |
|
Myalgia |
765/6332(12.1) |
640(83.7) |
125(16.3) |
<0.045 |
|
Loss of Smell |
130/5548(2.3) |
127(97.7) |
3(2.3) |
<0.001 |
|
Loss of Taste |
100/2599(3.8) |
97(9.7) |
3(3) |
<0.001 |
|
Abdominal Pain |
375/7030(5.3) |
331(82.3) |
44(17.7) |
0.034 |
|
Diarrhea |
910/7112(12.8) |
744(81.7) |
166(18.3) |
0.314 |
|
Nausea |
746/7334(10.2) |
649(87) |
89(13) |
0.062 |
|
Vomiting |
555/6921(8) |
461(83.1) |
94(16.9) |
0.911 |
|
Laboratory Parameters |
||||
|
Sodium (Low) |
1251/3210(39) |
1051(84) |
200(16) |
<0.001 |
|
Potassium (Low) |
594/3024(18.5) |
517(87.1) |
77(12.9) |
<0.001 |
|
Chloride (Low) |
886/3209(27.6) |
730(82.4) |
92(17.6) |
<0.001 |
|
WBC |
||||
|
Low |
716/2756(26) |
568(79.3) |
101(20.7) |
0.001 |
|
High |
321/2756(11.6) |
220(68.5) |
148(31.5) |
0.001 |
|
Lymphocyte count |
||||
|
Low |
3029/6063(50) |
2454(81) |
575(19) |
0.001 |
|
High |
650/6063(10.7) |
460(70.8) |
190(29.2) |
0.001 |
|
Hemoglobin (Low) |
1968/3516(56) |
1635(83.1) |
333(16.9) |
0.59 |
|
BUN (elevated) |
1566/3446(45.4) |
1138(72.7) |
428(27.3) |
<0.001 |
|
Creatinine (elevated) |
2616/6576(40.6) |
1605(61.3) |
765(38.7) |
<0.001 |
|
Blood Glucose |
||||
|
Low |
10/3127(0.3) |
5(50) |
5(50) |
0.001 |
|
High |
1086/3127(34.7) |
810(74.6) |
276(25.4) |
<0.001 |
|
ProBNP (elevated) |
363/804(45.1) |
257(70.8) |
106(29.2) |
0.01 |
|
CPK (elevated) |
1091/2305(47.3) |
870(79.7) |
221(20.3) |
0.003 |
|
Troponin (elevated) |
1558/5513(28.3%) |
1027(65.9) |
531(34.1) |
0.001 |
|
Ferritin (elevated) |
3126/4717(67) |
2497(79.9) |
665(20.1) |
<0.001 |
|
ALT (elevated) |
595/4204(14.2) |
464(78) |
131(28) |
0.051 |
|
AST (elevated) |
2653/5227(50.8) |
1995(75.2) |
658(24.8) |
<0.001 |
|
ALP (elevated) |
111/987(11.2) |
87(78.4) |
24(21.6) |
0.18 |
|
Albumin (low) |
361/2040(17.7) |
216(59.8) |
145(40.2) |
0.03 |
|
Total Bilirubin (elevated) |
439/3807(11.5) |
321(73.1) |
118(26.9) |
<0.001 |
|
Procalcitonin (elevated) |
1535/3558(43.1) |
1027(66.9) |
508(33.1) |
<0.001 |
|
LDH (elevated) |
2176/2741(79.4) |
1595(73.3) |
581(26.7) |
<0.001 |
|
CRP (elevated) |
4625/5095(90.8) |
3626(78.4) |
999(21.6) |
<0.001 |
|
D-dimer (elevated) |
3584/4426(81) |
2828(78.9) |
756(21.1) |
<0.001 |
|
Diagnostic Imaging |
||||
|
Chest X-ray (Abnormal) |
2356/2752(85.6) |
1787(75.8) |
569(24.1) |
<0.001 |
|
Splenomegaly on CT-Chest |
142/450(31.6) |
96(67.6) |
46(32.4) |
<0.01 |
Table 2: Baseline demographics and clinical characteristics of Covid-19 hospitalized patients.
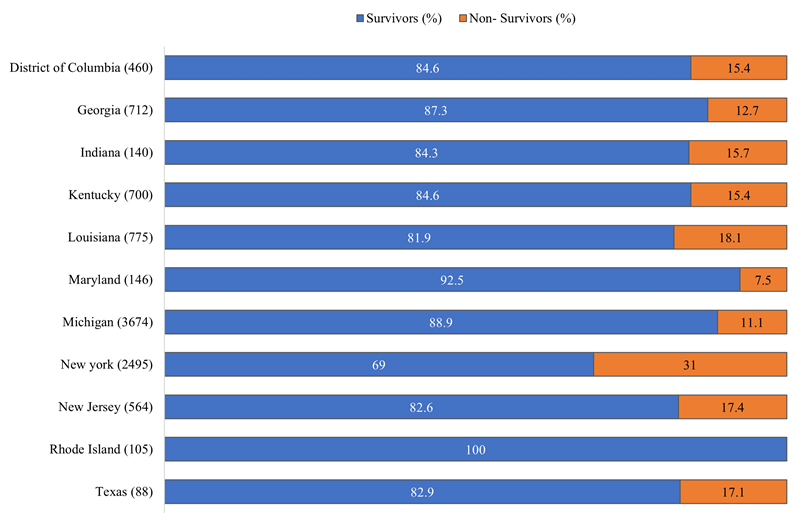
Our study includes patients from the North and Midwest, East and South geographical regions of the US (Table 1). Patients above 65 years were more likely to die from COVID-19 compared to <65 years. A large proportion of patients were from Michigan (n=3,674, 37.2%) and New York (n=2,505, 25.4%) (Table 1). Michigan had the highest number of African Americans, Caucasians, and Asians in this cohort. In comparison, New York had the highest number of Hispanic patients. The mean length of hospital stay was higher among COVID-19 non-survivors in Michigan (15.6 days) (Table 1). More patients in Louisiana and Texas were obese (Table 1). There were higher rates of ICU admission in Maryland (n= 60, 42%) and New York (n=663, 41.3%). COVID-19 patients requiring mechanical ventilation were more prevalent in Maryland (n=49, 33.7%). Mortality was significantly higher in New York (n=774, 31%, p= 0.001) (Table 1, eFigure 1).
3.2 Race/ethnicity specific patient demographics, clinical characteristics, and outcomes: There were significant differences in age and sex distribution across racial/ethnic groups (Table 3). The mean age, in years, was 60.7 for AA, 62.1 for Caucasians, 53.9 for Hispanics, and 56.8 for Asians. Most obese patients were AA. Hypertension (AA: 67.7%, Caucasian:50.1%, Hispanic: 46.9% and Asian: 45.7%) and diabetes mellitus (AA: 29.3%, Caucasian: 26.5%, Hispanic: 35.7%, and Asian: 31%) are the most common comorbidities (Table 3). Shortness of breath (45.4%) followed by cough (42.8%) and fever (42.5%) were the most common presenting symptoms for all participants in the cohort. CRP, LDH, ferritin and D-dimer were the most elevated inflammatory markers for all races/ethnicities (Table 4). African Americans had higher ICU admission rates (AA: 39.8%, Caucasian: 22.9%, Hispanic: 15.1% and Asian: 26.2%). Similarly, AAs had highest rates of mechanical ventilation (17.1%). The mortality rate was significantly higher in the AA (19.1%) compared to Caucasians (17.6%), Hispanics (16.2%), and Asians (14.1%) (Table 4).
|
African American |
Caucasian |
Latin American |
Asian |
|
|
Mean Age (N=9429) |
60.7 (5816) |
62.1 (2112) |
53.9 (905) |
56.80 (596) |
|
Length of hospital stay (mean, days) |
7.86 |
6.46 |
7.8 |
6.05 |
|
n (%) |
n (%) |
n (%) |
n (%) |
|
|
Sex (n=9421) |
||||
|
Female (n=4318) |
3061 (52.7) |
1083 (51.3) |
403 (44.6) |
271 (45.5) |
|
Male (n=4603) |
2749 (47.3) |
1029 (48.7) |
500 (55.5) |
325 (54.5) |
|
BMI |
||||
|
Normal (N=2017) |
1022 (23.6) |
644 (35.6) |
190 (24.8) |
161 (36.2) |
|
Overweight (N=2059) |
1151 (26.6) |
491(27.1) |
264(34.4) |
153(34.??) |
|
Obese (N=3270) |
2152(49.8) |
674(37.3) |
313(40.8) |
131(29.4) |
|
Co-Morbidities |
||||
|
COPD (N=7725) |
||||
|
Yes (793) |
447(10) |
273(13.7) |
54(6.8) |
20(4.1) |
|
No (6932) |
4011(90) |
1713(86.3) |
739(93.2) |
469(95.8) |
|
CKD (N=6060) |
||||
|
Yes (1113) |
764(21.4) |
227(15) |
88(13.9) |
34(11.2) |
|
No (4932) |
2840(78.6) |
1279(84.5) |
544(86.1) |
269(88.8) |
|
Cardiac Disease (N=8026) |
||||
|
Yes (1323) |
821(17.2) |
339(17) |
121(15.8) |
42(8.8) |
|
No (6703) |
3963(82.8) |
1658(83) |
644(84.2) |
438(91.3) |
|
Hypertension (N=8768) |
||||
|
Yes (5266) |
3567(67.7) |
1037(50.1) |
402(46.9) |
260(45.7) |
|
No (3502) |
1705(32.3) |
1032(49.9) |
456(53.1) |
309(54.3) |
|
Diabetes Mellitus N=8911) |
||||
|
Yes (3155) |
2110(29.3) |
555(26.5) |
311(35.7) |
179(31) |
|
No (5756) |
3257(60.7) |
1541(73.5) |
560(64.3) |
398(69) |
|
Asthma (N=8027) |
||||
|
Yes (1273) |
932(19.2) |
175(8.7) |
113(19.3) |
53(9.3) |
|
No (6754) |
3919(80.8) |
1844(91.3) |
473(80.7) |
518(90.7) |
|
Malignancy (N=6800) |
||||
|
Yes (611) |
343(9) |
202(10.4) |
49(8.2) |
17(3.8) |
|
No (6189) |
3467(91) |
1741 (89.6) |
546(91.8) |
435 (96.2) |
|
Chronic Liver Disease (N=4318) |
||||
|
Yes (598) |
322(12.8) |
80(10.1) |
180(22.9) |
16(7.2) |
|
No (3720) |
2191(87.2) |
716(89.9) |
606(77.1) |
207(92.8) |
|
Immunocompromised (N=5056) |
||||
|
Yes (310) |
210(6.2) |
49(5.9) |
43(7.3) |
8(3) |
|
No (4746) |
3153(93.8) |
785(94.1) |
548(92.7) |
260(97) |
Table 3: Demographics and comorbidities of covid-19 patients based on individual races
|
African American (n %) |
Caucasian (n %) |
Latin American (n %) |
Asian (n %) |
|
|
Clinical Manifestations |
||||
|
Fever (N=8737) |
||||
|
Yes (3721) |
2211(42.1) |
648 (31.5) |
601(69) |
261(47.1) |
|
No (5016) |
3041 (57.9) |
1412 (68.5) |
270 (31) |
293 (52.9) |
|
Shortness of Breath (N=7501) |
||||
|
Yes (3422) |
2129 (46.7) |
783 (40) |
262 (56.5) |
248 (47.7) |
|
No (4079) |
2431 (53.3) |
1174 (60) |
202 (43.5) |
272 (52.3) |
|
Cough (N = 9126) |
||||
|
Yes (3921) |
2422(43) |
673(32.2) |
580(68.8) |
246 (42.9) |
|
No (5205) |
3217(57) |
1417(67.8) |
243(29.5) |
328 (57.1) |
|
Headache (N=7268) |
||||
|
Yes (704) |
497(11) |
76 (4) |
104(27.7) |
27(5.4) |
|
No (6564) |
4007 (89) |
1816 (96) |
271 (72.3) |
470 (94.6) |
|
Chest Pain (N=6870) |
||||
|
Yes (600) |
311(8) |
132(6.7) |
130(22.5) |
27(5.9) |
|
No (6270) |
3562 (92) |
1827 (93.3) |
447 (77.5) |
434 (94.1) |
|
Rhinorrhea (N=5594) |
||||
|
Yes (394) |
281(8.7) |
38(2.2) |
68(28.7) |
7(1.7) |
|
No (5200) |
2956 (91.3) |
1676 (97.8) |
169 (71.3) |
399 (98.3) |
|
Fatigue (N=6821) |
||||
|
Yes (1165) |
707 (17.1) |
282 (15) |
90(28.1) |
86(18.1) |
|
No (5656) |
3438(82.9) |
1598(85) |
230(71.9) |
390(81.9) |
|
Altered Mental Status (N=1804) |
||||
|
Yes (280) |
183(16.7) |
77(13.8) |
- |
20(13.6) |
|
No (1524) |
910(83.3) |
480(86.2) |
- |
127(86.4) |
|
Myalgia (N=6807) |
||||
|
Yes (921) |
580(13.8) |
147(7.9) |
129(44) |
65(14.9) |
|
No (5886) |
3631(86.2) |
1720(92.1) |
164(56) |
371(85.1) |
|
Loss of Smell/ Anosmia (N=6807) |
||||
|
Yes (141) |
42(1.3) |
17(0.9) |
76(21.1) |
6(1.4) |
|
No (5800) |
3289(98.7) |
1789(99.1) |
284(78.9) |
438(98.6) |
|
Loss of taste/ Ageusia (N=3197) |
||||
|
Yes (113) |
69(3.5) |
17(2.6) |
16(4.9) |
11(5.1) |
|
No (3084) |
1929(96.5) |
638(97.4) |
312(95.1) |
205(94.9) |
|
Any GI symptoms (N=7421) |
||||
|
Yes (1632) |
688 (14.5) |
120 (6) |
182 (52.6) |
21 (5) |
|
No (5789) |
4380 (85.5) |
1540 (94) |
227 (47.4) |
344 (95) |
|
Abdominal Pain (N=6643) |
||||
|
Yes (342) |
220 (5.3) |
33 (2.2) |
61(10) |
28(7.2) |
|
No (6301) |
3931(94.7) |
1458(97.8) |
552(90) |
360(92.8) |
|
Nausea (N=7652) |
||||
|
Yes (856) |
545(11.7) |
154(7.8) |
102(19) |
55(11.2) |
|
No (6796) |
4106(88.3) |
1818(92.2) |
436(81) |
436(88.8) |
|
Vomiting (N=7321) |
||||
|
Yes (628) |
428(9.2) |
92(5) |
68(16.3) |
40(9.4) |
|
No (6693) |
4200(90.8) |
1756(95) |
350(83.7) |
387(90.6) |
|
Diarrhea (N=7421) |
||||
|
Yes (998) |
570 (13.2) |
173 (9.2) |
190(25.7) |
65(13.5) |
|
No (6423) |
3741(86.8) |
1715(90.8) |
549(74.3) |
418(86.5) |
|
GI Bleeding (N=4966) |
||||
|
Yes (16) |
12 (0.4) |
4 (0.3) |
- |
- |
|
No (4950) |
3216(99.6) |
1326(99.7) |
103(100) |
305(100) |
|
Laboratory Parameters |
||||
|
WBC (N=2556) |
||||
|
Elevated (288) |
154(10.6) |
37(10.6) |
63(10.6) |
34(21.5) |
|
Low (646) |
315(21.7) |
90(25.7) |
175(29.5) |
66(41.8) |
|
Lymphocyte Count (N=5802) |
||||
|
Elevated (602) |
328(8.8) |
78(6.3) |
173(30.6) |
23(8.3) |
|
Low (2922) |
1854(49.8) |
753(61) |
176(31.2) |
139(50.4) |
|
Platelets (N=1204) |
||||
|
Elevated (41) |
23(3.4) |
2(1) |
15(6.4) |
1(1.1) |
|
Low (355) |
251(37.3) |
44(21.9) |
41(17.4) |
19(20.2) |
|
CRP (N=4864) |
||||
|
Elevated (4411) |
2754(89.7) |
952(93.9) |
422(89.2) |
283(91.9) |
|
Troponin (N= 5199) |
||||
|
Elevated (1477) |
953 (30.2) |
351 (31) |
87 (14.7) |
86 (26.5) |
|
Pro BNP (N=780) |
||||
|
Elevated (344) |
226 (41.1) |
50(46.3) |
41(67.2) |
27(45) |
|
Procalcitonin (N=3410) |
||||
|
Elevated (1485) |
983 (46.6) |
278(35.6) |
126(39.1) |
98(49.2) |
|
CPK (N=2174) |
||||
|
Elevated (1034) |
701 (56.8) |
252 (35.1) |
24 (32) |
52 (35.4) |
|
Glucose (N=2970) |
||||
|
Elevated (1041) |
676 (35.7) |
268 (31.1) |
16 (64) |
81 (43.1) |
|
Low (9) |
9(0.5) |
- |
- |
- |
|
LDH (N=2615) |
||||
|
Elevated (1866) |
1219 (72.1) |
224 (67.5) |
297 (68.9) |
126 (78.3) |
|
D-Dimer (N=4209) |
||||
|
Elevated (3401) |
2068(81) |
771(81.5) |
341(81.8) |
221(75.2) |
|
Ferritin (N=4516) |
||||
|
Elevated (3022) |
1994(68.6) |
601(60.4) |
218(70.6) |
209(68.1) |
|
BUN (N=3288) |
||||
|
Elevated (1590) |
991(47.3) |
461(52.2) |
68(65.5) |
79(38.9) |
|
Creatinine (N=6232) |
||||
|
Elevated (1979) |
1329(34) |
404(31.2) |
164(23.7) |
82(24.6) |
|
Albumin (N=1942) |
||||
|
Elevated (368) |
227(23) |
32(6.8) |
66(22.9) |
43(21.8) |
|
Low (361) |
160(16.2) |
131(28) |
47(16.3) |
23(11.7) |
|
AST (N=5680) |
||||
|
Elevated (2814) |
1737(50.9) |
688(47.5) |
174(44.7) |
215(49.9) |
|
ALT (N=4652) |
||||
|
Elevated (737) |
343(13.1) |
197(14.6) |
108(37.1) |
90(21.8) |
|
ALP (N=896) |
||||
|
Elevated (100) |
41(8.5) |
7(7) |
46(20) |
6(7.4) |
|
Total Bilirubin (N=4250) |
||||
|
Elevated (552) |
273 (11.9) |
214 (17.6) |
25(6.4) |
40 (11.8) |
|
Hemoglobin (N=3352) |
||||
|
High (53) |
32(1.5) |
8(0.8) |
12(11.3) |
1(0.5) |
|
Low (1875) |
1182(56.7) |
544(56.1) |
43(40.6) |
106(55.2) |
|
Disease Severity |
||||
|
ICU Admission (N=3544) |
||||
|
Yes (1129) |
877(39.8) |
76(22.9) |
121(15.1) |
55(26.2) |
|
No (2415) |
1324(60.2) |
256(77.1) |
680(84.9) |
155 (73.8) |
|
Intubated in ER (N=7549) |
||||
|
Yes (1024) |
751(15.2) |
116(7.1) |
106(19.1) |
51(12.5) |
|
No (6525) |
4197 (84.8) |
1523 (92.9) |
449 (80.9) |
356 (87.5) |
|
Mechanical Ventilation (N=6210) |
||||
|
Yes (925) |
683(17.1) |
146 (10.5) |
45 (9.5) |
51 (14.4) |
|
No (5285) |
3318 (82.9) |
1238 (89.5) |
427 (90.5) |
302 (85.6) |
|
Outcome (N=8721) |
||||
|
Alive (7134) |
4524 (80.9) |
1425 (82.4) |
757 (83.8) |
428 (85.9) |
|
Death (1587) |
1067 (19.1) |
304(17.6) |
146(16.2) |
70(14.1) |
Table 4: Clinical manifestations, laboratory parameters, and disease severity of COVID-19 patients among different race/ethnicities.
3.3 Gastrointestinal manifestations were most common among hospitalized Hispanics and minorities: Diarrhea (12.8%) was the most common gastrointestinal (GI) symptom (Table 2). GI symptoms were most seen among Hispanics (44.5%, n=182) followed by AAs (14.5%, n=688), when compared to Caucasians (6%, n=120), and Asians (5%, n=21) (Table 4). Symptoms of abdominal pain (5.3%, p=0.03), anosmia (n=130, 2.3%, p=0.001)), and ageusia (n=100, 3.8%, p=0.001), as well as the presence of chronic liver disease (n=622, 16.3%, p=0.001), splenomegaly (n=142, 31.6%, p=0.01), elevated AST (n= 2653, 50.2%, p=<0.001, OR=2.18), bilirubin (n=577, 12.9%, p=0.01) and low albumin (n=361, 19.1%, p=0.03) were significantly associated with death (Table 2-5).
3.4 Predictors of mortality on multivariable modeling for the study cohort: Age was a significant predictor of mortality from COVID-19 (Table 5). Compared to individuals < 35 years of age, those who were >= 75 years were at substantially higher risk of death with an OR1 (95% CI) of 9.24 (6.24-15.0). This association remained strong after adjustment for comorbidities, with OR3 (95% CI) of 8.43 (4.98-10.84). Other demographic predictors of mortality were male sex (p= 0.001, OR=1.46, 1.31-1.62) and AA race (p=0.009). New York state had significantly higher mortality even after adjusting for demographics (p=<0.001, OR2: 3.51) (Fig 1). Michigan, Indiana, Rhode Island, and Maryland had significantly lower death rates (Table 5 and Supplemental Figure 1).
|
Predictors of Mortality |
Dead (N%) |
OR 1 (95% CI) |
OR 2 (95% CI) |
OR 3 (95% CI) |
|
Age (n = 9145) |
||||
|
< 35 (n = 875) |
27 (3.1) |
- |
- |
- |
|
35 to 44 (n = 931) |
58 (6.2) |
2.09 (1.61 - 2.82) |
1.38 (1.04 - 3.22) |
0.89 (0.56 - 1.67) |
|
45 to 54 (n = 1428) |
117 (8.2) |
2.81 (1,21 - 3.36) |
1.64 (1.68 - 2.36) |
1.09 (0.78 - 1.88) |
|
55 to 64 (n = 2001) |
266 (13.3) |
4.83 (2.94 - 5.02) |
2.84 (1.94 - 3.79) |
1.64 (1.12 - 2.08) |
|
65 to 74 (n = 1867) |
438 (23.5) |
9.67 (6.35 - 12.90) |
5.03 (3.15 - 8.03) |
2.13 (1.94 - 4.09) |
|
>= 75 (n = 2039) |
745 (36.5) |
18.16 (11.78 - 24.64) |
9.24 (6.40 - 15.0) |
5.16 (4.68 - 10.16) |
|
Sex (n = 9151) |
||||
|
Female (n = 4640) |
709 (42.9) |
- |
- |
- |
|
Male (n = 4511) |
942 (57.1) |
1.46 (1.31 - 1.62) |
1.51 (1.34 - 1.70) |
1.54 (1.27 - 1.86) |
|
Admitting Center (n = 9159) |
||||
|
New York (n =2495) |
774 (43.9) |
3.61 (3.15 - 4.12) |
3.51 (3.00 - 4.09) |
3.54 (3.03 - 4.14) |
|
Indiana (n = 140) |
22 (1.2) |
1.49 (0.93 - 2.38) |
1.48 (0.91 - 2.43) |
1.40 (0.85 - 2.28) |
|
Rhode Island (n = 105) |
0 (0) |
0.00 (0.00 - 0.00) |
0.00 (0.00 - 0.00) |
0.00 (0.00 - 0.00) |
|
Maryland (n = 146) |
11 (8.3) |
0.65 (0.35 - 1.22) |
0.74 (0.39 - 1.42) |
0.79 (0.41 - 1.54) |
|
New Jersey (n = 564) |
98 (5.7) |
1.68 (1.32 - 2.14) |
1.60 (1.26 - 2.12) |
1.59 (1.19 - 2.11) |
|
DC (n = 460) |
71 (4) |
1.53 (1.15 - 2.04) |
1.72 (1.28 - 2.12) |
- |
|
Georgia (n = 712) |
116 (6.6) |
1.56 (1.24 - 1.95) |
1.55 (1.22 - 1.98) |
- |
|
Kentucky (n = 700) |
108 (6.1) |
1.62 (1.21 - 2.01) |
1.58 (1.24 - 2.08) |
- |
|
Louisiana (n= 775) |
140 (7.9) |
1.77 (1.43 - 2.18) |
2.99 (1.56 - 3.65) |
- |
|
Texas (n= 88) |
15 (0.8) |
0.82 (0.56 - 1.91) |
1.77 (1.41 - 2.22) |
- |
|
Michigan (n=3674) |
407(23.1) |
- |
- |
- |
|
Ethnicity (n = 8721) |
||||
|
African American (n = 5591) |
1067 (67.2) |
- |
- |
- |
|
Caucasians (n = 1729) |
304 (19.2) |
1.12 (0.95 - 1.33) |
1.10 (0.94 - 1.64) |
1.21 (0.95 - 1.31) |
|
Hispanics (n = 903) |
146 (9.2) |
0.45 (0.29 - 0.71) |
0.75 (0.60 - 0.94) |
0.73 (0.58 - 0.92) |
|
Asian (n = 498) |
70 (4.4) |
0.12 (0.3 - 0.51) |
0.86 (0.65 - 1.15) |
0.89 (0.66 - 1.18) |
|
BMI (n = 7016) |
||||
|
Normal (n = 1746) |
210 (16.1) |
- |
- |
- |
|
Overweight (n = 2008) |
310 (29.9) |
1.40 (0.74 - 2.01) |
1.04 (0.87 - 1.23) |
1.03 (0.86 – 1.23) |
|
Obese (n = 3262) |
675 (51.7) |
2.65 (1.21 - 3.75) |
1.14 (0.96 - 1.34) |
1.11 (0.93 – 1.32) |
|
Asthma |
329 (26.3) |
1.86 (1.61 - 2.13) |
1.42 (1.19 - 1.69) |
|
|
COPD |
208 (29.1) |
2.09 (1.87 - 2.56) |
1.45 (1.19 - 1.76) |
|
|
Cardiac Disease |
338 (27.8) |
2.29 (1.99 - 2.65) |
1.32 (1.12 - 1.55) |
|
|
Hypertension |
1060 (2.8) |
2.31 (2.03 - 2.63) |
1.58 (1.39 - 1.43) |
|
|
Diabetes Mellitus |
701 (23) |
2.04 (1.81 - 2.28) |
1.20 (1.03 - 1.39) |
|
|
Malignancy |
158 (26.5) |
2.15 (1.77 - 2.61) |
1.50 (0.92 - 1.79) |
|
|
Immunocompromised |
88 (30.6) |
1.40 (1.08 - 1.82) |
1.46 (1.10 - 1.93) |
|
|
Fever |
625 (17.9) |
1.26 (1.12 -1.42) |
0.95 (0.82 - 1.10) |
|
|
Headache |
603 (19.3) |
1.75 (1.44 - 2.11) |
0.96 (0.75 - 1.23) |
|
|
Cough |
159 (24.9) |
1.96 (1.71 - 2.23) |
1.21 (0.87 - 1.16) |
|
|
Shortness of Breath |
737 (20.2) |
2.03 (1.52 - 2.69) |
1.73 (1.48 - 2.02) |
1.63 (1.33 - 1.99) |
|
Chest Pain |
67 (12.8) |
1.42 (1.10 - 2.13) |
0.73 (0.53 - 0.99) |
|
|
Fatigue |
162 (16.6) |
1.22 (1.01 - 1.47) |
0.59 (0.47 - 1.01) |
|
|
Lymphocyte Count |
||||
|
High |
190 (17.2) |
1.41 (1.22 - 1.64) |
1.21 (1.07 - 1.54) |
|
|
Low |
575 (52.1) |
2.50 (2.03 - 3.06) |
1.55 (1.30 - 1.86) |
|
|
Elevated CPK |
221 (54.8) |
1.43 (1.15 - 1.77) |
1.54 (1.20 - 1.98) |
|
|
Elevated CRP |
999 (97.2) |
4.19 (2.85 - 6.14) |
5.15 (3.38 - 7.84) |
4.59 (2.55 - 8.27) |
|
Elevated Troponin |
531 (47.1) |
2.91 (2.5 - 3.34) |
3.01 (2.58 - 3.53) |
|
|
Elevated Ferritin |
665 (74.1) |
1.88 (1.57 -2.25) |
1.90 (1.56 - 2.32) |
|
|
Elevated LDH |
451 (69.3) |
2.10 (1.60 - 2.76) |
2.69 (1.94 - 3.53) |
|
|
Elevated AST |
658 (66.1) |
2.18 (1.88 - 2.52) |
2.24 (1.90 -2.63) |
|
|
Elevated D-Dimer |
756 (92.3) |
2.75 (1.97 - 3.61) |
2.26 (1.68 - 3.03) |
2.76 (1.91 - 3.99) |
|
Glucocorticoid Treatment |
513 (51.6) |
1.49 (1.27 - 1.69) |
1.66 (1.40 - 1.97) |
|
|
Mechanical Ventilation |
524 (48.2) |
10.67 (9.15 - 12.45) |
9.78 (8.19 - 11.68) |
Table 5: Significant Predictors of mortality in hospitalized COVID-19 patients.
Several comorbidities were associated with increased risk of death, including presence of asthma (p=0.001, OR2=1.42), COPD (p=<0.001, OR2=1.45), diabetes mellitus (p=0.001, OR2=1.58), hypertension (p=0.001, OR2=1.20), cardiac disease (p=0.01, OR2=1.32), malignancy (p=<0.001, OR2= 1.50) and immunosuppression (p=0.01, OR2=1.46). Patients with BMI >30 was more than twice as likely to die from COVID-19 than normal BMI patients (OR1: 2.65), this association was no longer significant after adjusting for age, sex, and race.
Fever, (OR1=1.26), shortness of breath (OR1=2.03), headache (OR1=1.75), cough (OR1=1.96), chest pain (OR1=1.42), and fatigue (OR1=1.22) were significant predictors of mortality in univariate analysis (OR1). However, after adjusting for age, sex, and race, only shortness of breath (OR2=1.73) and cough (OR2=1.21) were significantly associated to death. After adjusting for demographics (OR2) and comorbidities (OR3), only shortness of breath was a significant predictor of death, with OR3 (95% CI) of 1.91 (1.61-2.28). Several laboratory markers were associated with increased risk of death, including lymphocytopenia (OR2=1.55, 1.30-1.86), lymphocytosis (OR2=1.21, 1.07-1.54), elevated CRP (OR=5.15, 3.38-7.84), CPK (OR2=1.54,1.20-1.98), troponin (OR2=3.00, 2.54-3.53), ferritin (OR2=2.18, 1.41-3.40), LDH (OR2=2.69, 1.94-3.53), D-dimer (OR2=2.26, 1.68-3.03), and elevated AST (OR2=2.63, 2.13-3.24) in univariate analysis (OR1) and after adjusting for the demographics (OR2) (Table 5). However, after adjusting for demographics and comorbidities, only CRP (OR3=4.59) and D-dimer (OR3=2.76) remained significant.
3.5 Treatment for hospitalized COVID-19 patients:
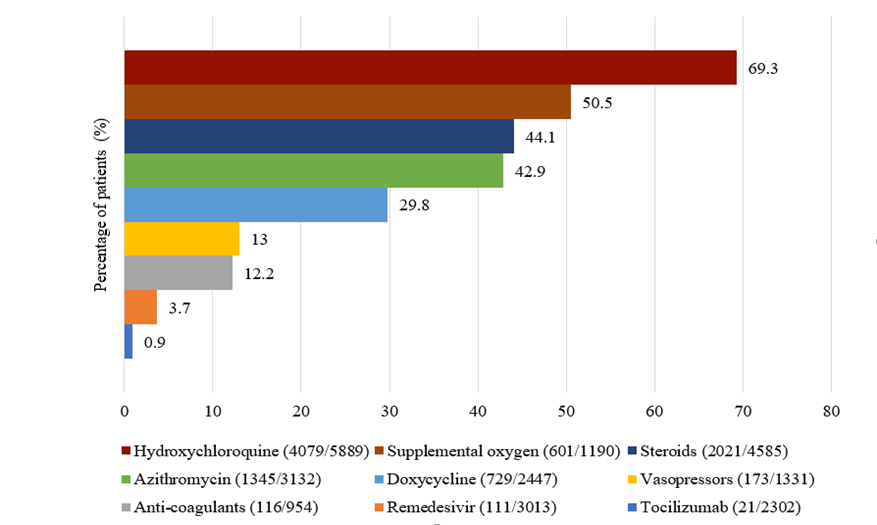
Hydroxychloroquine (69.3%) followed by supplemental oxygen (50.5%), and glucocorticoids (44.1%) were the most used treatments (Supplemental Figure 2). Patients taking hydroxychloroquine (n=4079, 69.3%, p=0.001), Tocilizumab (n=21, 0.9%, p=<0.001), and steroids (n=2021, 44.1%, p=0.001) displayed significant high in-hospital mortality. Glucocorticoid use was associated with an OR2 of 1.66 (1.40-1.97) for mortality after adjusting for age/gender/race/center. In patients receiving mechanical ventilation, the OR2 for death was 9.78 (8.19-11.68) (Table 5).
4. Discussion
Our study comprehensively analyzed demographics, clinical manifestations, and outcomes of COVID-19 patients hospitalized at 15 hospitals across the US. The SARS-CoV-2 positive rate was exceptionally high at the pandemic's start in New York State (NY), which emerged as a national epicenter for the disease in March 2020. New York had the highest odds to have in-hospital death than other states in the US. In contrast, hospital mortality was very low in Rhode Island, Michigan, and Maryland. Gastrointestinal symptoms were significantly more common among AA patients with diarrhea being the most prevalent symptom. Abdominal pain, anosmia, ageusia, and factors indicating hepatic dysfunction including the presence of chronic liver disease, elevated AST, bilirubin, and low albumin levels were significantly associated with death. Our findings are consistent with prior observations indicating older age and male sex were associated with the risk of death [12-15,8]. Gender differences in adaptive and innate immune systems as well as hormonal differences have been previously reported and may account for the female benefit in COVID-19. Females have a considerably higher number of CD4+ cells, more potent CD8+, increased B-cell immunoglobulin production, and more type 1 interferon (IFN), a potent anti-viral cytokine 14. More importantly, these gender and age differences in mortality could be due to a higher level of expression of Transmembrane Protease Serine 2 (TMPRSS2), and Angiotensin-Converting Enzyme 2 (ACE2) receptors which facilitate viral entry in to the host cells [16,8]. Our cohort consisted of 64.1% AA, 19.8% Caucasian, 10.4% Hispanic, and 5.7% Asian patients, with an overall mortality of 18.1%. AA showed the highest mortality compared to other races/ethnicities. However, after adjustment for age, sex, geographic location and comorbidities, there was no significant race-based difference in mortality, similar to the findings from Yehia et al [17]. We noticed higher prevalence rates of comorbid conditions such as diabetes mellitus, hypertension, chronic kidney disease, and obesity among AA patients, similar to prior studies [18,19]. AAs had higher ICU admission rates and required mechanical ventilation significantly more than other races/ethnicities [20]. The conjunction of social, economic, and biologic factors, concurrently with a higher prevalence of comorbidities resulted in a greater COVID-19 burden and worse outcomes among minority populations. The excessive burden of COVID-19 among Hispanics and AAs also may be partially explained by their overrepresentation as an essential workers, resulting in higher exposures [21,22]. Pre-existing conditions of asthma, COPD, cardiac disease, hypertension, diabetes, and malignancy were significantly associated with death in our study. Supporting our findings, Choi et al. identified that COVID-19 mortality rates of patients with diabetes, COPD, immunosuppression, hypertension, CKD, and cardiovascular diseases were approximately 2.5-4 times higher than those without underlying conditions [23]. Obesity has been described as a negative factor for COVID-19 patient outcome, primarily because of obesity-associated pro-inflammation, excessive oxidative stress, impaired immunity, and a trigger/stimulus of metabolic syndrome [24]. Our cohort was 45.6% obese, 28.6% overweight, and 24.9% with normal BMI. Obese patients were significantly more prone to death. However, this trend was not significant after age/sex/race adjustments. Shortness of breath was significantly associated with death after adjusting for demographics and comorbidities similar to previous studies [25]. This finding suggests that shortness of breath, should be given special attention in managing hospitalized COVID-19 patients. The ACE2 receptor, the target of the SARS-CoV-2 virus SPIKE protein, and TMPRSS2 that is required for its cleavage and entry, are expressed in the gastrointestinal track, suggesting that GI symptoms could be the consequences of a direct virus effect. Diarrhea was the most common presenting symptom consistent with previous studies [26]. Evidence of liver dysfunction such as low albumin, elevated AST and elevated total bilirubin was significantly associated with death. Other studies have reported similar results [27-29]. Our study additionally showed that COVID-19 patients with splenomegaly were more prone to death. The increase in spleen size is correlated with COVID-19 disease severity score calculated on the chest CT data in a study conducted by Tahtabasi et al. [30]. This pathological change is due to microthrombus-related end organ (kidneys, heart, spleen and central nervous system) damage mainly caused by impairment of coagulation mechanisms and immune response by the SARS-CoV-2 virus infection [30]. Alternatively, pre-existing splenomegaly may indicate prior liver compromise and portal hypertension. In our study, only a limited number of patient data regarding splenomegaly was available. Further studies related to splenomegaly as a predictor of mortality for COVID-19 may be required. Our study attempted to assess the association of specific laboratory biomarkers (CRP, LDH, ferritin and D-dimer) with outcome from COVID-19. Consistent with prior studies, serum inflammatory markers such as elevated D-dimer, LDH, CRP, ferritin, troponin were significantly associated with death [31,32]. Thus, the continuous search for markers associated with the course of the disease can aid a better assessment of the severity and management of the disease. Such attempts may help clinical decision-making. Thousands of patients have received hydroxychloroquine outside of clinical trials without evidence of its beneficial effects. A collaborative meta-analysis of 28 published and unpublished RCTs, that included 10319 patients by Axfors et al., showed that treatment with hydroxychloroquine was associated with increased mortality in COVID-19 patients, and there was no benefit from treatment 33. Similarly, treatment with hydroxychloroquine was associated with increased mortality in our COVID-19 cohort. Our study identified use of steroids for hospitalized COVID-19 patients was an independent predictor of death after adjusting for demographics and baseline comorbidities. However, in a prospective meta-analysis of clinical trials of critically ill patients with COVID-19, administration of systemic corticosteroids, compared with usual care or placebo, was associated with lower 28-day all-cause mortality [34]. In contrast, a metanalysis including 32 studies that included 14659 COVID-19 patients showed no significant decrease in all-cause mortality in critically ill COVID-19 patients treated with corticosteroids [35]. Our data reflects precautionary measures at the beginning of the pandemic when specific protocols including timing of glucocorticoids were not yet developed. Multivariate logistic regression models showed that patients on mechanical ventilation were nine times more prone to death. Mortality of patients with COVID-19 who required invasive mechanical ventilation was reported to be significantly high in many previous studies [36,37].
One of the main strengths of the study was our ability to collect comprehensive patient data from admission to the primary endpoints: discharge or death. Also, data were obtained by detailed medical records review. Our study included patients from the North and Midwest, East, and South of the US. However, despite valiant efforts, we were not successful in obtaining data from Western states. A review of COVID-19 literature from Western states showed similar results to ours. Male gender and older patients (>70 years) are more likely to die from COVID-19 [38-40].
Limitations of the study include its retrospective nature and the fact that it involves areas in the US that were hit by the pandemic asynchronously. This might have affected our findings as the New York area was hit first and very hard by the pandemic and as such displayed higher mortality than other regions that might have not matured in case saturation. There was not uniform data regarding some clinical manifestations and laboratory parameters from all the centers. However, our analyses are consistent with other published studies, and this consistency suggests that our data is robust and validated. In summary, analysis of hospitalized COVID-19 patients across 11 states in the US showed that older age, male sex, AA race, patients in the New York state have higher in-hospital mortality. Other predictors of mortality include the presence of comorbid conditions, shortness of breath, cough, elevated serum inflammatory markers, altered lymphocyte count, elevated AST, and low serum albumin. This study suggests that African Americans carried a disproportionate burden of COVID-19 death in the US in 2020.
Declarations
Funding
This project was supported (in part) by the National Cancer Institute RO1 CA258519, and National Institute on Minority Health and Health Disparities of the National Institutes of Health G12MD007597.
Conflict-of-interest statement
The authors declare no conflicts of interest.
Financial Disclosure
No Financial disclosure applied for this research.
Author contributions
HA designed the study, HA, HB and LGC wrote the manuscript, John M. Carethers & Farin Kamangar, Zaki A. Sherif, Fatimah Jackson reviewed and edited the paper; Antonio Pizuorno, Folake Adeleye, Maryam Mehdipour Dalivand, Suryanarayana Reddy Challa, Boubini Jones-Wonni, Sheldon Rankine, Camelita Thrift, Chiamaka Ekwunazu, Abigail Banson, Rachel Kim, Chandler Gilliard, Elizabeth Ekpe, Nader Shayegh, Constance Nyaunu, Chidi Martins, Ashley Slack, Princess Okwesili, Malachi Abebe, Yashvardhan Batta, Do, Ly, Ogwo Valarie, Tori Smith, Kyra Watson, Oluwapelumi Kolawole , Sarine Tahmazian, Sofiat Atoba, Myra Khushbakth, Gregory Riley, Warren Gavin, Areeba Kara, Manuel Hache-Marliere, Leonidas Palaiodimos, Vishnu R Mani, Aleksandr Kalabin, Vijay Reddy Gayam, Pavani Reddy Garlapati, Joseph Miller, Vinod Rustgi, and Lakshmi Gayathri Chirumamilla collected and analyzed the clinical data. Gholamreza Oskrochi performed statistical analysis. All authors read and approved the final manuscript.
Data availability statement
Data are available on reasonable request. Please contact corresponding author, Dr. Hassan Ashktorab, email: hashktorab@howard.edu
Acknowledgement
We would like to thank all covid patients who participated in this study. We appreciate the work of all healthcare providers in this COVID-19 pandemic. We would also like to thank the funding agency National Cancer Institute, and National Institute on Minority Health and Health Disparities of the National Institutes of Health. Hassan Ashktorab had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical statement
The study was approved by Howard University Intuitional Review Board 355 (IRB-20-MED-26).
Abbreviation list:
C-Reactive Protein (CRP); Lactose dehydrogenase (LDH); Creatine Phosphokinase (CPK); Intensive Care Unit (ICU); Aspartate Transaminase (AST); Odds Ratio (OD).
References
- Zhu N. A novel coronavirus from patients with pneumonia in China, 2019. New England journal of medicine 382 (2020): 727-733.
- The World Health Organisation COVID-19 (2022).
- The John Hopkins Coronavirus Resource Center (2022).
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China JAMA Intern Med 108 (2020): 934-943.
- Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, Morbidity and mortality weekly report 69 (2020): 458.
- Ashktorab H. COVID-19 among African Americans and Hispanics: Does gastrointestinal symptoms impact the outcome? World Journal of Clinical Cases 9 (2021): 8374.
- Ashktorab H. Elevated liver enzymes, ferritin, C-reactive protein, D-dimer, and age are predictive markers of outcomes among African American and Hispanic patients with coronavirus disease 2019. Gastroenterology 161 (2021): 345-349.
- Carethers JM. Insights into disparities observed with COVID-19. Journal of internal medicine 289 (2021): 463-473.
- COVID-19 Racial Data Tracker (2021).
- Anaele BI, Doran C, and McIntire R. Visualizing COVID-19 mortality rates and African-American populations in the USA and Pennsylvania. Journal of racial and ethnic health disparities 8 (2021): 1356-1363.
- The Statista- US Covid-19 Death By Race (2022).
- Griffith DM. Men and COVID-19: a biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Preventing chronic disease 17 (2020): 63.
- Nguyen NT. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. Plos One 16 (2021): 0254066.
- Peckham H. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature communications 11 (2020): 1-10.
- Jin JM. Gender differences in patients with COVID-19: focus on severity and mortality. Frontiers in public health 8 (2020): 152.
- Okwan-Duodu D, Lim E-C, You S, et al. TMPRSS2 activity may mediate sex differences in COVID-19 severity. Signal transduction and targeted therapy 6 (20221): 1-3.
- Yehia BR. Association of Race With Mortality Among Patients Hospitalized With Coronavirus Disease 2019 (COVID-19) at 92 US Hospitals. JAMA Network Open 3 (2020): 2018039-2018039.
- Qeadan F. Racial disparities in COVID-19 outcomes exist despite comparable Elixhauser comorbidity indices between Blacks, Hispanics, Native Americans, and Whites. Scientific reports 11 (2021): 1-11.
- Kabarriti R. Association of Race and Ethnicity With Comorbidities and Survival Among Patients With COVID-19 at an Urban Medical Center in New York. JAMA Network Open 3 (2020): 2019795-2019795.
- Muñoz-Price LS. Racial Disparities in Incidence and Outcomes Among Patients With COVID-19. JAMA Network Open 3 (2020): 2021892-2021892.
- Kalyanaraman MR. Characteristics and outcomes of COVID-19 patients in New York City’s public hospital system. Plos One 15 (2020): 0243027.
- Newman LA, Winn RA and Carethers JM. Similarities in risk for COVID-19 and cancer disparities. Clinical Cancer Research 27 (2021): 24-27.
- Choi W Y. Mortality Rate of Patients With COVID-19 Based on Underlying Health Conditions. Disaster medicine and public health preparedness (2021): 1-6.
- Caci G. COVID-19 and obesity: dangerous liaisons. Journal of clinical medicine 9 (2020): 2511.
- Shi L, Wang Y, Duan G, et al. Dyspnea rather than fever is a risk factor for predicting mortality in patients with COVID-19. Journal of Infection 81 (2020): 647-679.
- Schettino M. Clinical characteristics of COVID-19 patients with gastrointestinal symptoms in Northern Italy: a single-center cohort study. Official journal of the American College of Gastroenterology ACG 116 (2021): 306-310.
- Zhang L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Annals of oncology 31 (2020): 894-901.
- Wagner J. Elevated transaminases and hypoalbuminemia in Covid-19 are prognostic factors for disease severity. Scientific reports 11 (2021): 1-5.
- Liu Z. Bilirubin levels as potential indicators of disease severity in coronavirus disease patients: a retrospective cohort study. Frontiers in medicine 799 (2020).
- Tahtabasi M. Does COVID-19 cause an increase in spleen dimensions? Possible effects of immune activation, hematopoietic suppression and microthrombosis. Clinical imaging 79 (2021): 104-109.
- Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet 395 (2020): 1054-1062.
- Gao Y. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. Journal of medical virology 92 (2020): 791-796.
- Axfors C. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nature communications 12 (2021): 1-13.
- Sterne JAC, Murthy S, Diaz JV, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 324 (2020): 1330-1341.
- Sahilu T, Sheleme T and Melaku T. Severity and mortality associated with steroid use among patients with COVID-19: a systematic review and meta-analysis. Interdisciplinary Perspectives on Infectious Diseases 2021 (2021): 6650469
- King CS. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. Plos one 15 (2020): 0242651.
- Nicholson CJ. Estimating risk of mechanical ventilation and mortality among adult COVID-19 patients admitted to Mass General Brigham: the VICE and DICE scores. Medrxiv (2020): 100765.
- Bhatraju PK. Covid-19 in critically ill patients in the Seattle region—case series. New England Journal of Medicine 382 (2020): 2012-2022.
- Arentz M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. Jama 323 (2020): 1612-1614.
- Nuño M, García Y, Rajasekar G, et al. COVID-19 hospitalizations in five California hospitals: a retrospective cohort study. BMC infectious diseases 21 (2021): 1-9.