Adult Stem Cells Based Therapies in Veterinary Medicine
Article Information
Zuzana Vikartovska1, Filip Humenik1, Marcela Maloveska1, Jana Farbakova2, Lubica Hornakova2, Adriana-Natalia Murgoci3, Dasa Cizkova1,3,*
1Department of Anatomy, Histology and Physiology, University of Veterinary Medicine and Pharmacy in Kosice, Komenskeho 73, Kosice 041 81, Slovakia
2Small Animal Clinic, University of Veterinary Medicine and Pharmacy in Kosice, Komenskeho 73, 041 81 Kosice, Slovakia
3Institute of Neuroimmunology, Slovak Academy of Sciences, Dubravska cesta 9, Bratislava 84510, Slovakia
*Corresponding Author: Dr. Dasa Cizkova, Department of Anatomy, Histology and Physiology, University of Veterinary Medicine and Pharmacy in Kosice, Komenskeho 73, Kosice 041 81, Slovakia
Received: 16 May 2020; Accepted: 22 May 2020; Published: 16 June 2020
Citation: Zuzana Vikartovska, Filip Humenik, Marcela Maloveska, Jana Farbakova, Lubica Hornakova, Adriana-Natalia Murgoci, Dasa Cizkova. Adult Stem Cells Based Therapies in Veterinary Medicine. Archives of Veterinary Science and Medicine 3 (2020): 40-50.
View / Download Pdf Share at FacebookAbstract
Research into adult mesenchymal stem cells (MSCs) and their therapeutic potential for treating various diseases is nowadays on the rise, not only in human, but also in veterinary medicine. MSCs are easy to collect from most tissues and relatively safe with no ethical concerns. They can be used in veterinary medicine as autologous or allogenic cell-based therapies for orthopaedic injuries, cardiovascular, muscular and neurodegenerative disorders and wound healing. The therapeutic action of MSCs lies either in their differentiation towards cells which need to be replaced, or in their immunomodulatory and paracrine effects through which they support recovery of various tissues. Regarding these pro-regenerative properties considerable attention has to be paid to proper matching of MSC sources with respect to the nature of the diseases needing to be cured. Overall data indicate that particularly the umbilical cord MSC (UC MSC) therapy which involves the most primitive MSCs may bring considerable benefits to patients with a wide range of diseases. This review describes the pro-regenerative potential of MSCs with the main focus on advances in UC MSCs based therapy in veterinary medicine.
Keywords
MSC; Therapy; Umbilical cord
MSC articles, Therapy articles, Umbilical cord articles
Article Details
1. Introduction
Stem cells are specific cells with unique properties. They are divided into categories based on their origin and differentiation properties [1]. Embryonic stem cells are pluripotent, able to differentiate into all cells of the primary layers: mesoderm, endoderm and ectoderm [2-4]. Furthermore, they have unlimited capacity for self-renewal, which makes them a unique tool for research in regenerative medicine [4, 5]. However, due to ethical concerns associated with their isolation from blastocysts, tumorigenicity and problems with histocompatibility, their translation into clinical trials is being postponed. Induced pluripotent stem cells (iPSCs) present an alternative to embryonic stem cells [5]. Human iPSCs were first derived from skin fibroblasts, but now they can be established from diverse somatic cell types and various animal species [6]. Induced pluripotent stem cells are adapted by specific reprogramming procedure towards pluripotency, involving Oct4, Sox2, Klf4, and c-Myc genes, so they show characteristics similar to embryonic stem cells, but without the ethical dilemma [5]. Their greatest advantage lies in the fact that they are disease and patient specific, thus potentially could be enrolled in personalized therapies for almost all disorders [5]. Recent studies from experimental veterinary medicine have shown the ability to generate neural precursors from cells derived from adult canine skin. Skin-derived neural precursors (SD NPCs) are able to produce mature neural cells similar to neurons in the central nervous system [7]. SD NPCs seem to be a hot candidate for treatment of canine cognitive dysfunction (CDS) or Alzheimer’s disease (AD) in humans, as these cells with basic electrophysiological functionality are capable of replacing lost neurons in the brain [7]. During the last three decades considerable attention has been given to research into multipotent MSCs, which are clonogenic, non-haematopoietic and able to replicate extensively in vitro. In veterinary clinics in particular the numbers of companion animals treated with MSCs derived from various adult tissues have significantly increased and provide an important basis for assessing their effectiveness and potential translation to human medicine.
2. Sources and Characteristicts of MSCs
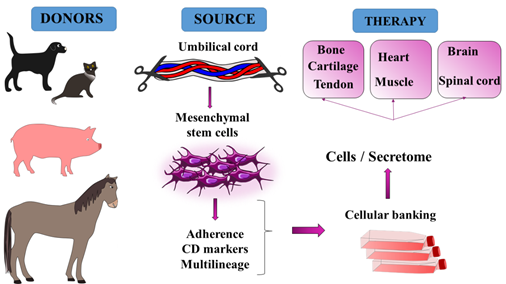
Although bone marrow and adipose tissues are the main sources of adult MSCs, stromal stem cells can also be obtained from synovium, dermis, periodontal ligament, dental pulp and other adult tissues [8-12]. However, the most primitive MSCs are nowadays isolated from umbilical cord blood and from umbilical cord tissue, known as Wharton’s jelly (Figure 1). In the veterinary field there are many species from which the umbilical cord can be collected [3, 13, 14]. The preferable method for acquiring an umbilical cord is by caesarean section, when the risk of contamination is reduced to a minimum. Canine umbilical cord blood and Wharton’s jelly can serve as good sources of MSCs, which is why many different isolation protocols have already been established [14-17]. On the other hand, the MSCs used in feline regenerative medicine are mostly isolated from bone marrow, adipose tissue or amniotic fluid through minimal manipulation, but not from umbilical cord up to now [18-20]. The first isolation of equine MSCs from umbilical cord blood was published in 2007 by Koch et al [13]. Since then many studies have described different application strategies for equine MSCs derived from both umbilical cord blood and Wharton´s jelly from horses or other larger species such as sheep, goats and cows [13, 21-27]. However, due to their anatomical, genetic and physiological similarities with humans, pigs serve as the best preclinical model for safety evaluation and clinical application of UC MSCs in veterinary practice as well as in translation studies for human medicine [28, 29]. Nowadays, standard protocols for effective isolation of Wharton´s jelly MSCs from different animals and tissues have been established, thus opening up possibilities for cell-based therapies in veterinary clinics [14, 15].
3. Characterization of MSCs
Mesenchymal stem cells are multipotent cells capable of differentiating into multiple lineages. According to the International Society for Cellular Therapy they have to meet three criteria, which are plastic adherence, expression of specific cell surface markers and three lineage differentiation potential [31]. Even though these criteria were firstly established for human MSCs, they also apply to animal MSCs [31, 32]. MSCs show spindle-like and fibroblastic morphology, and after the first two or three passages they express stromal markers CD105, CD73 and CD90, and they lacki hematopoietic factors such as CD45, CD34, CD14 or CD11b, CD79a or CD19 and HLA class [31, 33, 34]. According to the literature, there are also other CD markers expressed by umbilical cord-derived MSCs such as CD44 or CD29 [21]. MSCs are assumed to differentiate into osteoblasts, chondrocytes and adipocytes, proven by staining with Alizarin Red, Alcian blue and Oil Red O respectively [31]. Interestingly, some studies do not support UC MSC differentiation into adipocytes, while others demonstrate their adipogenic potential [21, 30, 33]. Three lineage differentiations of UC MSCs can also be proven by means of RT- PCR and specific gene expression [35]. Chondrogenic differentiation of UC MSCs is associated with expression of Sox9, COL1 and COL2 genes [36]. Genes specific for osteogenic differentiation are osteonectin and Runx2 [37], but there are also other markers used in human medicine such as ALP (alkaline phosphatase), PSAT1, HSP27, OAT and CRB1 [38]. Adipogenic differentiation is routinely demonstrated through the expression of lipoprotein lipase (LPL), leptin and fatty acid-binding protein 4 (FABP4) [16]. In addition, under specific experimental conditions MSCs can give rise to other cell types, such as hepatocytes, which was indicated through the expression of albumin and cytokeratin 18. Neural differentiation potential was confirmed with glial fibrillary acidic protein (GFAP) and microtubule-associated protein 2 (MAP2) [16, 39]. As a result of the effort to establish what particular tissue-specific cells can differentiate into in vitro or in vivo, there is a need to analyse the gene-expression patterns of differentiated MSCs together with their protein content.
4. Preclinical and Clinical Studies
As the idea of using MSC-based therapy developed, animals were first selected for safety testing. Very soon MSCs made their way out from laboratories, from mouse and rat experimental studies into veterinary clinics, where pets are “tested” and treated side-by-side [3]. Developed veterinary approaches give us opportunities for safety studies and for researching the most appropriate MSC application strategies in disorders which pets and humans have in common, whether identical or similar [3].
|
Donors |
Sources |
Stem cells |
Disease |
|
Dog |
Skin Bone marrow Adipose tissue Umbilical cord |
iPSCs, NPCs MSC More primitive > MSC |
Kidney failure Neurologic disorders Cutaneous wound Bone and cartilage defects |
|
Cat |
Bone marrow Adipose tissue |
MSC |
Kidney failure Neurologic disorders |
|
Horse |
Bone marrow Adipose tissue Umbilical cord |
MSC More primitive > MSC |
Tendon cyst Bone and cartilage defect |
|
Pig |
Umbilical cord |
More primitive > MSC |
Bone and cartilage defects Animal model |
Table 1: Donors, sources and types of stem cells used in veterinary medicine with most common treated diseases.
There are many disorders in veterinary medicine where MSCs can be indicated, but many others still remain under research (Table 1). The most common diseases treated with MSCs are musculoskeletal, cardiovascular, ophthalmological, neurological and urological disorders. Companion animals such as dogs and cats may present with bone problems, mainly with hereditary background or caused by malformation due to inappropriate diet. Horses subjected to high-impact sports often struggle with injuries to their soft tissues, tendons, cartilages and bones. Other animals such as mice, rats or pigs are still used as small or large experimental animal models for human medicine [40].
4.1 Musculoskeletal disorders
Musculoskeletal disorders such as tendonitis, osteoarthritis, muscular dystrophy or bone defects caused by trauma, cancer or inflammation are the most common indications for UC MSC administration [17, 41-44]. The repair of bone defects caused by injury, chronic inflammation and tumor presents a challenge in veterinary regenerative medicine [45]. Newly-developed biocompatible bone-forming scaffolds seeded with MSCs seem to be a promising form of therapy [41]. Although the differentiation potential of MSCs varies with respect to the tissue source, UC MSCs have shown higher osteogenic potential than bone marrow MSCs in vitro [42]. Recent studies indicate that regeneration of fractures can be achieved by injection of UC MSCs with beta-tricalcium phosphate (β-TCP) included as a supportive implant [41]. By means of this therapy, enhanced bone remodelling leading to accelerated fracture healing was found after just six weeks [41]. Furthermore, the paracrine effect of UC MSCs may play a role in bone regeneration as well [46]. The curative properties of UC MSCs have also been documented in the case of canine osteoarthritis, with the regeneration of cartilages and surrounding tissues being observed [44]. Impressive results have been attained in the treatment of superficial digital flexor tendinitis, which is the most common disorder in race horses [23]. UC MSC injection in combination with regular physical therapy demonstrated locomotor improvement just one month after the initiation of treatment [23]. Spontaneously-occurring muscular dystrophy in golden retriever dogs is an excellent model for studying the progressive muscle weakness and atrophy in humans known as Duchenne muscular dystrophy (DMD). Recently-developed pharmacological, genetic and UC MSC-based therapies may partially improve the muscle function of those affected by muscular dystrophy. However, after single administration of UC-MSCs in a dog clinical study, no dystrophin was detected in the affected muscles [17]. Therapy including multiple injections is therefore now being considered.
4.2 Neurological disorders
Neurological disorders are usually associated with irreversible processes due to the limited regeneration capability in the central nervous system. They can be classified into traumatic injuries of the brain (TBI) and spinal cord (SCI) and age-related degenerations. Recent findings indicate that MSCs derived from different sources are able to survive and migrate towards injury sites and contribute to axonal survival, thus promoting functional recovery after SCI and TBI [47-51]. Moreover, UC MSCs have been associated with more neuroprotection, nerve regeneration and less inflammation than other MSCs following SCI [52]. Surprisingly, no enhanced sparing of spinal cord tissue was demonstrated in a study using MRI [50].
Preclinical studies have shown possibilities of treating neurodegenerative disorders such as Parkinson's and Alzheimer's disease, multiple sclerosis, or stroke and brain tumours [53]. Both MSCs and NPCs seem to have promising therapeutic impact, as they decrease neuroinflammation, support neurogenesis, synaptogenesis and differentiation of neural cells [54]. Furthermore, they are able to suppress cell death caused by tau protein and Aβ deposit accumulation [54]. Thus their biological activity seems to consist in neuroprotection and the ability to release immune modulatory factors [54]. MSC-based therapy may decrease depression states in rats with subarachnoid haemorrhage, although the mechanism is not yet clear. Because MSCs are able to cross the blood-brain barrier [54], the administration of MSCs can be provided intravenously, intra-arterially or intranasally, which are easy procedures that can be carried out on awake animals. Although this treatment appears promising, there is a need for further research also in the field of veterinary medicine [53].
4.3 Myocardial disorders and wound healing
Several stem-cell populations, including adult MSCs from various organs and tissues, have been tested for cardiac repair potential with encouraging pre-clinical and clinical results [55]. For a long time the heart has been considered a post-mitotic organ, although this view has recently changed with the identification of stem/progenitor cells residing within the adult heart [56]. Stem cells in the dog heart are self-renewing, clonogenic and multipotent, capable of regenerating infarcted myocardium, and improving cardiac function [57]. They regenerate the injured heart either directly via differentiation into cardiomyocytes and vascular cells, or indirectly through paracrine effects (modulating angiogenesis and inflammation and preserving the Cx43 gap junction). Another recent study involving porcine acute myocardial infarction indicates that intravenous injection of UC MSCs is a feasible and effective procedure for preserving left ventricle function and augmenting myocardial remodelling [28]. Although promising results in restoring cardiac function in patients with heart failure have been achieved, the efficacy of UC MSCs should be further explored through large randomized controlled trials. Finally, accumulating evidence indicates the beneficial properties of UC MSCs in wound healing (cuts, burns, and ulcers) and in non-healing wounds (diabetes). This depends on their ability to differentiate into different skin-cell types such as keratinocytes, endothelial cells, pericytes and monocytes, regulating collagen I and III and ECM, and decreasing the TIMP/MMP ratio [58, 59]. UC MSCs have also been tested in dogs with keratoconjuctivitis sicca, also known as dry eye syndrome, and in patients with ocular injury or retinal detachments with promising results [60, 61].
5. Conclusion
MSCs are able to differentiate into a wide range of specialized cells with the capacity to renew or rebuild damaged tissues. MSCs collected from bone marrow seems to be the best source for treating patients with musculoskeletal disorders (tendinitis, osteoarthritis, muscular dystrophy or bone defects). However, despite being considered as more primitive, umbilical cord (UC) MSCs show more pluripotent and genetically-flexible features with more enhanced immunomodulatory and paracrine action than other adult stem cells. Thus they can be enrolled in therapies for neurological and myocardial disorders, or even for difficult wound healing conditions. Due to these facts, special effort is needed for matching the most convenient sources of MSCs with specific disorder conditions in order to promote the best therapeutic benefit to patients. These results are still preliminary, however, and we need more extensive clinical trials for safety and efficacy studies. Nowadays, stem cell-based therapy plays a crucial role in the development of veterinary regenerative medicine and in the translation process into human medicine.
Acknowledgements
The authors acknowledge grants: APVV 15-0613, APVV-19-0193, IGA UVLF 06/2018 "Influence of Regeneration Capacity of Nervous Tissue in In vitro Conditions through Adult Stem Cells", VEGA 1/0376/20, 2/0146/19, IGA UVLF 02/2019 „Stratification of patients with canine cognitive dysfunction, application of innovative stem cell therapy “ .
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Rep (2015): 35.
- De Schauwer C, Meyer E, Van de Walle GR, et al. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology 75 (2011): 1431-1443.
- Stem cells in veterinary medicine. Stem Cell Res. Ther 2 (2011): 9.
- Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. Biosci. Bioeng 100 (2005): 12-27.
- Nordin N. Induced Pluripotent Stem Cells: History, Properties and Potential Applications 66 (2011): 6.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 (2006): 663-676.
- Duncan T, Lowe A, Sidhu K, et al. Replicable Expansion and Differentiation of Neural Precursors from Adult Canine Skin. Stem Cell Rep 9 (2017): 557-570.
- Anker PS in `t, Scherjon SA, Keur CK der, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102 (2003): 1548-1549.
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. J. Haematol 109 (2000): 235-242.
- Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Natl. Acad. Sci. U. S. A 97 (2000): 13625-13630.
- Prockop DJ. Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues. Science 276 (1997): 71-74.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7 (2001): 211-228.
- Koch TG, Heerkens T, Thomsen PD, et al. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol 7 (2007): 26.
- Seo MS, Jeong YH, Park JR, et al. Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells. Vet. Sci 10 (2009): 181.
- Groza I, Pop RA, Cenariu M, et al. Canine Wharton’s Jelly Derived Mesenchymal Stem Cells Isolation. Agric. Sci. Procedia 10 (2016): 408-411.
- Seo MS, Park SB, Kang, KS. Isolation and Characterization of Canine Wharton’s Jelly-Derived Mesenchymal Stem Cells. Cell Transplant 21 (2012): 1493-1502.
- Zucconi E, Vieira NM, Bueno CR, et al. Preclinical Studies with Umbilical Cord Mesenchymal Stromal Cells in Different Animal Models for Muscular Dystrophy. Biomed. Biotechnol (2011): 1-9.
- Martin DR, Cox NR, Hathcock TL, et al. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Hematol 30 (2002): 879-886.
- Quimby JM, Borjesson DL. Mesenchymal stem cell therapy in cats: Current knowledge and future potential. Feline Med. Surg 20 (2018): 208-216.
- Vidane AS, Souza AF, Sampaio RV, et al. Cat amniotic membrane multipotent cells are nontumorigenic and are safe for use in cell transplantation. Stem Cells Cloning Adv. Appl 7 (2014): 71-78.
- De Schauwer C, Meyer E, Cornillie P, et al. Optimization of the Isolation, Culture, and Characterization of Equine Umbilical Cord Blood Mesenchymal Stromal Cells. Tissue Eng. Part C Methods 17 (2011): 1061-1070.
- Jäger M, Bachmann R, Scharfstädt A. et al. Ovine Cord Blood Accommodates Multipotent Mesenchymal Progenitor Cells. In Vivo 11 (2006).
- Kang JG, Park SB, Seo MS, et al. Characterization and clinical application of mesenchymal stem cells from equine umbilical cord blood. Vet. Sci 14 (2013): 367.
- Kumar K, Agarwal P, Das K, et al. Isolation and characterization of mesenchymal stem cells from caprine umbilical cord tissue matrix. Tissue Cell 48 (2016): 653-658.
- Nazari-Shafti TZ, Bruno IG, Martinez RF, et al. High Yield Recovery of Equine Mesenchymal Stem Cells from Umbilical Cord Matrix/Wharton’s Jelly Using a Semi-automated Process (2015): 131-146. In: Stem Cell Protocols, (Rich, Ivan N. eds.) Springer New York, New York, NY.
- Qiu P, Bai Y, Liu C, et al. A dose-dependent function of follicular fluid on the proliferation and differentiation of umbilical cord mesenchymal stem cells (MSCs) of goat. Cell Biol 138 (2012): 593-603.
- Raoufi MF, Tajik P, Dehghan MM, et al. Isolation and Differentiation of Mesenchymal Stem Cells From Bovine Umbilical Cord Blood. Domest. Anim 46 (2011): 95-99.
- Lim M, Wang W, Liang L, et al. Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res. Ther 9 (2018).
- Weiss ML, Mitchell KE, Hix JE, et al. Transplantation of porcine umbilical cord matrix cells into the rat brain. Neurol 182 (2003): 288-299.
- Lee KS, Nah JJ, Lee BC, et al. Maintenance and characterization of multipotent mesenchymal stem cells isolated from canine umbilical cord matrix by collagenase digestion. Vet. Sci 94 (2013): 144-151.
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 (2006): 315-317.
- de Bakker E, Van Ryssen B, De Schauwer C. et al. Canine mesenchymal stem cells: state of the art, perspectives as therapy for dogs and as a model for man. Q 33 (2013): 225-233.
- Kern S, Eichler H, Stoeve J, et al. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 24 (2006): 1294-1301.
- Ryu HH, Kang BJ, Park SS, et al. Comparison of Mesenchymal Stem Cells Derived from Fat, Bone Marrow, Wharton’s Jelly, and Umbilical Cord Blood for Treating Spinal Cord Injuries in Dogs 14.
- Arufe MC, De la Fuente A, Fuentes I, et al. Umbilical cord as a mesenchymal stem cell source for treating joint pathologies. World J. Orthop 2 (2011): 43-50.
- de Crombrugghe B, Lefebvre V, Nakashima, K. Regulatory mechanisms in the pathways of cartilage and bone formation. Opin. Cell Biol 13 (2001): 721-728.
- Reed SA, Johnson SE. Equine umbilical cord blood contains a population of stem cells that express Oct4 and differentiate into mesodermal and endodermal cell types. Cell. Physiol 215 (2008): 329-336.
- Kim J, Shin JM, Jeon YJ, et al. Proteomic Validation of Multifunctional Molecules in Mesenchymal Stem Cells Derived from Human Bone Marrow, Umbilical Cord Blood and Peripheral Blood. PLoS ONE 7 (2012).
- Cizkova D, Devaux S, Le Marrec-Croq F, et al. Modulation properties of factors released by bone marrow stromal cells on activated microglia: an in vitro Sci. Rep 4 (2014): 7514.
- Sultana T, Lee S, Yoon HY, et al. Current Status of Canine Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Veterinary Medicine. Stem Cells Int (2018): 1-14.
- Jang BJ, Byeon YE, Lim JH, et al. Implantation of canine umbilical cord blood-derived mesenchymal stem cells mixed with beta-tricalcium phosphate enhances osteogenesis in bone defect model dogs. Vet. Sci 9 (2008): 387.
- Kang BJ, Ryu HH, Park SS, et al. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton’s jelly for treating bone defects. Vet. Sci. 13 (2012): 299.
- Volk SW, Theoret C. Translating stem cell therapies: the role of companion animals in regenerative medicine. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc 21 (2013): 382-394.
- Zhang B, Wang B, Li S, et al. Evaluation of the Curative Effect of Umbilical Cord Mesenchymal Stem Cell Therapy for Knee Arthritis in Dogs Using Imaging Technology. Stem Cells Int (2018): 1-12.
- Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28 (2007): 4240-4250.
- Byeon YE, Ryu HH, Park SS, et al. Paracrine effect of canine allogenic umbilical cord blood-derived mesenchymal stromal cells mixed with beta-tricalcium phosphate on bone regeneration in ectopic implantations. Cytotherapy 12 (2010): 626-636.
- Blaško J, Szekiova E, Slovinska L, et al. Axonal outgrowth stimulation after alginate/mesenchymal stem cell therapy in injured rat spinal cord. Acta Neurobiol. Exp. (Warsz.) 77 (2017): 337-350.
- Cizkova D, Cubinkova V, Smolek T, et al. Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury. J. Mol. Sci 19 (2018): 870.
- Krupa P, Vackova I, Ruzicka J, et al. The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. J. Mol. Sci 19 (2018).
- Lim JH, Byeon YE, Ryu HH, et al. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. Vet. Sci 8 (2007): 275.
- Weston NM, Sun D. The Potential of Stem Cells in Treatment of Traumatic Brain Injury. Neurol. Neurosci. Rep 18 (2018): 1.
- Park SS, Byeon YE, Ryu HH, et al. Comparison of Canine Umbilical Cord Blood-Derived Mesenchymal Stem Cell Transplantation Times: Involvement of Astrogliosis, Inflammation, Intracellular Actin Cytoskeleton Pathways, and Neurotrophin-3. Cell Transplant 20 (2011): 1867-1880.
- Riordan NH, Morales I, Fernández G, et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. Transl. Med 16 (2018).
- Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther 8 (2017).
- du Pré, BC, Doevendans PA, van Laake LW. Stem cells for cardiac repair: an introduction. Geriatr. Cardiol. JGC 10 (2013): 186-197.
- Le T, Chong J. Cardiac progenitor cells for heart repair. Cell Death Discov 2 (2016): 16052.
- Linke A, Müller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Natl. Acad. Sci. U. S. A 102 (2005): 8966-8971.
- Azari O, Babaei H, Derakhshanfar A, et al. Effects of transplanted mesenchymal stem cells isolated from Wharton’s jelly of caprine umbilical cord on cutaneous wound healing; histopathological evaluation. Res. Commun 35 (2011): 211-222.
- Sasaki M, Abe R, Fujita Y, et al. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. Immunol 180 (2008): 2581-2587.
- Barros SD, Dehez S, Arnaud E, et al. Aging-related Decrease of Human ASC Angiogenic Potential Is Reversed by Hypoxia Preconditioning Through ROS Production. Ther 21 (2013): 399-408.
- Mead B, Tomarev S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med 6 (2017): 1273-1285.