Adult Granulosa Cell Tumor of the Ovary: Initial Evaluation and Current Treatment Paradigm
Article Information
Melissa Hodeib1, Ilene Tsui1, Abed Sinno1,2, Nisha Bansal1 and Joshua G. Cohen1*
1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of California Los Angeles, Los Angeles, CA, USA
2Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Olive View-UCLA Medical Center, Sylmar, CA, USA
*Corresponding Author: Joshua G. Cohen, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of California Los Angeles, Los Angeles, CA 90095, USA
Received: 27 October 2017; Accepted: 24 November 2017; Published: 04 December 2017
View / Download Pdf Share at FacebookAbstract
Adult granulosa cell tumor (AGCT) is a sex cord stromal tumor (SCST) which constitutes 2-5% of all ovarian cancers. Initial treatment of early stage disease includes primary surgical resection with or without adjuvant treatment. Late stage disease and recurrent disease management involves multimodality treatment with surgical resection as the mainstay. Given the rarity of this tumor and its relative chemoresistance, future study is needed to better guide adjuvant treatment and individualize therapy for patients. Further evaluation of diagnostic and potential therapeutic implications involving FOXL2 mutations in AGCT is warranted.
Keywords
Adult granulosa cell tumor, Fertility preservation, Sex cord stromal tumor, Ovarian cancer
Adult granulosa cell tumor articles Adult granulosa cell tumor Research articles Adult granulosa cell tumor review articles Adult granulosa cell tumor PubMed articles Adult granulosa cell tumor PubMed Central articles Adult granulosa cell tumor 2023 articles Adult granulosa cell tumor 2024 articles Adult granulosa cell tumor Scopus articles Adult granulosa cell tumor impact factor journals Adult granulosa cell tumor Scopus journals Adult granulosa cell tumor PubMed journals Adult granulosa cell tumor medical journals Adult granulosa cell tumor free journals Adult granulosa cell tumor best journals Adult granulosa cell tumor top journals Adult granulosa cell tumor free medical journals Adult granulosa cell tumor famous journals Adult granulosa cell tumor Google Scholar indexed journals Fertility preservation articles Fertility preservation Research articles Fertility preservation review articles Fertility preservation PubMed articles Fertility preservation PubMed Central articles Fertility preservation 2023 articles Fertility preservation 2024 articles Fertility preservation Scopus articles Fertility preservation impact factor journals Fertility preservation Scopus journals Fertility preservation PubMed journals Fertility preservation medical journals Fertility preservation free journals Fertility preservation best journals Fertility preservation top journals Fertility preservation free medical journals Fertility preservation famous journals Fertility preservation Google Scholar indexed journals Sex cord stromal tumor articles Sex cord stromal tumor Research articles Sex cord stromal tumor review articles Sex cord stromal tumor PubMed articles Sex cord stromal tumor PubMed Central articles Sex cord stromal tumor 2023 articles Sex cord stromal tumor 2024 articles Sex cord stromal tumor Scopus articles Sex cord stromal tumor impact factor journals Sex cord stromal tumor Scopus journals Sex cord stromal tumor PubMed journals Sex cord stromal tumor medical journals Sex cord stromal tumor free journals Sex cord stromal tumor best journals Sex cord stromal tumor top journals Sex cord stromal tumor free medical journals Sex cord stromal tumor famous journals Sex cord stromal tumor Google Scholar indexed journals Ovarian cancer articles Ovarian cancer Research articles Ovarian cancer review articles Ovarian cancer PubMed articles Ovarian cancer PubMed Central articles Ovarian cancer 2023 articles Ovarian cancer 2024 articles Ovarian cancer Scopus articles Ovarian cancer impact factor journals Ovarian cancer Scopus journals Ovarian cancer PubMed journals Ovarian cancer medical journals Ovarian cancer free journals Ovarian cancer best journals Ovarian cancer top journals Ovarian cancer free medical journals Ovarian cancer famous journals Ovarian cancer Google Scholar indexed journals malignant articles malignant Research articles malignant review articles malignant PubMed articles malignant PubMed Central articles malignant 2023 articles malignant 2024 articles malignant Scopus articles malignant impact factor journals malignant Scopus journals malignant PubMed journals malignant medical journals malignant free journals malignant best journals malignant top journals malignant free medical journals malignant famous journals malignant Google Scholar indexed journals chemoresistance articles chemoresistance Research articles chemoresistance review articles chemoresistance PubMed articles chemoresistance PubMed Central articles chemoresistance 2023 articles chemoresistance 2024 articles chemoresistance Scopus articles chemoresistance impact factor journals chemoresistance Scopus journals chemoresistance PubMed journals chemoresistance medical journals chemoresistance free journals chemoresistance best journals chemoresistance top journals chemoresistance free medical journals chemoresistance famous journals chemoresistance Google Scholar indexed journals therapeutic articles therapeutic Research articles therapeutic review articles therapeutic PubMed articles therapeutic PubMed Central articles therapeutic 2023 articles therapeutic 2024 articles therapeutic Scopus articles therapeutic impact factor journals therapeutic Scopus journals therapeutic PubMed journals therapeutic medical journals therapeutic free journals therapeutic best journals therapeutic top journals therapeutic free medical journals therapeutic famous journals therapeutic Google Scholar indexed journals epithelial tumor articles epithelial tumor Research articles epithelial tumor review articles epithelial tumor PubMed articles epithelial tumor PubMed Central articles epithelial tumor 2023 articles epithelial tumor 2024 articles epithelial tumor Scopus articles epithelial tumor impact factor journals epithelial tumor Scopus journals epithelial tumor PubMed journals epithelial tumor medical journals epithelial tumor free journals epithelial tumor best journals epithelial tumor top journals epithelial tumor free medical journals epithelial tumor famous journals epithelial tumor Google Scholar indexed journals luteinizing hormone articles luteinizing hormone Research articles luteinizing hormone review articles luteinizing hormone PubMed articles luteinizing hormone PubMed Central articles luteinizing hormone 2023 articles luteinizing hormone 2024 articles luteinizing hormone Scopus articles luteinizing hormone impact factor journals luteinizing hormone Scopus journals luteinizing hormone PubMed journals luteinizing hormone medical journals luteinizing hormone free journals luteinizing hormone best journals luteinizing hormone top journals luteinizing hormone free medical journals luteinizing hormone famous journals luteinizing hormone Google Scholar indexed journals articles Research articles review articles PubMed articles PubMed Central articles 2023 articles 2024 articles Scopus articles impact factor journals Scopus journals PubMed journals medical journals free journals best journals top journals free medical journals famous journals Google Scholar indexed journals
Article Details
1. Epidemiology/Prognostic Factors
Adult granulosa cell tumor (AGCT) is a sex cord stromal tumor (SCST) which constitutes 2-5% of all ovarian cancers and approximately 70% of malignant SCSTs [1]. Despite late recurrences occurring up to 37 years after initial diagnosis, patients typically have a good prognosis since tumors tend to follow an indolent course [2]. Most AGCTs present at an earlier stage compared to epithelial tumors, often with signs of estrogen excess as they express aromatase activity and promote estrogen synthesis [3]. Symptoms include virilization, abnormal uterine bleeding, abdominal distention or pain due to the tumor size, and ascites in 1-2% of the cases [4, 5].
Given the rarity of this tumor risk factors for the disease have not yet been well elucidated. In the past, it was thought fertility promoting agents and oral contraceptive use may increase the risk of development of AGCTs however this has not been proven to be the case [6]. AGCTs also characteristically produce inhibin, estradiol, anti-mullerian hormone (AMH), and express receptors for follicle stimulating hormone (FSH) and luteinizing hormone (LH) [3].
Non-tumoral risk factors include obesity, non-white race, and diabetes [7], which may predispose patients to AGCT and recurrence [7] (Table 1). In one patient population, on multivariate analysis diabetes mellitus was the strongest predictor of recurrent disease with a hazard ratio (HR) of 3.58 and confidence interval (CI) of 1.10-9.03 [7]. Counseling and treatment of diabetes mellitus has therefore been a complementary approach to disease prevention and AGCT recurrence risk (RR) modification.
Significant non-modifiable prognostic factors for recurrence include stage at diagnosis, tumor size, and mitotic index greater than four [8, 9] (Table 1). Stage is considered the most important prognostic factor; patients with stage II disease have a greater than 7-fold increased risk for recurrence than patients with stage IA disease [8]. Mitotic index greater than 4 confers a 4.9x greater hazard for recurrence [8]. Residual disease after initial surgery [7, 10, 11] and residual disease after secondary debulking significantly affect overall survival (OS) [12].
|
Risk Factors* |
Prognostic Factors+ |
|
Diabetes Mellitus |
Stage at diagnosis |
|
Obesity |
Optimal tumor debulking |
|
Non-white race |
Tumor size (>5 cm) |
|
Mitotic index (>4) |
*Oral contraceptives and fertility drugs have not been associated with greater risk of AGCT and there has been no known inherited predisposition that increases risk; +Unkila-Kallio, et al (1998); +Suri, et al (2013); +Thomakos, et al (2016).
Table 1: Adult Granulosa Cell Tumors.
2. Molecular Biology/Genetics/Pathology
More than 95% of ovarian AGCTs harbor a 402C>G missense somatic point mutation in the transcription factor FOXL2 gene which codes for granulosa cell differentiation and maintenance of ovarian function. This mutation causes a partial loss of apoptotic function in AGCT-derived cells [13]. In fact, the 402C>G mutation may reduce expression of GnRH receptors, making cells resistant to GnRH-induced apoptosis [14]. There has been some interaction studied between FOXL2, SMAD3 and GATA4 in regulating CCND2, which plays a role in cell cycle progression and granulosa cell proliferation [13].
Recent evaluation of tumors has identified molecular differences between early and late stage disease [15], which suggests the need for different therapeutic targets in managing these two molecularly unique clinicopathologic stages. A transcriptome analysis of AGCT cells with a positive FOXL2 mutation identified 24 genes whose expression differs significantly between stage 1 and stage 3 disease [15]. The impact of differing molecular signatures in early versus late stage disease remains to be determined. Future characterization is needed to determine if molecular signatures lead to better prediction of tumor response and further therapeutic intervention.
AGCTs are often large (10-15cm in diameter) and multicystic [16]. They can have an edematous exterior, causing them to be adherent to other pelvic organs [16]. Within the interior, these tumors are comprised of solid components mixed with hemorrhagic and necrotic or cystic areas that are filled with serosanguinous fluid [17]. Microscopically, they are composed predominantly of granulosa cells with coffee bean nuclei and Call-Exner bodies, a rosette arrangement of cells around eosinophilic fluid space [16]. Cells are crowded and contain scant, pale cytoplasm [16].
3. Evaluation
Upon the initial presentation of a patient with suspected AGCT, evaluation should include imaging with a pelvic ultrasound, a computer tomography (CT) scan of the abdomen and pelvis, and tumor marker levels, including inhibin A, inhibin B, and possibly mullerian inhibiting substance (MIS) [19, 20, 21] (Figure 1). Magnetic resonance imaging (MRI) of the abdomen and pelvis is another option for preoperative imaging. If final pathology confirms cancer, imaging of the chest with CT, MRI, or chest x-ray to evaluate for metastatic disease is reasonable.
Inhibin, a peptide hormone produced by granulosa cells in response to follicle stimulating hormone (FSH), can be inappropriately elevated in women with ovarian granulosa cell tumors [22]. Studies have shown an inappropriate rise 5-20 months before a clinical recurrence suggesting its utility as a marker for early detection of disease [22]. Anti-mullerian hormone (AMH), like inhibin, is also produced by granulosa cells and typically undetectable in post-menopausal women. An elevated AMH level is highly specific for granulosa cell tumors and can become elevated 11 months before a recurrence [19]. A recent study has shown that AGCT recurrence may be detected non-invasively from circulating plasma cell free DNA in patients with clinical disease via a liquid biopsy for the FOXL2 mutation [23]. This may have particular efficacy if tumor biopsy samples cannot otherwise be obtained. Results suggest that the mutation can be detected prior to elevation of serum markers and clinical symptoms [23]. Further testing of this technology is needed before this becomes routine.
An endometrial biopsy is another component in the initial evaluation for AGCT due to the prolonged exposure of the endometrium to high levels of estradiol produced by granulosa cells. The incidence of endometrial hyperplasia ranges from 21-60% and endometrial carcinoma, from 1.3-12.8%, with almost all of these women being older than 45 years of age [24-27]. A preoperative endometrial biopsy is especially important in women who desire fertility preservation in order to appropriately manage their risk of cancer.
3. Treatment of Early Stage Disease
Surgery has been the mainstay of treatment for AGCTs with the goal of achieving no gross residual disease [28]. Residual disease at the time of completion surgery is associated with poor prognosis and recurrence [9-11].
Complete surgical resection and limited staging include a total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), exploration of the peritoneal cavity, omentectomy, peritoneal biopsies and washings. Nodal dissection is of limited value and typically not recommended, especially in suspected early stage 1A disease, as sex cord stromal tumors have a very low incidence of lymph node metastasis [29]. A recent study performed by Wilson et al further validated the importance of upfront surgical resection with optimal cytoreduction followed by adjuvant therapy if indicated [26]. This study reported patients who received neoadjuvant chemotherapy had a median recurrence almost two times earlier than those who underwent upfront surgical resection [26]. For young premenopausal patients desiring fertility sparing surgery, a unilateral salpingo-oophorectomy (USO) is feasible if disease is confined to one ovary (stage 1A) [27, 28]. Pelvic washings, omental sampling, and peritoneal biopsies are recommended as part of evaluation for metastatic disease. An endometrial biopsy is recommended to rule out concomitant endometrial cancer (Figure 1).
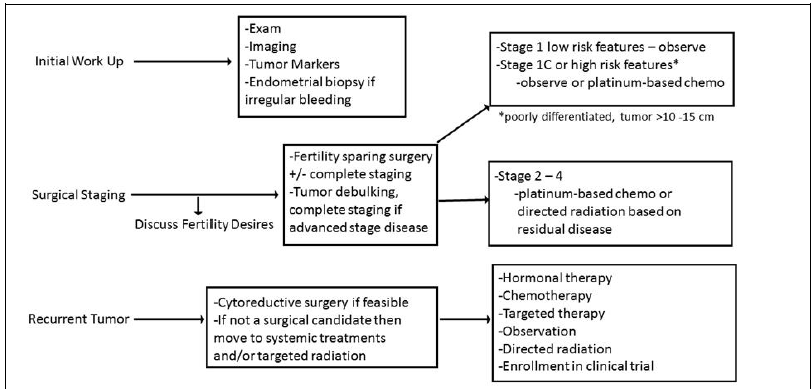
Figure 1: Adult Granulosa Cell Tumor.
* Schumer et al (2003); * NCCN - Ovarian Cancer (2017).
The efficacy of adjuvant chemotherapy in the setting of AGCT remains unclear for several reasons. Indications for use include an unresectable tumor at initial diagnosis or recurrent tumor, extra ovarian spread (advanced stage) or suboptimal cytoreduction. Consideration for chemotherapy in high risk stage 1 patients is recommended through the National Comprehensive Cancer Network (NCCN), though the definition of a high risk group remains unclear [28]. High risk features may include large tumor size (>10cm), poorly differentiated tumor, high mitotic index, tumor rupture, and stage 1C as these factors are associated with a higher risk of relapse [30]. Additional risk factors that may also warrant adjuvant therapy include obesity, diabetes, and residual disease [7, 26].
Adjuvant chemotherapy was not significantly associated with DFS in early stage AGCT (p=0.193) [29]. A retrospective study of 40 patients with stage IC primary granulosa cell tumor of the ovary recently found no improvement in disease free survival with adjuvant chemotherapy [31].The relative chemoresistance of this tumor and its predisposition for late recurrence makes the role of chemotherapy in early stage, low risk patients unclear.
There is variability in the cytotoxic treatment regimens for AGCT. The BEP regimen has been the most commonly used and widely studied regimen [32]. Several other regimens have been evaluated including cisplatin, vinblastine, bleomycin [33], cisplatin, doxorubicin, cyclophosphamide [34], taxanes with or without platinum [35]. Given the toxicity associated with chemotherapy, particularly with BEP, use of adjuvant treatment in early stage AGCT should involve a discussion with the patient regarding prognostic factors in the context of treatment toxicity.
4. Treatment of Advanced Stage and Recurrent AGCT
With metastatic or recurrent disease the mainstay of treatment is surgical cytoreduction with the goal to achieve no visible residual disease. In the setting of recurrent disease, a multi-modality approach is recommended. In non-resectable disease, radiation, chemotherapy, and hormonal therapy are reasonable approaches (Figure 1). Most AGCTs recur in the abdominal cavity and complete resection of recurrent tumor offers the best chance for survival [36]. Greater than 80% of recurrent patients can be optimally cytoreduced, though it is frequently technically challenging and can increase morbidity [10, 37]. Either paclitaxel/carboplatin or BEP is preferred for patients with recurrent AGCT of the ovary following complete surgical resection [28, 30].
In a study of 57 patients, 84% of whom had sex cord stromal tumors, only 69% of patients with advanced stage disease and 51% of patients with recurrent disease remained progression free with the BEP regimen [35]. Grade 4 myelotoxicity occurredin 61% of patients and grade 4 gastrointestinal toxicity in 14%. Two bleomycin-related deaths occurred in the first six patients and a dose reduction from 20 units/m ^ 2 weekly for 9 weeks to 20 units/m ^ 2 every three weeks for four in the remaining study population. If BEP is the regimen chosen for use, the bleomycin dose reduction should be used at 20 units/m ^ 2 every three weeks. Pulmonary function tests (PFTs) should be obtained prior to initiation of any treatment which includes bleomycin with low threshold to repeat and compare PFTs during treatment.
In inoperable recurrent disease, a response rate of only 22% was reported with moderate to severe toxicity [38]. A review of fifteen studies that assessed response rates to chemotherapy found in a total of 224 patients with residual or recurrent disease, a measured response rate of 50% was demonstrated (95% CI; 44-57%) [38]. A recent phase two study in patients with malignant stromal tumors evaluated paclitaxel (175mg/m2) over 3 hours as second line therapy [39]. Only one patient (3.2%) had a complete response and 8 patients (25.8%) had a partial response with this regimen [39]. Median PFS in this limited study group was 10.0 months with an OS of 73.6 months. Myelosuppression was also a common side effect, impacting more than a third of participants[39]. Given the small number of complete responses, further study of single agent paclitaxel in the setting of recurrent malignant ovarian stromal tumors with measurable disease was not warranted [39].
Data comparing the efficacy of paclitaxel/carboplatin to standard BEP is still lacking and an ongoing randomized phase 2 trial led by the Gynecological Oncology Group is evaluating this question [40]. One study demonstrated that at 4 years, the OS and event-free survival were 58% and 30% respectively with the BEP. Toxicity included 35% of patients with grade 4 neutropenia, 20% with febrile neutropenia, and 25% with a low bleomycin pulmonary toxicity [41]. Other studies have shown that while there is an overall response to the BEP regimen reported as high as 83%, its effect may be short lived with a mean PFS of only 14 months [42]. The improved toxicity profile associated with paclitaxel/carboplatin compared to BEP make it important to further elucidate if there is a difference in efficacy between these regimens. Prospective clinical trials are needed to further compare the efficacy of carboplatin and paclitaxel vs BEP (Table 2).
*BEP = Belomycin Etoposide and Cisplatin; PFS = Progress Free Survival; SCT = sex cord tumors;
OS = overall survival; AGCT = adult granulosa cell tumor; TRC102 = methoxyamine HCl.
Table 2: Ongoing clinical trials.
Chemotherapy in the recurrent setting appears to have clinical benefit (either complete remission, partial remission or stable disease after 6 months) [27, 29, 43, 44]. Other studies evaluating the role of chemotherapy in the recurrent setting have called into question the clinical benefit with recurrent AGCT with some studies suggesting no benefit [9-12]. Limitations of these studies include the small number of enrolled patients, the various stages of disease, and the variation in chemotherapy regimens used.
The role for radiation therapy with residual disease or in the recurrent setting remains unclear. Response rates are variable across the studies as imaging studies were not as precise at the time the original research was conducted [45-51]. With improvement in targeted radiation, further research is warranted in the role of radiation for treatment of oligometastatic disease.
5. Hormone Therapy/Targeted Therapy/Immunotherapy
The toxicity of BEP or carboplatin/paclitaxel regimens, including pulmonary fibrosis, myelosuppression, and neuropathy, demonstrate the importance of further evaluation of hormonal therapy and targeted agents. Hormonal therapy (HT) is a treatment option for recurrent metastatic or surgically unresectable AGCT [28]. Various agents that have been studied include medroxyprogesterone acetate, megestrol acetate, tamoxifen, aromatase inhibitors and GnRh agonists [28]. Progestins inhibit the production of pituitary gonadotropin, thereby reducing estrogen secretion and interfering with the amount of available estrogen [52]. In one small study, all 22 patients with AGCT were progesterone receptor (PR) positive, suggesting that one additional mechanism for the effectiveness of continuous progesterone is to downregulate receptors on the tumors themselves [53]. GnRH agonists inhibit gonadotropin release, thereby decreasing stimulation of granulosa cells [52]. As most published reports on hormone therapy (HT) are case reports or small studies there are unclear guidelines about patient selection for HT and unclear guidelines for HT regimens. In general, there has been a widely reported range of response to HT in patients with measurable disease: 18% pooled objective response rate (ORR) with a range of 2-34 months PFS to 71% ORR with median PFS of 18 months [28, 54, 55]. The efficacy of HT is further complicated by the wide range of HT combinations showing various results among a limited number of patients who receive HT as an adjunctive therapy. In one small study of 25 patients, only 4 patients who received HT had progressive disease, defined as a greater than 20% increase in the longest lesion diameter [55].
The therapeutic role of aromatase inhibitors (AI) continues to be an area of active study. The proposed mechanism of action is that the FOXL2 missense point binds the aromatase promoter region and increases the activation of aromatase compared to wild type and endogenous estrogen production [56]. Recent data indicates the preservation of the c.402C>G FOXL2 mutation in recurrent AGCTs [57]. Two commonly prescribed AIs for HT are anastrozole (1 mg daily) or letrozole (2.5 mg daily). Switching to a different AI when there is disease progression on the initial AI may provide some clinical benefit [58].
Within the last decade, the development of anti-angiogenic and immunogenic agents may impact the treatment of AGCT. VEGF-A is a mediator of angiogenesis and is intimately involved with tumor growth, migration, and metastasis and is overexpressed in 94% of AGCTs [59]. Bevacizumab, is a monoclonal antibody that blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A). A recent study by Brown et al. demonstrated that 77.8% of patients achieved stable disease with bevacizumab therapy alone [60]. One study showed that inhibin A and B appear to be markers of bevacizumab therapy response as they were significantly lower post-treatment in patients that did respond to bevacizumab [60]. Further study is needed to determine if inhibin A and B could be used as prognostic markers to select patients for response to anti-VEGF therapy. Additionally, several potential immunotherapeutic targets are now available and warrant further study. Given the presence of FOXL2 upregulation in AGCT, this may represent a potential therapeutic target in vaccines [61].
6. Surveillance
Due to the late recurrence of this disease, long term follow-up is necessary. Monitoring inhibin A and B levels can be helpful in detecting recurrence. A study of 27 patients showed that serum inhibin levels increased to 10 times the upper limit of normal for postmenopausal women with evidence of disease [25]. Other studies have confirmed a 60-fold elevation in median inhibin B concentrations with active disease, reporting that disease usually becomes manifest within one year from marker elevation [62]. Inhibin B had a 89% sensitivity and 100% specificity rate and seemed to more accurately reflect disease status than inhibin A and thus may be the preferred method of follow-up [62]. Both inhibin B and AMH seem to predict earlier recurrence of disease compared to monitoring clinical symptoms and no evidence-based preference for either tumor marker exists [19, 20, 23]. Importantly AMH will not become elevated in epithelial ovarian cancers therefore making it an option to be used for follow up [23].
Imaging should be limited to patients who are symptomatic or have concerning findings on physical exam. Serum tumor markers and a physical exam should be assessed every 2-4 months for the first 2 years followed by every 6 months thereafter [63].
7. Survival
Early stage AGCT has an OS rate of 87 and 76% after 5 and 10 years [65]. In another study of 160 early stage patients, 5 and 10-year OS was 98.5 and 91.6% [26]. In comparison, OS for advanced stage disease is worse with 5-year survival rates between 22-50% [17] and 10-year survival rates for stage III and stage IV at 10% and 0%, respectively [65]. AGCT has been described as a very indolent clinical course well known for late recurrence with recurrence reported even 37 years after initial diagnosis [2].
More than 70% of women with recurrence die from their disease [17]. Recurrence varies based on median follow-up period; 7% in 9.2 years, 21% in a median time of 57.6 months, 25% in 4-6 years; 32% in 12 years; 43% in 3.2 years [8, 9, 27, 65]. The pelvis is the most common site of recurrence followed by the liver, bowel and retroperitoneum [37]. Overall mortality rates of patients with recurrent AGCT was 30.3% with a median post-recurrent survival of 55.8 months (4.6-193.7 months) [36]. DFS alone contributed to OS in this study; if patients had DFS > 61.5 months, the risk of disease-related death was significantly decreased [36].
8. Conclusion
AGCT is a rare subtype of ovarian cancer, however, this tumor accounts for over 70% of malignant sex cord stromal tumors. Initial treatment of early stage disease includes primary surgical resection followed with or without adjuvant treatment. Fertility preservation is an option for patients with early stage disease. Late stage disease and recurrent disease management involves multimodality treatment with surgical resection as the mainstay followed by chemotherapy, hormonal therapy, or observation. AGCT classically present with late recurrences which can be managed surgically, with chemotherapy, radiation, hormonal therapy, or a combination of these. Given the rarity of this tumor subtype and its relative chemoresistance future research is warranted to better guide our approach and individualize therapy for patients in the post-surgical phase of treatment. Molecular characterization shows promise however no definitive treatment modalities exist at this time based on these studies. Further evaluation of FOXL2 mutations in AGCT and potential therapeutic interventions represents an important future area of study. Enrollment in clinical trials is encouraged when feasible to further elucidate better treatment options for these patients.
9. Acknowledgements
Patients and families impacted by this cancer.
10. Disclosure of Interests
None declared. Completed disclosure of interests form available to view online as supporting information.
11. Contribution To Authorship
MH and IT performed the initial literature review,MH and JC conceived the ?gure, and MH, IT, AS, NB, and JC participated in the development and revisions of the manuscript.
12. Details of Ethics Approval
Not applicable.
13. Funding
The study was supported solely by departmental funds
References
- Evans III AT, Gaffey TA, Malkasian GD. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol 55 (1980): 231-238.
- Hines JF. Recurrent granulosa cell tumor of the ovary 37 years after inital diagnosis: A case report and review of the literature. Gynecol Oncol 60 (1995): 484-488.
- Jamieson SFP. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr Review 33 (2012): 109-144.
- Malmstrom H, Hogberg T, Risberg B. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol 52 (1994): 50-55.
- Azad K. Cytomorphology of adult granu- losa cell tumor in ascitic fluid. Indian J Pathol Microbiol 53 (2010): 119-121.
- Unkila-Kallio L, Leminen A, Tiitinen A, et al. Nationwide data on falling incidence of ovarian granulosa cell tumours concomitant with increasing use of ovulation inducers. Hum. Reprod 13 (1998): 2828-2830.
- Suri A. Factors associated with an increased risk of recurrence in women with ovarian granulosa cell tumors. Gynecol Oncol 131 (2013): 321-324.
- Thomakos N. Prognostic factors for recurrence in early stage adult granulosa cell tumor of the ovary. Arch Gynecol Obstet 294 (2016): 1031-1036.
- Sun HD, Jao MS, et al. A long-term follow-up study of 176 cases with adult- type ovarian granulosa cell tumors. Gynecol Oncol 124 (2012): 244-249.
- Lee YK, et al. Characteristics of recurrence in adult-type granulosa cell tumor. Int J Gynecol Cancer 18 (2008): 642-647.
- Al-Badawi IA BP, Ghatage P, Nation JG, et al. Postoperative chemotherapy in advanced ovarian granulosa cell tumors. Int J Gynecol Cancer 12 (2002): 119-123.
- Mangili G. Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br J Cancer 109 (2013): 29-34.
- Mancari R. Adult granulosa cell tumours of the ovary. Curr Opin Oncol 26 (2014): 536-541.
- Cheng JC, Klausen C, Leung PC. Oberexpression of wild-type but not C134W mutant FOXL2 enhances GnRH-induced cell apoptosis by increasing GnRH receptor expression in human granulose cell tumors. PLoS One 8 (2013): e55099.
- Alexiadis M, Chu S, Leung D, et al. Transcriptomic analysis of stage 1 versus advanced adult granulosa cell tumors. Oncotarget 7 (2016): 14207-14219.
- Hoffman B, Schorge J, et al. Williams Gynecology, McGraw-Hill Education. (2016).
- Colombo N, Zanagnolo V. Management of ovarian stromal cell tumors. J Clin Oncol 25 (2007): 2944.
- Rey RA, Marcillac I. Antimüllerian hormone as a serum marker of granulosa cell tumors of the ovary: comparative study with serum alpha-inhibin and estradiol. Am J Obstet Gynecol 174 (1996): 958-965.
- Lane AH, Fuller A-FJ, Kehas DJ, et al. Diagnostic utility of Müllerian inhibiting substance determination in patients with primary and recurrent granulosa cell tumors. Gynecol Oncol 73 (1999): 51-55.
- Chang HL, Halpern EF, MacLaughlin DT. Serum Müllerian Inhibiting Substance/anti-Müllerian hormone levels in patients with adult granulosa cell tumors directly correlate with aggregate tumor mass as determined by pathology or radiology. Gynecol Oncol 114 (2009): 57-60.
- Lappöhn RE, Bouma J, Bangah M, et al. Inhibin as a marker for granulosa-cell tumors. N Eng J Med 321 (1989): 790-793.
- Geerts I, Neven P, Billen J. The role of inhibins B and antimüllerian hormone for diagnosis and follow-up of granulosa cell tumors. Int J Gynecol Cancer 19 (2009): 847-855.
- Farkkila. Pathogenesis and treatment of adult-type granulose cell tumor of the ovary. Ann Med 49 (2017): 435-447.
- Ayhan AS, Velipasaoglu M, Sakinci M, et al. Prognostic factors in adult granulose cell tumors of the ovary: a retrospective analysis of 80 cases. J Gynecol Oncol 20 (2009): 158-163.
- van Meurs, et al. Hormone therapy in ovarian granulosa cell tumors: a systematic review. Gynecol Oncol 134 (2013): 196-205.
- Wilson MK. Stage I granulosa cell tumours: A management conundrum? Results of long-term follow up. Gynecol Oncol 138 (2015): 285-291.
- Schumer ST. Granulosa Cell Tumor of the Ovary. J Clin Oncol 21 (2003): 1180-1189.
- NCCN. NCCN - Ovarian Cancer. (2017).
- Park JY. Surgical staging and adjuvant chemotherapy in the management of patients with adult granulosa cell tumors of the ovary. Gynecol Oncol 125 (2012): 80-86.
- Khosla D, Dimri K, Pandey AK, et al. Ovarian granulose cell tumor: clinical features, treatment, outcome, and prognostic factors. N Am J Med Sci 6 (2014): 133-138.
- Mangili G et al. Adjuvant chemotherapy does not improve disease-free survival in FIGO stage 1C ovarian granulose cell tumors: The MITO-9 study. Gynecol Oncol 143 (2016): 276-280.
- Homesley. Bleomycin, Etoposide, and Cisplatin Combination Therapy of Ovarian Granulosa Cell Tumors and Other Stromal Malignancies: A Gynecologic Oncology Group Study. Gynecol Oncol 72 (1999): 131-137.
- Pecorelli SHW, IB Vergote, D Curran, et al. Cisplatin (P), vinblastine (V) and bleomycin (B) combination chemotherapy in recurrent or advanced granulosa(-theca) cell tumours of the ovary. An EORTC Gynaecological Cancer Cooperative Group study. Eur J Cancer 35 (1999): 1331-1337.
- Gershenson DM, Kavanagh JJ, Stringer CA, et al. Treatment of metastatic stromal tumors of the ovary with cisplatin, doxorubicin, and cyclophosphamide. Obstet Gynecol 70 (1987): 765-769.
- Brown J, DeaversMT, Burke TW, et al. The activity of taxanes in the treatment of sex cord-stromal ovarian tumors. J Clin Oncol 22 (2004): 3517-3523.
- Wang PH, et al. Outcome of patients with recurrent adult-type granulosa cell tumors--a Taiwanese Gynecologic Oncology Group study. Taiwan J Obstet Gynecol 54 (2015): 253-259.
- Fotopoulou C, et al. Adult granulosa cell tumors of the ovary: tumor dissemination pattern at primary and recurrent situation, surgical outcome. Gynecol Oncol 119 (2010): 285-290.
- van Meurs HS, Buist MR, Westermann AM, et al. Effectiveness of chemotherapy in measurable granulosa cell tumors: a retrospective study and review of literature. Int J Gynecol Cancer 24 (2014): 496-505.
- Burton ER. A phase II study of paclitaxel for the treatment of ovarian stromal tumors: An NRG Oncology/ Gynecologic Oncology Group Study. Gynecol Oncol 140 (2016): 48-52.
- Gurumurthy M. Effectiveness of different treatment modalities for the management of adult-onset granulosa cell tumours of the ovary (primary and recurrent). Cochrane Database Syst Rev 4 (2014): CD006912.
- Pautier P. Combination of bleomycin, etoposide, and cisplatin for the treatment of advanced ovarian granulosa cell tumors. Int J Gynecol Cancer 18 (2008): 446-452.
- Gershenson DM, Burke TW, Levenback C, et al. Treatment of poor-prognosis sex cord-stromal tumors of the ovary with the combination of bleomycin, etoposide, and cisplatin. Obstet Gynecol 87 (1996): 527-531.
- Uygun K, Saip P, et al. Clinical parameters and treatment results in recurrent granulosa cell tumor of the ovary. Gynecol Oncol 88 (2003): 400-403.
- Meisel J. The role of systemic chemotherapy in the management of granulosa cell tumors. Gynecol Oncol 136 (2015): 505-511.
- Choan E, Samant R, Fung M, et al. Palliative radiotherapy for recurrent granulosa cell tumor of the ovary: a report of 3 cases with radiological evidence of response. Gynecol Oncol 102 (2006): 406-410.
- Engle EB. Roentgen treatment of granulosa cell carcinoma of the ovary. Amer J Roentgenol 80 (1958): 793.
- Hauspy J, Beiner ME, Harley I, et al. Role of adjuvant radiotherapy in granulosa cell tumors of the ovary. International Journal of Radiation Oncology, Biology, Physics 79 (2011): 770-774.
- Lee I, Levin W, Chapman W, et al. Radiotherapy for the treatment of metastatic granulosa cell tumor in the mediastinum: a case report. Gynecol Oncol 73 (1999): 455-460.
- Ohel G, Kaneti H, Schenker J. Granulosa cell tumors in Israel: a study of 172 cases. Gynecol Oncol 15 (1983): 278-286.
- Savage P, Constenla D, Fisher C, et al. Granulosa cell tumours of the ovary: demographics, survival and the management of advanced disease. Clinical Oncology (Royal College of Radiology) 10 (1998): 242-245.
- Wolf J, Mullen J, Eifel P, et al. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecologic Oncology 73 (1999): 35-41.
- Teoh D, Freedman R, Soliman PT. Nearly 30 years of treatment for recurrent granulosa cell tumor of the ovary: a case report and review of the literature. Case Rep Oncol 3 (2010): 14-18.
- Hardy RD, et al. Hormonal treatment of a recurrent granulosa cell tumor of the ovary: case report and review of the literature. Gynecol Oncol 96 (2005): 865-869.
- van Meurs HS. Development and internal validation of a prognostic model to predict recurrence free survival in patients with adult granulosa cell tumors of the ovary. Gynecol Oncol 134 (2014): 498-504.
- van Meurs HS. Evaluation of response to hormone therapy in patients with measurable adult granulose cell tumors of the ovary. Acta Obstet Gynecol Scand 94 (2015): 1269-1275.
- Fleming NI, Knower KC, Lazarus KA, et al. Aromatase is a direct target of FOXL2: C134W in granulosa cell tumors via a single highly conserved binding site in the ovarian specific promoter. PLoS One 5 (2010) :e14389.
- Yanagida S. Clinical and genetic analysis of recurrent adult-type granulosa cell tumor of the ovary: Persistent preservation of heterozygous c.402C>G FOXL2 mutation. PLoS One 12 (2017): e0178989.
- Schwartz M, Huang GS. Retreatment with aromatase inhibitor therapy in the management of granulosa cell tumor. Gynecol Oncol Rep 15 (2016): 20-21.
- Tsoi M. Anti-VEGFA Therapy Reduces Tumor Growth and Extends Survival in a Murine Model of Ovarian Granulosa Cell Tumor. Transl Oncol 6 (2013): 226-233.
- Brown JBW, Schink J. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: results of a phase 2 trial of the GynecologicOncology Group. Cancer 120 (2014): 344-351.
- University of Pennsylvania School of Medicine (2016).
- Mom CH. Granulosa cell tumors of the ovary: the clinical value of serum inhibin A and B levels in a large single center cohort. Gynecol Oncol 105 (2007): 365-372.
- Salani. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. AJOG 204 (2011): 466-478.
- Sehouli J. Granulosa Cell Tumor of the Ovary: 10 Years Follow-up Data of 65 Patients. Anticancer Research 24 (2004): 1223-1230.
- Mangili G. Recurrent granulosa cell tumors (GCTs) of the ovary: a MITO-9 retrospective study. Gynecol Oncol 130 (2013): 38-42.
