Additional Treatment for Digital Ulcers in Patients with Systemic Sclerosis A Prospective Open-Label Multi-Arm Study for the use of Platelet Rich Plasma Lipofilling and Ultrasound-Based Treatments
Article Information
Pirrello R1, Schinocca C2, Scaturro D3*, Rizzo C2, Terrana P3, Tumminelli L3, Ruscitti P4, Cordova A1, Giacomelli R5, Ciccia F6, Letizia Mauro G3, Guggino G2
1Department of Oncological, Stomatological and Surgical Disciplines - Sezione di Chirurgia Plastica e Ricostruttiva- University Hospital "P. Giaccone", Palermo, Italy
2Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, Rheumatology section - University Hospital “P. Giaccone”, Palermo, Italy
3Department of surgical, Oncological, Stomatological Disciplines, Rehabilitation unit, University of Palermo, Italy
4Department of Biotechnological and Applied Clinical Science, Rheumatology Unit, School of Medicine - University of L'Aquila, Italy
5Unit of Allergology, Immunology, Rheumatology, Department of Medicine - University “Campus Bio-Medico”, Rome, Italy
6Department of Precision Medicine - University of Campania "Luigi Vanvitelli”, Naples, Italy
*Corresponding Author: Dalila Scaturro, Department of surgical, Oncological, Stomatological Disciplines, Rehabilitation unit, University of Palermo, Italy
Received: 16 June 2021; Accepted: 22 June 2021; Published: 13 July 2021
Citation:
Pirrello R, Schinocca C, Scaturro D, Rizzo C, Terrana P, Tumminelli L, Ruscitti P, Cordova A, Giacomelli R, Ciccia F, Letizia Mauro G, Guggino G, Additional Treatment for Digital Ulcers in Patients with Systemic Sclerosis: A Prospective, Open-Label, Multi-Arm Study for the use of Platelet-Rich Plasma-Lipofilling and Ultrasound-Based Treatments. Journal of Biotechnology and Biomedicine 4 (2021): 84-107.
View / Download Pdf Share at FacebookAbstract
Background: Local treatments such as ultraviolet-A (UVA) phototherapy, topical calcitriol, injection of autologous fat grafting, Platelet-Rich Plasma (PRP), hyaluronic acid (HA) and local ultrasound (US) treatment are considered alternative approaches for skin involvement in Systemic sclerosis (SSc). The aim of our study was to evaluate the efficacy of PRP injection and lipofilling or local ultrasound in the treatment of SSc-related digital ulcers (DUs).
Methods: We enrolled 28 patients with SSc. At baseline time (T0), all patients were treated with Iloprost intravenous infusions. Then, twelve patients (group 1) received a first inoculation of PRP, after 15 days a second inoculation of PRP and after 15 days a third of lipofilling. Six patients continued only the Iloprost therapy (controls). Ten patients (group 2) underwent medical sessions with ultrasound treatment for 10 days. Clinical evaluation was assessed at baseline, after 3 and 12 months of treatment for all patients.
Results: In our study have shown an improvement in cutaneous and microvascular level, in quality of life, in mobility of extremities of upper limbs and a reduction of administration of Iloprost after PRP-Lipofilling or US treatment.
Conclusions: Our findings suggest that PRP coupled with lipofilling or ultrasound treatment in SSc patients, can be considered additional procedures in the management of DUs.
Keywords
Systemic sclerosis; Digital ulcers; Lipofilling; PRP; Ultrasound
Systemic sclerosis articles Systemic sclerosis Research articles Systemic sclerosis review articles Systemic sclerosis PubMed articles Systemic sclerosis PubMed Central articles Systemic sclerosis 2023 articles Systemic sclerosis 2024 articles Systemic sclerosis Scopus articles Systemic sclerosis impact factor journals Systemic sclerosis Scopus journals Systemic sclerosis PubMed journals Systemic sclerosis medical journals Systemic sclerosis free journals Systemic sclerosis best journals Systemic sclerosis top journals Systemic sclerosis free medical journals Systemic sclerosis famous journals Systemic sclerosis Google Scholar indexed journals Digital ulcers articles Digital ulcers Research articles Digital ulcers review articles Digital ulcers PubMed articles Digital ulcers PubMed Central articles Digital ulcers 2023 articles Digital ulcers 2024 articles Digital ulcers Scopus articles Digital ulcers impact factor journals Digital ulcers Scopus journals Digital ulcers PubMed journals Digital ulcers medical journals Digital ulcers free journals Digital ulcers best journals Digital ulcers top journals Digital ulcers free medical journals Digital ulcers famous journals Digital ulcers Google Scholar indexed journals Lipofilling articles Lipofilling Research articles Lipofilling review articles Lipofilling PubMed articles Lipofilling PubMed Central articles Lipofilling 2023 articles Lipofilling 2024 articles Lipofilling Scopus articles Lipofilling impact factor journals Lipofilling Scopus journals Lipofilling PubMed journals Lipofilling medical journals Lipofilling free journals Lipofilling best journals Lipofilling top journals Lipofilling free medical journals Lipofilling famous journals Lipofilling Google Scholar indexed journals PRP articles PRP Research articles PRP review articles PRP PubMed articles PRP PubMed Central articles PRP 2023 articles PRP 2024 articles PRP Scopus articles PRP impact factor journals PRP Scopus journals PRP PubMed journals PRP medical journals PRP free journals PRP best journals PRP top journals PRP free medical journals PRP famous journals PRP Google Scholar indexed journals Ultrasound articles Ultrasound Research articles Ultrasound review articles Ultrasound PubMed articles Ultrasound PubMed Central articles Ultrasound 2023 articles Ultrasound 2024 articles Ultrasound Scopus articles Ultrasound impact factor journals Ultrasound Scopus journals Ultrasound PubMed journals Ultrasound medical journals Ultrasound free journals Ultrasound best journals Ultrasound top journals Ultrasound free medical journals Ultrasound famous journals Ultrasound Google Scholar indexed journals Mesenchymal stem cells articles Mesenchymal stem cells Research articles Mesenchymal stem cells review articles Mesenchymal stem cells PubMed articles Mesenchymal stem cells PubMed Central articles Mesenchymal stem cells 2023 articles Mesenchymal stem cells 2024 articles Mesenchymal stem cells Scopus articles Mesenchymal stem cells impact factor journals Mesenchymal stem cells Scopus journals Mesenchymal stem cells PubMed journals Mesenchymal stem cells medical journals Mesenchymal stem cells free journals Mesenchymal stem cells best journals Mesenchymal stem cells top journals Mesenchymal stem cells free medical journals Mesenchymal stem cells famous journals Mesenchymal stem cells Google Scholar indexed journals micro vasculopathy articles micro vasculopathy Research articles micro vasculopathy review articles micro vasculopathy PubMed articles micro vasculopathy PubMed Central articles micro vasculopathy 2023 articles micro vasculopathy 2024 articles micro vasculopathy Scopus articles micro vasculopathy impact factor journals micro vasculopathy Scopus journals micro vasculopathy PubMed journals micro vasculopathy medical journals micro vasculopathy free journals micro vasculopathy best journals micro vasculopathy top journals micro vasculopathy free medical journals micro vasculopathy famous journals micro vasculopathy Google Scholar indexed journals monocentric articles monocentric Research articles monocentric review articles monocentric PubMed articles monocentric PubMed Central articles monocentric 2023 articles monocentric 2024 articles monocentric Scopus articles monocentric impact factor journals monocentric Scopus journals monocentric PubMed journals monocentric medical journals monocentric free journals monocentric best journals monocentric top journals monocentric free medical journals monocentric famous journals monocentric Google Scholar indexed journals microcirculation articles microcirculation Research articles microcirculation review articles microcirculation PubMed articles microcirculation PubMed Central articles microcirculation 2023 articles microcirculation 2024 articles microcirculation Scopus articles microcirculation impact factor journals microcirculation Scopus journals microcirculation PubMed journals microcirculation medical journals microcirculation free journals microcirculation best journals microcirculation top journals microcirculation free medical journals microcirculation famous journals microcirculation Google Scholar indexed journals visualization articles visualization Research articles visualization review articles visualization PubMed articles visualization PubMed Central articles visualization 2023 articles visualization 2024 articles visualization Scopus articles visualization impact factor journals visualization Scopus journals visualization PubMed journals visualization medical journals visualization free journals visualization best journals visualization top journals visualization free medical journals visualization famous journals visualization Google Scholar indexed journals
Article Details
Abbreviations:
ACD- Acid citrate dextrose; ADSCs- Adipose-derived stromal cells; bFGF- Fibroblast growth factor; BM-MSC- Bone marrow-MSC; CTGF- Connective tissue growth factor; DHI- Duruoz Hand Index; DS- Standard deviation; DUs- Digital ulcers; EGF- Epidermal growth factor; EULAR- European League Against Rheumatism; EUSTAR- EULAR Scleroderma Trials and Research; HA-Hyaluronic acid; HAQ- Health Assessment Questionnaire; HGF- Hepatocyte growth factor; IGF- Insulin-like growth factor; MSC- Mesenchymal stem cells; MRSS- Modified Rodnan Skin Score; NVC- Nailfold video capillaroscopy; PDGF- Platelet-derived growth factor; PGI2- Prostaglandin I-2;PPP- Platelet-poor plasma; PRP- Platelet-Rich Plasma; RBC- Red blood cells; SSc- Systemic sclerosis; SVF- Stromal vascular fraction; TGF: Transforming growth factor; US- Ultrasound; UVA- Ultraviolet-A; VAS- Visual Analogue Scale; VEGF- Vascular endothelial growth factor; WB- Whole blood.
1. Introduction
Systemic sclerosis (SSc) is a chronic connective tissue disease caused by microvascular damage, inflammation and excessive deposition of collagen in the skin and internal organs [1].
Etiology of SSc is still largely unknown but both genetic and environmental factors contribute to disease development [2].
The initial stage of the pathogenetic process seems to be injury to endothelial cells, followed by aberrant vascular and immune responses that lead to the excessive deposition and accumulation of extracellular matrix [3, 4]. The resultant progressive tissue remodeling can subvert tissue architecture with the consequent possible loss of organ function in end stage disease [5].
Incidence of SSc is estimated between 4 and 43/million person years with a prevalence between 88 and 443/million [6-8]. SSc affects prevalently women with a female: male ratio of 3-6:1 [9].
The main clinical features are thickening and fibrosis of the skin and internal organs as results of progressive vasculopathy [10]. Most of patients with SSc experience marked disability, especially of hands, because of puffy digits, digital ulcers (DUs), skin sclerosis, cutis calcinosis and pruritus [11]. In particular DUs are the most common complication derived from SSc-associated micro vasculopathy and more than 50-70% of SSc patients report a history of DUs. These lesions are recurrent and serve as biomarkers of overall disease severity and organ involvement. Their presence reflects the ongoing severe ischemic damage that affects vessels in SSc. DUs are characterized by loss of continuity and depth in the skin, that can be covered by an eschar or necrotic tissue [12, 13]. Patients presenting with DUs experience extreme pain which causes hand dysfunction and negatively impacts on daily activities and work production with a consequent marked disability. In addition, the presence of DUs requires a tight follow up with continuous assessment for possible complications, such as infections that can involve both skin and bone resulting in osteomyelitis [14, 15].
Currently, SSc treatment is focused on different pharmacological interventions based upon evidence-based recommendations published by European League Against Rheumatism
(EULAR) and EULAR Scleroderma Trials and Research (EUSTAR) groups in 2009 [16].
Although there are no specific pharmacological treatments for skin involvement in SSc, many strategies have been tried [17-19], such as corticosteroids and immunomodulators [20], UVA phototherapy [21-23], topical calcitriol [24] and retinoids [25]. Moreover, several local therapies have also been investigated to guarantee a satisfactory aesthetic and functional result, especially for facial fibrotic skin changes, such as injection of autologous fat grafting and Platelet-Rich Plasma (PRP) [26, 27] or of a combination of adipose-derived stromal cells (ADSCs) in hyaluronic acid (HA) solution [28] or of HA and PRP [29].
Up to date, the management of DUs relies on systemic treatment such as prostanoids or vasodilator drugs and local therapies, which mainly consist in surgical debridement and application of ointment or specific wound dressing such as hydrocolloids. Severe refractory cases or untreatable osteomyelitis may still require amputation. In rheumatological clinical practice defining new procedures to treat DUs represents a major need and a growing interest on different techniques is emerging. The aim of our study was to compare the clinical response on SSc-related DUs in patients receiving local therapy with PRP injection and lipofilling or local ultrasound treatment. All the patients were treated with conventional therapy (Iloprost).
2. Material and Methods
2.1 Patients
This prospective, open-label, monocentric, multi-arm, randomized study was conducted on patients with SSc recruited from the Rheumatology Section of Policlinico “Paolo Giaccone” - University Hospital of Palermo.
We enrolled 28 consecutive patients (19 females, 9 males) aged between 38-55 years, with SSc diagnosed according to the 2013 American College of Rheumatology/European League Against Rheumatism classification criteria for SSc [30-32]. All patients showed skin ulcers.
Concomitant infectious disease (i.e., HIV, HBV, HCV, syphilis), cancer, neurodegenerative or chronic heart disease were considered exclusion criteria.
This study was approved by the Ethical Committee of the University Hospital of Palermo and informed consent was obtained from each patient in accordance with the Helsinki Declaration. (Registration number n.9/2016,19102016).
All patients were treated with Iloprost intravenous (iv) infusions, the conventional therapy for DUs. Iloprost is a synthetic prostacyclin analogue characterized by more stability and longer half-life compared to epoprostenol, that is the pharmacological name for the prostaglandin I-2 (PGI2), which is a natural metabolite of arachidonic acid and is produced by endothelial cells. Iloprost acts mimicking the pharmacodynamic properties of PGI2, mainly consisting in inhibition of platelet aggregation and vasodilatation [33]. 75% of patients received treatment with 6-hours Iloprost infusion for 5 consecutive days every 4 weeks, while 25% of patients received the 6-hours infusion treatment every 2 weeks.
2.2 Physical assessment
Cutaneous sclerosis and adhesion of the skin to underlying planes, skin elasticity, skin texture, skin appendages, dyschromia, skin sensitivity, functionality of microcirculation, pain, mobility, and functionality of extremity of upper limbs and quality of life were assessed at baseline (T0), after 3 months (T1) and after 12 months (T2) of treatment for all patients (group 1 and controls).
Group 2 patients were evaluated at baseline (T0) and 3 months after the last ultrasound session (T1). The appearance of new ulcers, skin sclerosis and adhesion of the skin to the underlying planes, the mobility and functionality of the upper limbs, the extent of pain, and quality of life were the parameters taken into consideration in this group of patients.
Furthermore, DUs evaluation was performed at baseline (T0), after 3 months (T1) and after 12 months (T2) of treatment for all patients (group 1, group 2 and controls).
Patients’ clinical characteristics are described in Table 1.
|
Patients n. 28 |
Value |
|
Women, n. (%) |
19 (67.9) |
|
Raynaud, n. (%) |
28 (100) |
|
Digital ulcers, n. (%) |
28 (100) |
|
Scleroderma pattern early/active/late n. (%) |
16 (57.1)/5 (17.9)/ 7 (25) |
|
mRSS (mean value) |
12±2 |
|
VAS pain (mean value) |
5.75±1.5 |
|
HAQ (mean value) |
1,8±0.4 |
|
Iloprost therapy/2 weeks, n. (%) |
7 (25) |
|
Iloprost therapy/4 weeks, n. (%) |
21 (75) |
Table 1: Clinical characteristics of patients at baseline.
mRSS: modified Rodnan skin score.
VAS: visual analogue scale.
HAQ: health assessment questionnaire.
2.3 Cutaneous sclerosis and adhesion of the skin to underlying planes
Cutaneous fibrosis causes adhesion of the skin to underlying planes, this results in the difficulty to lift the skin of phalanges in folds. The Modified Rodnan Skin Score (MRSS) was calculated to assess the extension of cutaneous sclerosis [34].
In MRSS skin thickening was assessed by palpation of the skin in 17 areas of the body (fingers, hands, forearms, arms, feet, legs and thighs, face, chest, and abdomen) using a 0–3 scale, where 0 = normal, 1 = mild thickness, 2 = moderate thickness and 3 = severe thickness. Total skin score can range from 0 (no thickening) to 51 (severe thickening) [35].
2.4 Skin elasticity
Skin elasticity was measured with a Skin Elastometer device, a hand-held device that permit, using a probe, a non-invasive quantification of elastic properties of skin.
The probe was placed on the left cheek and above the left upper lip of patients. The skin was aspirated for 3 seconds with a negative pressure of 400 mbar, and after other 3 seconds were needed for the skin to return to its initial position. The value, shown as a percentage, has to be related to patient age because skin elasticity could decrease with increasing age.
2.5 Microcirculation, Raynaud phenomenon, Digital ulcers
The presence of Raynaud phenomenon and/or DUs, their extension, associated pain and onset frequency were assessed by clinical examination. Microcirculation was assessed by nailfold video capillaroscopy (NVC), a highly sensitive, non-invasive imaging technique used to analyze and quantify capillary abnormalities in the nailfold area [36]. NVC was performed by using an optical probe videocapillaroscope equipped with 100x and 200x contact lenses and connected to image analysis software (Videocap; DS MediGroup, Milan Italy). The naifolds of fingers were examined in each patient; a drop of immersion oil was placed on the naifold bed to allow the proper visualization of the microcirculation. Capillary abnormalities were classified, accordingly to Cutolo et al., into three patterns called scleroderma pattern "early", "active" and "late" respectively [37].
2.6 Hypomobility and functionality of upper limbs extremity
Mobility and functionality of acral upper limbs were assessed by physical examination and Duruoz Hand Index (DHI). DHI is a self-report questionnaire designed to evaluate the capacity to carry out manual tasks with no assistance or aids [38, 39]. It consists of 18 questions regarding manual activities which are grouped in 5 principal domains: kitchen, dressing, hygiene, work and other activities. The patient is asked to evaluate the degree of difficulty he/she experiences in completing these tasks (each item can be scored from 0 = no difficulty to 5 = impossible) [40].
2.7 Pain
Pain was assessed by the pain Visual Analogue Scale (VAS). The pain VAS is a straight, 100-mm line (10 cm) that represents continuous pain intensity, where the extremities indicate “no pain” and “pain as bad as it could possibly be.” Patients were asked to indicate their level of pain (in mm), by marking a single point on the line [41].
2.8 Quality of life
Quality of life was assessed by Health Assessment Questionnaire (HAQ). HAQ includes 8 sections: dressing, arising, eating, walking, hygiene, reach, grip, and activities. There are 2 or 3 questions for each section. Scoring within each section ranges from 0 (without any difficulty) to 3 (unable to do). The 8 scores of the 8 sections are summed and divided by 8 to obtain the final HAQ score [42].
2.9 Platelet-rich plasma (PRP)
Platelet-rich plasma or platelet gel (PRP) is an autologous thrombocyte concentrate derived from whole blood. PRP not only contains high levels of platelets but is also enriched with growth factors, cytokines, chemokines, and several plasma proteins. This specific composition accounts for PRP pleiotropic effects, especially in regenerative medicine. In recent years, PRP injections have gained considerable attention as a treatment option for musculoskeletal conditions due to their safety and ability to potentially improve soft tissue healing [43].
PRP stimulates recruitment, proliferation and differentiation of cells involved in tissue regeneration through the release from platelets of different growth factors, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), transforming growth factor (TGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), fibroblast growth factor (bFGF) and connective tissue growth factor (CTGF).
In addition, platelets produce cytokines and chemokines which prevent the massive recruitment of leukocytes, thus contributing to the regulation of inflammatory responses and immunological aspects of tissue healing. PRP also contains proteins known to act as cell adhesion molecules, such as fibrin, fibronectin and vitronectin [44].
Considering the properties of platelets and in particular their regenerative potential, it was decided to use a platelet gel for wound healing process. In our study PRP was prepared by centrifuge (EBA 200, Hettic), accordingly to the following procedure [45]:
-Obtain WB (whole blood) by venipuncture in acid citrate dextrose (ACD) tubes.
-Do not chill the blood at any time before or during platelet separation.
-Centrifuge the blood using a ‘soft’ spin, to separate red blood cells (RBC) from the remaining WB volume.
-Transfer the supernatant plasma containing platelets into another sterile tube (without anticoagulant).
-Centrifuge tube at a higher speed (a hard spin) to obtain a platelet concentrate.
-The lower 1/3rd is PRP and upper 2/3rd is platelet-poor plasma (PPP). At the bottom of the tube, platelet pellets are formed.
-Remove PPP and suspend the platelet pellets in a minimum quantity of plasma (2-4 mL) by gently shaking the tube.
About 15 minutes before injection, anesthetic cream or lidocaine spray was used on the patient hands to reduce pain. We gave injections in digital areas and, at the end of each treatment, an antibacterial cream containing gentamicin was used to prevent infection; when necessary, the additional application of a specific cream for bruises was advised. Injections were given in the clinic. No major side effects or complication occurred; the only side effect reported were some bruises
2.10 Lipofilling
Lipofilling is a surgical protocol standardized by Coleman in 1997 [46] which can be used to correct soft tissue defects of the face, trunk and extremities, with minimal discomfort for patients [47]. It consists in removing adipose tissue from donor areas such as the abdomen, thighs or hip area and subsequently transplant it to the receiving areas. Adipose tissue stands out as a “perfect filler” as it is autologous, hypoallergenic, biocompatible, and abundantly available. In addition, there are no risks of rejection or need for immunosuppressive therapy which make this procedure appealing and promising for patients affected by autoimmune systemic disease, such as SSc [48].
The lipoaspirate derived from adipose tissue is made up for 2/3 of mature adipocytes, while the remaining 1/3 is composed by the stromal vascular fraction (SVF) which consists of a heterogeneous population of cells, including endothelial cells, erythrocytes, fibroblasts , pericytes, lymphocytes, monocytes/macrophages and an abundant quote of adipose derived stem cells (ADSCs) [49].
These latter are a population of adult stem cells, multipotent and able to differentiate into different cell types [50, 51].
ADSCs, in addition, have a high angiogenic and anti-apoptotic potential, as they secrete multiple growth factors, which work synergistically for pro-angiogenic purposes. In particular, ADSCs secrete VEGF, HGF and TGF [44]. These molecules, which are crucial for tissue regeneration, allow a remodeling of the environment in which they are transplanted thanks to a fine regulation of several processes: proliferation and differentiation of stem cells, promotion of angiogenesis and growth of microcirculatory tree, modulation of immune activity of self-reactive T and B lymphocytes, decrease in production of pro-inflammatory cytokines and increase in anti-inflammatory counterpart that leads to a generalized reduction of inflammation [52-55].
The surgical procedure adopted in our center consisted in small skin incisions in the donor areas, through which lipoaspiration cannulas with a tip of 3 mm diameter (10 gauge) were inserted. Lipoaspiration was carried out manually in order to preserve as much as possible the integrity of adipocytes and stem cells present in the adipose tissue. The lipoaspirate was left for 20 minutes inside syringes in a vertical position to facilitate the separation of the fat components from the liquid components (oily, blood), according to gravity.
Sedimented fat was extracted and placed in a 1 ml syringe with Luer Lock type attack connected to a long lipofilling cannula of the caliber of 1.5 mm (17 gauge), that was used to graft into the subcutaneous plane of the hands (Figure 1).
Before the grafting, a loco-regional anesthesia was performed with adrenaline and 2% mepivacaine. Four skin entrances were prepared to allow the cannula to pass through the skin.
The first incision was obtained by performing a punch biopsy in the proximal portion of the back of the first phalanx and fat was delivered through the cannula in the form of thin, parallel, and contiguous filaments.
The next three incisions were practiced using a scalpel blade on dorsal interdigital skin of each finger. Fat was then injected linearly in contiguous and non-communicating tunnels in the subcutis in the back of the hand.
Finally, the incisions were closed with stitches and none of the patients developed scars.
The procedure was performed in the operating room through assisted local anesthesia. Only minor side effect such as seromas, hematomas and non-severe infections were reported.
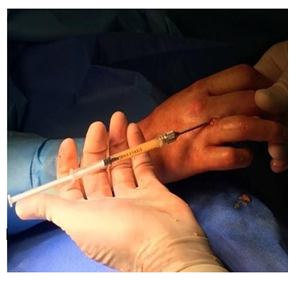
Figure. 1: Representative lipofilling execution.
2.11 Ultrasound
Ultrasounds (US) are mechanic sound waves with frequencies higher than the upper audible limit of human hearing, recently applied as treatment for wounds healing. US work through a complex interaction between thermic, mechanic, chemical and cavitation stimuli. They exert a wide range of biologic effects and can contribute to decrease inflammation, increase cellular recruitment and proliferation, boost collagen synthesis and promote angiogenesis, wounds contraction and fibrinolysis [56-58].
Moreover, US lower frequencies, applied at wound level, determine a decrease in the bacterial count suggesting a potential additional antibacterial effect [59].
In fact, several parameters related to US device can be modulated, such as frequency, expressed in kHz or MHz; capacity in terms of intensity W/cm2; release mode that can be pulsed or continuous and treatment interval time period. Via frequency modulation, the impact of US into tissues can be strictly controlled [60, 61].
In our study I-Tech UT2, a medical device, CE0476 certified, intended for medical qualified personal only was used. The US-dipping technique, with a frequency of 1 MHz, capacity of 1 W/cm2, duty cycle of 60% for 15 minutes a day, was employed. The aim of this US treatment, set with the described parameters, was to increase vasodilatation [56].
This technique requires the hand immersion in a sanitized steel bowl of 90 cm diameter, containing 4 litres of water at a temperature between 37-37,5° CA handpiece (5 cm²) is located inside the bowl, 2 cm from the body surface area.
2.12 Study protocol
Our therapeutic protocol was based on the infiltration of distal extremities of the upper limbs, with two different autologous components: PRP and adipose tissue (lipofilling).
Standard protocol consisted in three consecutive injections, performed every 15 days (first inoculation of the PRP, second inoculation of the PRP and execution of lipofilling) associated with conventional iv Iloprost therapy.
No pain or discomfort were reported at the end of each treatment and patients came back home after the procedure with no need for observation and/or hospitalization.
All patients (group 1) received the standard protocol, while six patients continued only the Iloprost therapy (controls).
Ten patients (group 2) were allocated to the US-based treatment coupled with Iloprost infusions. They underwent ten daily medical sessions with US treatment, from Monday to Friday in two consecutive weeks.
There were no substantial differences between the groups at baseline.
No dropout was recorded.
2.13 Statistical analysis
Results are expressed as mean ± standard deviation (DS). To analyze differences between outcomes measured at different time period, we used both parametric and non-parametric analysis.
Parametric analysis was conducted using standard one-way repeated measures ANOVA. Non-parametric analysis was implemented using the Friedman test. The null hypothesis for the Friedman test was that there were no differences between the outcomes measured at different time period. Finally, to investigate whether treatment was persistent, we also relied on the non-parametric Wilcoxon signed-rank test to compare mean ranks of each period with the baseline measured at time 0. p < 0.05 was considered significant.
3. Results
3.1 Cutaneous modification after PRP-Lipofilling treatment
At T0 the evaluation of skin thickness through the lifting of the skin was impossible in 37.5% of patients, difficult in 37.5% and possible with only mild difficulty in 25%.
After treatment (T2) the skin of all patients (group 1) appeared more elastic and less rigid, despite adhesion to the underlying planes still remained, but as shown in figure 2A mRSS values were not statistically different between groups at different time points.
At T0 skin elasticity (evaluated on the back of the hand and on the fingers) was reduced in all patients and there were no substantial differences among groups and controls at baseline. Data obtained by elastometry, as shown in figure 2B-C, failed to show an improvement at T2, after standard protocol in all patients when compared to baseline, although elastometry showed an increase in skin elasticity already from the day after the administration which lasted for the following weeks and then gradually returned to a stable condition until the next inoculation.
All patients had alterations in the skin appearance of distal extremity of the upper limb at baseline.
An improvement in skin texture was found at T1 in 65% of patients (group 1) and at T2 in 100% of patients (group 1) and in 20% of controls (Figure 3A). Skin appeared smoother, softer, more resistant to insults or trauma, more toned and showed an improvement in atrophy without evident signs of chaps and abrasions.
Skin appendages, normally located on the whole back of the hand including the phalanges were reduced and thinned in patients examined at T0.
A generalized regrowth of skin appendages was evidenced in 70% of patients (group 1) at T1 and in 100% of the patients (group 1) at T2 respectively. Only 30 % of controls showed an improvement. These results could be related to an increase in the micro vascularization related to local treatment (Figure 3B).
Chromatic alterations of the distal extremity of the upper limb were evidenced in all patients at baseline. In particular, 60% of patients showed only a slight chromatic variation (Figure 3C) while 30% had a salt and pepper type dyschromia. At T1 and T2 we observed a slight improvement of chromatic skin alterations of patients. Skin appeared pinker and vital, because of the increased blood supply, even if the "salt and pepper" aspects persisted.
At baseline 100% of patients reported alterations of thermal and tactile sensitivity and 25% of these could not even perceive objects on their skin.
Sixty % of patients at T1 (group 1) and 100% of patients at T2 (group 1) reported an improvement in terms of skin sensitivity at the distal extremities (figure 3D).
Seventy-five % of controls did not show any improvement.
3.2 Evaluation of microvascular alteration after PRP-Lipofilling treatment: Raynaud phenomenon, NVC and DUs
At baseline all patients experienced Raynaud phenomenon and NVC examination showed a scleroderma pattern "late" in 37.5% , "active" in 37.5% and "early" in 25% of patients respectively.
All patients (group 1), despite the Raynaud phenomenon continuing to occur, found a marked improvement at T1 and T2 in terms of reduction in frequency, duration, and intensity of attacks.
In 100% of patients (group 1), already after 3 months (T1), there was a significant increase in the density of the capillary tree and a decrease in the number and severity of various aberrant microcirculatory changes (e.g. capillary chains were more regular, capillary ectasias and microhemorrhages in some cases disappeared) (figure 4A).
At T0 all patients had DUs, that appeared with extreme frequency. DUs course was long with an average duration of 3-6 months, their presence was associated with severe pain, disability, and risk of infection.
As shown in Figure 4B, although only a reduction in the number of DUs was observed at T1, the total number of new ulcers in all patients (group 1) was not statistically different after 12 months from PRP and lipofilling treatment. Specially, 25% of T1 patients, 90% of T2 patients and 90% of controls experienced new ulcers (data not shown).
However, the new lesions were fewer, smaller, shallower, less painful, did not tend to become infected, and showed rapid resolution compared to lesions present at T0. There were no substantial differences between the groups at baseline.
All patients at baseline reported the presence of pain in the extremities of upper limbs that was associated mainly with DUs, but also with Raynaud phenomenon or arthralgias. As shown in figure 4C, pain improved significantly in almost all T2 patients (group 1) respect to controls. The improvement can be attributed to the reduction of the Raynaud phenomenon and ulcers occurrence.
3.3 Quality of life
Before treatment, all patients reported a significant physical and emotional discomfort, which compromised their social and working life. After treatment, a gradual and statistically significant improvement of quality of life was shown in patients respect to controls as demonstrated by the reduction of HAQ score values (figure 4D).
3.4 Hypomobility and functionality of extremity of upper limbs - DHI
At baseline we found in almost all patients examined showed hypomobility with difficulty in performing various daily manual activities. In particular 37.5% of patients had severe hypomobility, related to ankylosis of joint capsules or contracture in flexion of ligaments and tendons of hands due to fibrosis; while 62.5% of patients had only minimal loss of normal hand functions without marked signs of structural damage. At T1 90% of patients (group 1) reported an improvement in mobility. In particular, the 37.5% of patients who suffered from more serious hand impairment, found a marked improvement, with a partial recovery in the ability to open fingers and an increase in phalangeal and interphalangeal mobility. At T2 63 % of all patients (group 1) reported persistence of improvement over time. Sixty % of controls showed improvement at different time points. (Figure 5A).
3.5 In fusional therapy with Iloprost
As shown in figure 5B at baseline all patients (group 1) and controls were treated with Iloprost, in particular the 75% of subjects received iv infusion for 5 consecutive days every 4 weeks, while the 25% of subjects underwent iv treatment every 2 weeks.
Following the study protocol treatment, all patients needed the drug once every four weeks at T2 while controls did not change the infusion frequency.
3.6 US-based treatment
All patients (group 2) at the end of ten medical sessions of US treatment (T1) showed a significant improvement of DUs. In fact, during treatment, a decrease in depth and diameter of DUs was evidenced, with a complete resolution of lesions at the end of treatment in 100% of patients enrolled, as demonstrated by photographic records (Figure 5C).
No cases of new ulcers development or side effects were reported. Phalangeal and inter-phalangeal mobility improved together with hand functionality as demonstrated by the reduction of 69,2% in the DHI compared to T0 (72,3 ± 3,2 vs 22,2 ± 4,3; p<0,05) (Figure 6A). Even the Rodnan Skin Score showed significant improvement (41 ± 5,2 vs 18 ± 3,4; p<0,05) (Figure 6B), with the evidence of more elastic skin, although underlying adhesion to deeper planes remained. Pain decreased remarkably; VAS reduced from 4.5 (moderate) at T0 to 2.33 (mild) at T1 (p<0,05) (Figure 6C). A decrease of 72% in the HAQ at T1 demonstrated an improvement in quality of life too (2,57 ± 0,31 vs 0,72 ± 0,18; p<0,05) (Figure 6D).
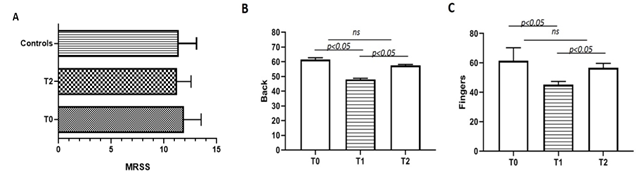
Figure. 2: (A) mRSS mean value of group 1 patients at baseline (T0) and at 12 months (T2) from therapy (p > 0,05) and control group; (B-C) mean value of elastometry of back hands and fingers of group 1 patients at baseline (T0), after 3 months (T1) and after 12 months (T2) from treatment.
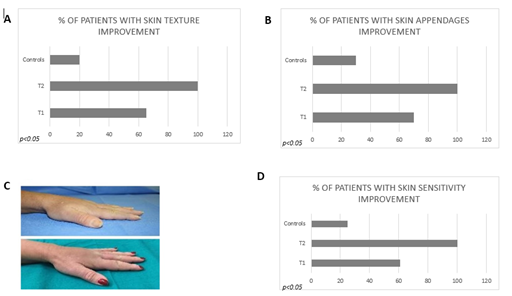
Figure. 3: (A) Percentage of group 1 patients with skin texture improvement after treatment (T1 and T2) and percentage of control group; (B) percentage of group 1 patients with skin appendages improvement after treatment (T1 and T2) and percentage of control group; (C) dyschromia pre- and post-treatment at baseline (T0) and after 3 months (T1) from treatment; (D) percentage of group 1 patients with skin sensitivity improvement after treatment (T1 and T2) and percentage of control group.
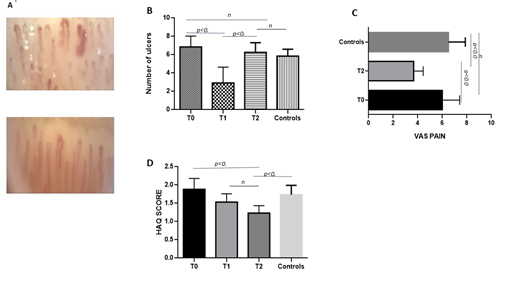
Figure. 4: (A) NVC images of IV finger pre- and after treatment (T1); (B) number of ulcers (mean values) at T0 at T2 of group 1 patients and control group; (C) VAS Pain score (mean values) modification at baseline (T0) and after 12 months from treatment (T2) in group 1 patients and in control group; (D) HAQ score (mean values) at different time points (T0, T1 and T2) and control group.
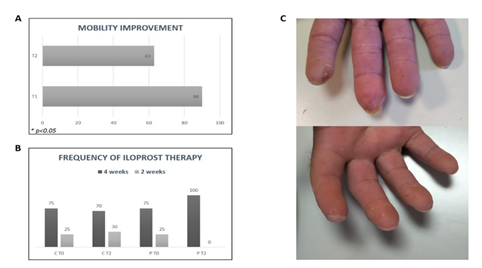
Figure. 5: (A) Percentage of group 1 patients with mobility improvement after treatment (T1 and T2) and control group; (B) frequency of Iloprost infusions (percentage) at baseline and after 12 months from treatment (T2). C = control group; P = patients (group 1); (C) US Representative image of patient’s fingers before and after US treatment.
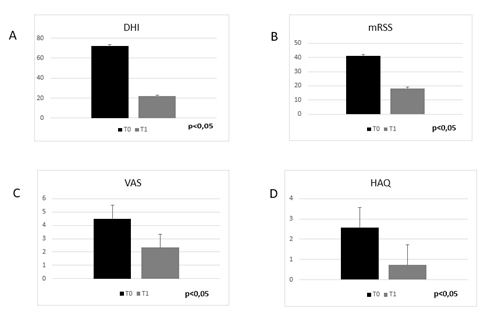
Figure. 6: (A) DHI mean value of group 2 patients at baseline (T0) and after treatment (T1);(B) mRSS mean value of group 2 patients at baseline (T0) and after treatment (T1); (C) VAS Pain score (mean values) modification at baseline (T0) and treatment (T1) in group 2 patients; (D) HAQ score (mean values) at baseline (T0) and treatment (T1) in group 2 patients.
4. Discussion
DUs are a significant complication of SSc, their presence reflects the almost complete desertification of the capillary bed located at fingertips. DUs are associated with severe pain, impairment in hand functionality and important aesthetic impact on patient’s quality of life that can be deeply deteriorated. DUs healing can be a long process which requires high intensity care with consequently increased healthcare resource utilization and associated costs [62].
Up to date standard treatments, involving general and local therapeutic strategies, are not sufficient to grant a significant improvement in DUs natural history, healing time and recurrence rate.
Our study clearly demonstrated that the additional treatment of DUs with PRP-lipofilling injection or ultrasound was more effective than vasoactive therapy alone in DUs healing. Patients experienced a prompt reduction in pain with a significant improvement in mobility and in their quality of life. In addition, the skin appeared smoother and more elastic after treatment. The regrowth of skin appendages as well as the reduction in Raynaud phenomenon attacks rate were related to the revascularization of the capillary bed at fingertip level as demonstrated by NVC.
At the end of treatment, the DUs although they recurred, were fewer, smaller, shallower, less painful, did not tend to become infected and showed rapid resolution. All patients at the end of the protocol required only a daily infusion every 4 weeks.
The role of ancillary procedures, involving PRP and fat transplantation, to treat DUs in SSc patients has yet been investigated in the last decade but clear information on how these biological products exert their regenerative activities is still lacking [63].
The rational beneath the use of lipofilling in SSc relies on the encouraging data that have already demonstrated its beneficial properties in wound care, as for example in diabetes foot, severe burns, recalcitrant venous ulcers, or ischemic ulcers not suitable for revascularization [64, 65]. The adipose tissue is an easily accessible source of mesenchymal stem cells (MSC), known as ADSC. ADSC share common features with bone marrow-MSC (BM-MSC) but they are easier to obtain and expand in vitro. Moreover, ADSC compared to BM-MSC from SSc, present a preserved phenotype and functional behavior. In particular, ADSC are able to differentiate in several cell lineages involved in tissue remodeling, they exert a marked proangiogenic activity, especially in hypoxia conditions, and exhibit immunosuppressive properties. On the other hand, BM-MSC from SSc patients show a defective proliferation and immunosuppressive potential as well as a more senescent phenotype [66]. The surgical procedure to obtain adipose tissue was standardized in 1997 and is now considered a safe and easy procedure [46]. Beside ADSC, the lipoaspirate contain a wide range of other different cells such as pericytes, fibroblasts, immune system cells that can all contribute to tissue healing [67].
The regenerative approach to SSc with fat grafting has been investigated in several previous reports mainly focused on the treatment of facial and perioral lesions, such as microstomia and microcheilia [68, 69]. However, researchers have also evaluated the role of adipose tissue transfer on DUs with impressive results. In particular Granel et al in 2015 demonstrated the safety and tolerability of finger lipofilling that was capable of improving skin elasticity, Raynaud’s phenomenon and vascularization of upper extremities [70]. Other reports, in line with our results, confirmed the positive effect of fat grafting on accelerating the healing time of DUs [71-73].
PRP is rich in platelets, cytokines, and growth factors. This autologous gel is able to enhance MSC proliferation boosting the tissue regeneration process [74]. The synergistic effect of lipofilling and PRP was evaluated in breast reconstruction surgery. In SSc, Virzì et al. in 2017 investigated the potential of this combination therapy for facial-skin lesions. Authors observed a significant improvement in microcheilia, skin elasticity and vascularization [75]. In the present paper, for the first time, we assessed the positive effect of consecutive injections of PRP and lipofilling at hand level in SSc patients demonstrating that these techniques can grant a more rapid healing of DUs and an improvement in skin appearance as well as in fingers mobility. Patients experienced a rapid and sustained decrease in pain level, as demonstrated by the VAS data. The prompt reduction in pain may suggest that ADSC secrete neuromodulator molecules, such as neurotrophic factors, that are able to interfere with pain genesis and transmission [76].
Although the good results obtained with injection treatment with PRP or adipose tissue, DUs recur, even if lesions tend to be less severe in term of size and associated pain. The described techniques require highly trained operators to be performed and are burdened by high sanitary costs, so there is a constant need to investigate even other approaches that could be less expensive but effective. In particular, in the last few years, US-based procedures have emerged as alternative approaches for difficult-to-treat, chronic wounds [77]. Several preclinical studies in vitro and on animal models have demonstrated the physiological effects of US that are able to improve cell proliferation, collagen production, bone formation, and angiogenesis. A decrease in pain perception has even been observed in clinical trials [78, 79]. Promising outcomes were obtained in treating tendon injuries, bone fractures, surgical wounds, and indolent ulcers. For these purposes, and in particular for superficial skin lesions, US devices are usually set to low frequency noncontact modalities, as the one used in our study, to ensure beneficial effects without the risk of skin burning [80, 81]. A previous case report in 2008 demonstrated the effectiveness of a 10-weeks low frequency, noncontact, nonthermal US therapy (acoustic pressure wound therapy) in granting the complete resolution of a severe, complicated DU in a male patient with SSc. The patient at 6 weeks had no longer need of analgesic drugs. In addition, the procedure was painless compared to classical surgical debridement with optimal patient’s compliance [82]. In our group of 10 SSc patients with DUs we obtained a complete healing of lesions with ten US sessions over 2 weeks. Pain improved remarkably and we evidenced even a significant decrease in DHI and HAQ scores. Skin appeared softer and smoother.
Up to date US-based treatment still lack guidelines on dose range and possible side effects for each specific lesion to treat. Further studies on larger cohorts are needed to standardize the technique but, in our opinion, US represent a promising adjunctive treatment for DUs and for the overall hand involvement in SSc.
5. Conclusion
To our knowledge this is the first study that evaluated the effect of PRP coupled with lipofilling, or ultrasound-based treatment in SSc patients who present DUs. Our results suggest that these techniques, classically applied to regenerative medicine and wound care, can become useful ancillary procedures in the rheumatologist toolbox. To obtain good results, a holistic approach to the patient is mandatory and the collaboration with highly trained colleagues, such as plastic surgeons with special interest in hand surgery and physiatrists with expertise in ultrasound treatment of soft tissues is required.
The improvement in patient quality of life stands out as the major success of our protocol and notably, the reduction of patients accesses to the hospital to receive Iloprost infusions underlines the importance of standardizing local therapy techniques to treat SSc patients. All changes evidenced in our study, in term of improvement in DUs, pain, hand mobility and skin appearance were maintained up to 12 months after treatment, by the way DUs recurred in 90% of patients but were characterized by more favorable course, being smaller and less painful. These procedures may play a major role especially in patients who suffer from indolent, severe DUs.
Future studies, involving larger cohorts of patients, are needed to further assess the impact and the cost-effectiveness of PRP, lipofilling and ultrasound treatment in SSc complicated with ischemic DUs.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the University Hospital of Palermo and informed consent was obtained from each patient in accordance with the Helsinki Declaration.
Consent for publication
Consent for publication has been obtained from all patients.
Availability of data and materials
All data generated or analysed during this study are included in this published article. The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
Not applicable
Authors' contributions
G.G., G.L.M., F.C., and R.G. conceptualized the study and study design.
R.P., C.S., and D.S. performed the experiments and wrote the paper. C.R., P.T., L.T., and A.C. were responsible for the patients’ enrollment and follow-up. P.R., and C.S. ran the statistical analysis of the data. All authors contributed to the manuscript review and read and approved the final manuscript.
Acknowledgements
Not applicable
References
- Hinchcliff M, Varga J. Systemic sclerosis/scleroderma: a treatable multisystem disease. Am Fam Physician 78 (2008): 961-968.
- Hoffmann-Vold AM, Midtvedt O, Molberg O, et al. Prevalence of systemic sclerosis in south-east Norway. Rheumatology (Oxford) 51 (2012):1600-1605.
- Di Benedetto P, Ruscitti P, Liakouli V, et al. The Vessels Contribute to Fibrosis in Systemic Sclerosis. Isr Med Assoc J 21 (2019): 471-474.
- Liakouli V, Cipriani P, Di Benedetto P, et al. The role of extracellular matrix components in angiogenesis and fibrosis: Possible implication for Systemic Sclerosis. Mod Rheumatol 28 (2018): 922-932.
- Ho YY, Lagares D, Tager AM, et al. Fibrosis--a lethal component of systemic sclerosis. Nat Rev Rheumatol 10 (2014): 390-402.
- Kaipiainen-Seppänen O, Aho K. Incidence of rare systemic rheumatic and connective tissue diseases in Finland. Journal of internal medicine 240 (1996): 81-84.
- Lo Monaco A, Bruschi M, La Corte R, et al. Epidemiology of systemic sclerosis in a district of northern Italy. Clin Exp Rheumatol 29 (2011): S10-S14.
- Bernatsky S, Joseph L, Pineau CA, et al. Scleroderma prevalence: demographic variations in a population-based sample. Arthritis Rheum 61 (2009):400-404.
- Nikpour M, Stevens WM, Herrick AL, et al. Epidemiology of systemic sclerosis. Best Pract Res Clin Rheumatol 24 (2010): 857-869.
- Denton CP, Khanna D. Systemic sclerosis. Lancet 390 (2017): 1685-1699.
- Pearson DR, Werth VP, Pappas-Taffer L. Systemic sclerosis: Current concepts of skin and systemic manifestations. Clin Dermatol 36(2018): 459-474.
- Abuowda Y, Almeida RS, Oliveira AA, et al. Treatment of digital ulcers in systemic sclerosis: Case series study of thirteen patients and discussion on outcome. Rev Assoc Med Bras (1992). 63 (2017): 422-426.
- LeRoy EC, Medsger TA, Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol. 28 (2001): 1573-1576.
- Meijs J, Voskuyl AE, Bloemsaat-Minekus JP, et al. Blood flow in the hands of a predefined homogeneous systemic sclerosis population: the presence of digital ulcers and the improvement with bosentan. Rheumatology (Oxford) 54 (2015): 262-269.
- Valentini G, Marcoccia A, Cuomo G, et al. The concept of early systemic sclerosis following 2013 ACR\EULAR criteria for the classification of systemic sclerosis. Curr Rheumatol Rev 10 (2014): 38-44.
- Kowal-Bielecka O, Landewe R, Avouac J, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis 68 (2009): 620-628.
- Giacomelli R, Afeltra A, Alunno A, et al. International consensus: What else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren's syndrome)?: The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun Rev 16 (2017): 911-924.
- Brown M, O'Reilly S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin Exp Immunol. 195 (2019): 310-321.
- Albilia JB, Lam DK, Blanas N, et al. Small mouths ... Big problems? A review of scleroderma and its oral health implications. J Can Dent Assoc 73 (2007): 831-836.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford) 48 (2009): 213-221.
- Ghoreschi K, Rocken M. Phototherapy of sclerosing skin diseases. Dermatology 205 (2002): 219-220.
- El-Mofty M, Mostafa W, Esmat S, et al. Suggested mechanisms of action of UVA phototherapy in morphea: a molecular study. Photodermatol Photoimmunol Photomed 20 (2004): 93-100.
- Kreuter A, Hyun J, Stucker M, et al. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol 54 (2006): 440-447.
- Elst EF, Van Suijlekom-Smit LW, Oranje AP. Treatment of linear scleroderma with oral 1,25-dihydroxyvitamin D3 (calcitriol) in seven children. Pediatr Dermatol 16 (1999): 53-58.
- Mizutani H, Yoshida T, Nouchi N, et al. Topical tocoretinate improved hypertrophic scar, skin sclerosis in systemic sclerosis and morphea. J Dermatol 26 (1999): 11-17.
- Gheisari M, Ahmadzadeh A, Nobari N, et al. Autologous Fat Grafting in the Treatment of Facial Scleroderma. Dermatol Res Pract 6568016 (2018).
- Griffin MF, Almadori A, Butler PE. Use of Lipotransfer in Scleroderma. Aesthet Surg J 37 (2017): S33-S37.
- Scuderi N, Ceccarelli S, Onesti MG, et al. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant 22 (2013): 779-795.
- Pirrello R, Verro B, Grasso G, et al. Hyaluronic acid and platelet-rich plasma, a new therapeutic alternative for scleroderma patients: a prospective open-label study. Arthritis Res Ther 21 (2019): 286.
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 72 (2013): 1747-1755.
- Jordan S, Maurer B, Toniolo M, et al. Performance of the new ACR/EULAR classification criteria for systemic sclerosis in clinical practice. Rheumatology (Oxford) 54 (2015): 1454-1458.
- Asano Y, Jinnin M, Kawaguchi Y, et al. Diagnostic criteria, severity classification and guidelines of systemic sclerosis. J Dermatol 45 (2018): 633-691.
- Grant SM, Goa KL. Iloprost. A review of its pharmacodynamic and pharmaco-kinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs 43 (1992): 889-924.
- Khanna D, Furst DE, Clements PJ, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2 (2017): 11-18.
- Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol 20 (1993): 1892-1896.
- Etehad Tavakol M, Fatemi A, Karbalaie A, et al. Nailfold Capillaroscopy in Rheumatic Diseases: Which Parameters Should Be Evaluated? Biomed Res Int (2015): 974530.
- Cutolo M, Sulli A, Pizzorni C, et al. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 27 (2000): 155-160.
- Duruoz MT, Poiraudeau S, Fermanian J, et al. Development and validation of a rheumatoid hand functional disability scale that assesses functional handicap. J Rheumatol 23 (1996): 1167-1172.
- Brower LM, Poole JL. Reliability and validity of the Duruoz Hand Index in persons with systemic sclerosis (scleroderma). Arthritis Rheum 51 (2004): 805-809.
- Sezer N, Yavuzer G, Sivrioglu K, et al. Clinimetric properties of the Duruoz hand index in patients with stroke. Arch Phys Med Rehabil 88 (2007): 309-314.
- Flaherty SA. Pain measurement tools for clinical practice and research. AANA J 64 (1996): 133-140.
- Pincus T, Summey JA, Soraci SA, et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum 26 (1983): 1346-1353.
- Mohammed S, Yu J. Platelet-rich plasma injections: an emerging therapy for chronic discogenic low back pain. J Spine Surg 4 (2018): 115-122.
- Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109 (2004): 1292-1298.
- Dhurat R, Sukesh M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author's Perspective. J Cutan Aesthet Surg 7 (2014): 189-197.
- Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg 24 (1997): 347-367.
- Simonacci F, Bertozzi N, Grieco MP, et al. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg (Lond) 20 (2017): 49-60.
- Maria AT, Maumus M, Le Quellec A, et al. Adipose-Derived Mesenchymal Stem Cells in Autoimmune Disorders: State of the Art and Perspectives for Systemic Sclerosis. Clin Rev Allergy Immunol. 52 (2017): 234-259.
- Han J, Koh YJ, Moon HR, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood 115 (2010): 957-964.
- Dromard C, Bourin P, André M, et al. Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres. Experimental cell research 317 (2011): 770-780.
- Lindroos B, Boucher S, Chase L, et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy11 (2009): 958-972.
- Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy 16 (2014): 245-257.
- Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 4 (2013): 201.
- Bianco P. Back to the future: moving beyond "mesenchymal stem cells". J Cell Biochem 112 (2011): 1713-1721.
- Nakagami H, Morishita R, Maeda K, et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb 13 (2006): 77-81.
- Samuels JA, Weingarten MS, Margolis DJ, et al. Low-frequency (<100 kHz), low-intensity (<100 mW/cm(2)) ultrasound to treat venous ulcers: a human study and in vitro experiments. J Acoust Soc Am. 134 (2013): 1541-1547.
- Lai J, Pittelkow MR. Physiological effects of ultrasound mist on fibroblasts. Int J Dermatol 46 (2007): 587-593.
- Karau MJ, Piper KE, Steckelberg JM, et al. In vitro activity of the Qoustic Wound Therapy System against planktonic and biofilm bacteria. Adv Skin Wound Care 23 (2010): 316-320.
- Serena T, Lee SK, Lam K, et al. The impact of noncontact, nonthermal, low-frequency ultrasound on bacterial counts in experimental and chronic wounds. Ostomy Wound Manage 55 (2009): 22-30.
- Kavros SJ, Coronado R. Diagnostic and Therapeutic Ultrasound on Venous and Arterial Ulcers: A Focused Review. Adv Skin Wound Care 31 (2018): 55-65.
- Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 53 (2011): 2S-48S.
- Morrisroe K, Stevens W, Sahhar J, et al. Digital ulcers in systemic sclerosis: their epidemiology, clinical characteristics, and associated clinical and economic burden. Arthritis Res Ther 21 (2019): 299.
- Lebedoff N, Frech TM, Shanmugam VK, et al. Review of local wound management for scleroderma-associated digital ulcers. J Scleroderma Relat Disord 3 (2018): 66-70.
- Lee HC, An SG, Lee HW, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circulation journal : official journal of the Japanese Circulation Society 76 (2012): 1750-1760.
- Han SK, Kim HR, Kim WK. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 18 (2010): 342-348.
- Capelli C, Zaccara E, Cipriani P, Di Benedetto P, Maglione W, Andracco R, et al. Phenotypical and Functional Characteristics of In Vitro-Expanded Adipose-Derived Mesenchymal Stromal Cells From Patients With Systematic Sclerosis. Cell Transplant 26 (2017): 841-854.
- Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Translational research : the journal of laboratory and clinical medicine163 (2014): 399-408.
- Daumas A, Magalon J, Delaunay F, et al. Fat Grafting for Treatment of Facial Scleroderma. Clin Plast Surg 47 (2020): 155-163.
- Blezien O, D'Andrea F, Nicoletti GF, et al. Effects of Fat Grafting Containing Stem Cells in Microstomia and Microcheilia Derived from Systemic Sclerosis. Aesthetic Plast Surg 41 (2017): 839-844.
- Granel B, Daumas A, Jouve E, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis 74 (2015): 2175-2182.
- Bene MD, Pozzi MR, Rovati L, Mazzola I, Erba G, Bonomi S. Autologous fat grafting for scleroderma-induced digital ulcers. An effective technique in patients with systemic sclerosis. Handchir Mikrochir Plast Chir 46 (2014): 242-247.
- Del Papa N, Di Luca G, Andracco R, et al. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: results of a monocentric randomized controlled study. Arthritis Res Ther 21 (2019): 7.
- Guillaume-Jugnot P, Daumas A, Magalon J, et al. State of the art. Autologous fat graft and adipose tissue-derived stromal vascular fraction injection for hand therapy in systemic sclerosis patients. Curr Res Transl Med 64 (2016): 35-42.
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J 4 (2014): 52-62.
- Virzì F, Bianca P, Giammona A, et al. Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients. Stem cell research & therapy 8 (2017): 236.
- Brini AT, Amodeo G, Ferreira LM, et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci Rep 7 (2017): 9904.
- Alkahtani SA, Kunwar PS, Jalilifar M, et al. Ultrasound-based Techniques as Alternative Treatments for Chronic Wounds: A Comprehensive Review of Clinical Applications. Cureus 9 (2017): e1952.
- Maan ZN, Januszyk M, Rennert RC, et al. Noncontact, low-frequency ultrasound therapy enhances neovascularization and wound healing in diabetic mice. Plastic and reconstructive surgery 134 (2014): 402e-411e.
- Gehling ML, Samies JH. The effect of noncontact, low-intensity, low-frequency therapeutic ultrasound on lower-extremity chronic wound pain: a retrospective chart review. Ostomy Wound Manage 53 (2007): 44-50.
- Miller DL, Smith NB, Bailey MR, et al. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med 31 (2012): 623-634.
- Kavros SJ, Miller JL, Hanna SW. Treatment of ischemic wounds with noncontact, low-frequency ultrasound: the Mayo clinic experience, 2004-2006. Adv Skin Wound Care 20 (2007): 221-226.
- Fleming CP. Acoustic pressure wound therapy in the treatment of a vasculopathy-associated digital ulcer: a case study. Ostomy Wound Manage 54 (2008): 62-65.
