Acyclovir Improves the Efficacy of Chemoradiation in Nasopharyngeal Cancer Containing the Epstein Barr Virus Genome
Article Information
Aditya Thandoni1, Andrew Zloza2, Devora Schiff3, Malay Rao4, Kwok-wai Lo5, Bruce G Haffty3, Sung Kim3, and Sachin R Jhawar6*
1Department of Orthopedic Surgery, Alleghany Health Network, Pittsburgh, PA
2Department of Internal Medicine, Division of Hematology, Oncology and Cell Therapy, Rush Medical College, Chicago, IL
3Department of Radiation Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
4Northeast Georgia Physicians Group Radiation Oncology, Gainesville, GA
5Department of Anatomical and Cellular Pathology, State Key Laboratory in Oncology in South China and Li Ka Shing Institute of Health Science, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong. 6Department of Radiation Oncology, Arthur G. James Comprehensive Cancer Center, The Ohio State University, Columbus, OH
*Corresponding author: Sachin R Jhawar, Department of Radiation Oncology, Arthur G. James Comprehensive Cancer Center, The Ohio State University, Columbus, USA.
Received: 18 August 2023; Accepted: 28 August 2023; Published: 29 September 2023
Citation:
Aditya Thandoni, Andrew Zloza, Devora Schiff, Malay Rao, Kwok-wai Lo, Bruce G Haffty, Sung Kim, and Sachin R Jhawar. Acyclovir Improves the Efficacy of Chemoradiation in Nasopharyngeal Cancer Containing the Epstein Barr Virus Genome. Journal of Bioinformatics and Systems Biology. 6 (2023): 286-288.
View / Download Pdf Share at FacebookAbstract
Nasopharyngeal carcinoma (NPC) is a malignancy endemic to East Asia and is caused by Epstein-Barr Virus (EBV)-mediated cancerous transformation of epithelial cells. The standard of care treatment for NPC involves radiation and chemotherapy. While treatment outcomes continue to improve, up to 50% of patients can be expected to recur by five years, and additional innovative treatment options are needed. We posit that a potential way to do this is by targeting the underlying cause of malignant transformation, namely EBV. One method by which EBV escapes immune surveillance is by undergoing latent phase replication, during which EBV expression of immunogenic proteins is reduced. However, chemoradiation is known to drive conversion of EBV from a latent to a lytic phase. This creates an opportunity for the targeting of EBV-infected cells utilizing antiviral drugs. Indeed, we found that combining acyclovir with cisplatin and radiation significantly decreases the viability of the EBV-infected C666- 1 cell line. Western blot quantification revealed a resultant increase of thymidine kinase (TK) and apoptosis-inducing mediators, cleaved PARP (cPARP) and phosphorylated ERK (pERK). These studies suggest that the addition of anti-viral drugs to frontline chemoradiation may improve outcomes in patients treated for EBV-related NPC and future in vivo and clinical studies are needed.
Keywords
Nasopharyngeal Cancer, Epstein Barr Virus, Acyclovir
Nasopharyngeal Cancer articles; Epstein Barr Virus articles; Acyclovir articles
Nasopharyngeal Cancer articles Nasopharyngeal Cancer Research articles Nasopharyngeal Cancer review articles Nasopharyngeal Cancer PubMed articles Nasopharyngeal Cancer PubMed Central articles Nasopharyngeal Cancer 2023 articles Nasopharyngeal Cancer 2024 articles Nasopharyngeal Cancer Scopus articles Nasopharyngeal Cancer impact factor journals Nasopharyngeal Cancer Scopus journals Nasopharyngeal Cancer PubMed journals Nasopharyngeal Cancer medical journals Nasopharyngeal Cancer free journals Nasopharyngeal Cancer best journals Nasopharyngeal Cancer top journals Nasopharyngeal Cancer free medical journals Nasopharyngeal Cancer famous journals Nasopharyngeal Cancer Google Scholar indexed journals Epstein Barr Virus articles Epstein Barr Virus Research articles Epstein Barr Virus review articles Epstein Barr Virus PubMed articles Epstein Barr Virus PubMed Central articles Epstein Barr Virus 2023 articles Epstein Barr Virus 2024 articles Epstein Barr Virus Scopus articles Epstein Barr Virus impact factor journals Epstein Barr Virus Scopus journals Epstein Barr Virus PubMed journals Epstein Barr Virus medical journals Epstein Barr Virus free journals Epstein Barr Virus best journals Epstein Barr Virus top journals Epstein Barr Virus free medical journals Epstein Barr Virus famous journals Epstein Barr Virus Google Scholar indexed journals Acyclovir articles Acyclovir Research articles Acyclovir review articles Acyclovir PubMed articles Acyclovir PubMed Central articles Acyclovir 2023 articles Acyclovir 2024 articles Acyclovir Scopus articles Acyclovir impact factor journals Acyclovir Scopus journals Acyclovir PubMed journals Acyclovir medical journals Acyclovir free journals Acyclovir best journals Acyclovir top journals Acyclovir free medical journals Acyclovir famous journals Acyclovir Google Scholar indexed journals drug concentrations articles drug concentrations Research articles drug concentrations review articles drug concentrations PubMed articles drug concentrations PubMed Central articles drug concentrations 2023 articles drug concentrations 2024 articles drug concentrations Scopus articles drug concentrations impact factor journals drug concentrations Scopus journals drug concentrations PubMed journals drug concentrations medical journals drug concentrations free journals drug concentrations best journals drug concentrations top journals drug concentrations free medical journals drug concentrations famous journals drug concentrations Google Scholar indexed journals 1-way ANOVA articles 1-way ANOVA Research articles 1-way ANOVA review articles 1-way ANOVA PubMed articles 1-way ANOVA PubMed Central articles 1-way ANOVA 2023 articles 1-way ANOVA 2024 articles 1-way ANOVA Scopus articles 1-way ANOVA impact factor journals 1-way ANOVA Scopus journals 1-way ANOVA PubMed journals 1-way ANOVA medical journals 1-way ANOVA free journals 1-way ANOVA best journals 1-way ANOVA top journals 1-way ANOVA free medical journals 1-way ANOVA famous journals 1-way ANOVA Google Scholar indexed journals cell articles cell Research articles cell review articles cell PubMed articles cell PubMed Central articles cell 2023 articles cell 2024 articles cell Scopus articles cell impact factor journals cell Scopus journals cell PubMed journals cell medical journals cell free journals cell best journals cell top journals cell free medical journals cell famous journals cell Google Scholar indexed journals DNA articles DNA Research articles DNA review articles DNA PubMed articles DNA PubMed Central articles DNA 2023 articles DNA 2024 articles DNA Scopus articles DNA impact factor journals DNA Scopus journals DNA PubMed journals DNA medical journals DNA free journals DNA best journals DNA top journals DNA free medical journals DNA famous journals DNA Google Scholar indexed journals NPC articles NPC Research articles NPC review articles NPC PubMed articles NPC PubMed Central articles NPC 2023 articles NPC 2024 articles NPC Scopus articles NPC impact factor journals NPC Scopus journals NPC PubMed journals NPC medical journals NPC free journals NPC best journals NPC top journals NPC free medical journals NPC famous journals NPC Google Scholar indexed journals
Article Details
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignancy in most of the world, but is endemic in southern China and parts of Southeast Asia [1, 2]. Almost all cases of endemic NPC are associated with mucosal epithelial cell infections with the Epstein-Barr Virus (EBV)[1], thus establishing a crucial association between the virus and the induction of transformed and invasive cancer [2]. EBV is a latency type II gamma herpes virus containing a genome of nearly 100 genes, with a majority of gene expression present during the lytic phase, compared to only eleven viral genes present during the latent phase [3]. Although naturally immunogenic, the ability of EBV to transition to latent phase replication provides a means of escape from immune surveillance through the decrease in expression of immunogenic proteins via epigenetic mechanisms [4]. While nucleoside-analogue anti-herpesvirus drugs, like acyclovir, can work in the lytic phase, leading to the death of infected cells (including tumor cells), these drugs remain in their inactive prodrug form in the latent phase, making them ineffective as primary therapy for EBV-associated malignancies [5, 6]. The role of EBV in NPC is important, as plasma titers of EBV DNA in NPC patients pre and post treatment correlate with the stage of NPC, tumor burden in patients, and changes in titers can predict outcomes [7].
The backbone of treatment of advanced NPC is the utilization of chemotherapy and radiation. Studies have shown that each of these two therapies may individually function as a lytic phase inducer of EBV. Therefore, we sought to determine whether targeting EBV in the context of chemoradiation may augment in vitro signatures of treatment response, including decreased tumor cell viability and increased anti-tumor pathway signaling.
2. Methodology
Tumor cell lines
C666-1, an EBV-infected nasopharyngeal cancer (NPC) human cell line, and HK-1, a non-EBV infected human NPC cell line were utilized in cell viability and western blot studies. Dr. Kwok-wai Lo and The Chinese University of Hong Kong generously donated both cell lines to The Cancer Institute of New Jersey.
2.1 Cell viability assay
The non-radioactive colorimetric tetrazolium (MTT) assay, measuring cellular metabolic activity, was utilized as an indicator of cell viability, as per Sigma-Aldrich instructions. C666-1 cells were placed at 10,000 cells per each well of a 96-well flat-bottom plate. Experimental arms were repeated in 8 wells per treatment. The cells were cultured for 12-15 hours in Gibco’s RPMI media supplemented with 1x Glutamax, 10% FBS, and 1% penicillin -steptomycin. Cells were left untreated (no treatment; NT) or treated with acyclovir (200ug/ml), cisplatin (cis; 1ug/ml), and radiation (RT; 0-8 Gy) alone and in combinations, as described within the results, figures, and figure legends for each experiment shown. Cells were dosed with their respective drug concentrations 24 hours after initially plating. RT was conducted 24 hours after the administration of drug treatment using the Gamma Cell 40 Exactor (MDS Nordion) irradiator. Colorimetric changes in the media were detected using the Tecan Infintie M200 Pro plate reader at a wavelength of 570nm.
2.2 Western blot analysis
Western blot analysis, as described by the protocol set by Invitrogen, was used to determine the expression of proteins of interest, including, cleaved PARP (cPARP; 1uL:2ml in PBS), phosphorylated AKT (pAKT; 1uL:2ml in PBS) phosphorylated ERK (pERK; 1uL:2ml in PBS), and thymidine kinase (TK; 1uL:2ml in PBS). GAPDH was utilized as a loading control. Quantification was conducted utilizing ImageJ software (version 1.53a; NIH), using the GELS analysis feature, as previously described8. All primary antibodies were obtained from CellSignaling. Secondary anti-rabbit antibody (1uL:5mL in PBS) was also obtained from CellSignaling.
2.3 Statistical Analysis
To determine statistical significance for data comparisons, 1-way ANOVA with Tukey correction (for comparing the means of each group to every other group within an experiment) or Dunnet correction (for focused comparisons of one group to all other groups). Prism version 8.0 (GraphPad) was used for generation of all graphs and performance of statistical analyses. Bar graphs display the mean, and error bars represent the standard error of the mean (S.E.M.). Statistical significance is denoted as ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3. Results
The cell viability assays demonstrate a significant increase in cell death of the C666-1 line in the presence of both acyclovir and cis compared to cells that received no treatment, acyclovir alone, and cis alone (Figure 1). Cell viability in the combination group decreased significantly as radiation fraction size increased. Western blot analysis established the greatest increase in expression of thymidine kinase in cells exposed to combination cis and radiation, consequently leading to high levels of cleaved PARP and decreased levels of phosphorylated ERK in cells exposed to the combination of cis, radiation, and acyclovir (Figure 2). In contrast, the control cell line, HK-1, did not express the enzymatic variation on western blot analysis seen with C666-1. HK-1 also failed to show a difference in cell death.
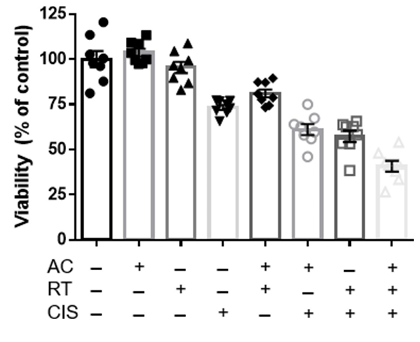
Figure 1: MTT cell viability analysis of EBV-infected C666-1 cells exposed to combinations of no treatment (NT), cisplatin, acyclovir, and 2Gy radiation (RT). The combination of acyclovir and cisplatin reduces cell viability at the 2Gy radiation dosage.
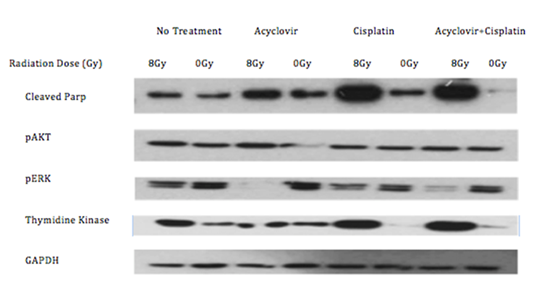
Figure 2: Western blot of the C666-1 cells after receiving no treatment, acyclovir, cisplatin, or radiation. Combination of radiation and cisplatin increases thymidine kinase. Addition of acyclovir increases cParp and decreases pERK.
4. Discussion
Recommended treatment for advanced NPC at the time this study was conducted was concurrent chemoradiation with cis followed by adjuvant cis and 5-FU [5]. New data has established induction gemcitabine and cis, followed by cis-RT as another standard of care [9]. This does not alter the relevance of the current study, however, since the backbone is still cis-RT based.
It has been well-established that plasma EBV DNA titers correlate with NPC stage and outcomes. In fact, the HN-001 randomized phase II/III clinical trial assessing risk-adapted treatment of patients with NPX based on EBV DNA titers, is currently in progress (NCT02135042). Targeting virally infected cells has emerged as a novel therapeutic strategy [10]. Although a promising approach, obstacles in efficacy arise as nucleoside-analogues are pro-drugs that can only be activated in the lytic phase by virus specific protein kinases not present in the latent phase [11]. While induction of the lytic phase is possible, data on the efficacy of this technique and synergy with nucleoside analogues is limited in the literature. We strove to establish the critical role of chemoradiation in inducing a lytic switch in latent type II EBV cells, along with the synergestic effect with acyclovir in inducing cell death [10]. Our data shows that the addition of acyclovir to standard of care chemoradiation leads to maximal tumoricidal effects in the C666-1 cells while sparing the control HK-1 line. Increases in TK expression demonstrates a switch into the lytic phase with resultant efficacy of acyclovir as shown by increased levels of cPARP and decreased levels of pERK and subsequent cell death via apoptosis. This synergistic interaction was consistent in enhancing in vitro anti-tumor effects across multiple experiments in the C666-1 cells, while sparing the control HK-1 cell line. The synergistic anti-tumor effects of acyclovir, cisplatin, and radiation combination should next be studied with in vivo mouse models.
References
- Yu MC, Yuan J-M. Epidemiology of nasopharyngeal carcinoma. Seminars in Cancer Biology 12 (2002): 421-429.
- Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer cell 5 (2004): 423-428.
- Ghosh SK, Perrine SP, Faller DV. Advances in virus-directed therapeutics against Epstein-Barr virus-associated malignancies. Advances in virology 2012 (2012).
- Hutajulu SH, Kurnianda J, Tan IB, et al. Therapeutic implications of Epstein-Barr virus infection for the treatment of nasopharyngeal carcinoma. Therapeutics and clinical risk management 10 (2014): 721.
- Ernberg I, Andersson J. Acyclovir efficiently inhibits oropharyngeal excretion of Epstein-Barr virus in patients with acute infectious mononucleosis. Journal of general virology 67 (1987): 2267-2272.
- Wakisaka N, Yoshizaki T, Paganoet J, et al. “Ribonucleotide reductase inhibitors enhance cidofovir induced apoptosis in EBV positive nasopharyngeal carcinoma xenografts.” International journal of cancer 116 (2005): 640-645.
- Lo YD, Chan LY, Lo K-W, et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer research 59 (1999): 1188-1191.
- Schneider Caroline A, Wayne Rasband S, and Kevin Eliceiri W. “NIH Image to ImageJ: 25 years of image analysis.” Nature methods 9 (2012): 671-675.
- Zhang Y, Chen L, Zhang N, et al. “Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma.” The New England journal of medicine 381 (2019): 1124.
- Lin J-C, Chen KY, Wang W-Y, et al. Detection of Epstein-Barr virus DNA in the peripheral-blood cells of patients with nasopharyngeal carcinoma: relationship to distant metastasis and survival. Journal of clinical oncology 19 (2001): 2607-2615.
- Chung Y-L, Lee Y-HW, Yen S-H, et al. A novel approach for nasopharyngeal carcinoma treatment uses phenylbutyrate as a protein kinase C modulator: implications for radiosensitization and EBV-targeted therapy. Clinical Cancer Research 6 (2000): 1452-1458.
