Acute Pericarditis Revealing Eosinophilic Granulomatosis with Polyangiitis Maria
Article Information
Maria Gloria Aversano1*, Giovanni Lasagni2, Fabrizio Colombo1, Yuri Alessandro Arguello3, Luca Giuseppe Balossi2
1Department of Internal Medicine, ASST Grande Ospedale Metropolitano Niguarda, Piazza Ospedale Maggiore 3, 20162, Milan, Italy
2Department of Allergology and Immunology, ASST Grande Ospedale Metropolitano Niguarda, Piazza Ospedale Maggiore 3, 20162, Milan, Italy
3Department of General Medicine, Immunology and Allergology, IRCCS Foundation Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
*Corresponding Author: Maria Gloria Aversano, Department of Internal Medicine, ASST Grande Ospedale Metropolitano Niguarda, Piazza Ospedale Maggiore 3, 20162, Milan, Italy.
Received: 25 April 2022; Accepted: 05 May 2022; Published: 20 May 2022
Citation: Maria Gloria Aversano, Giovanni Lasagni, Fabrizio Colombo, Yuri Alessandro Arguello, Luca Giuseppe Balossi. Acute Pericarditis Revealing Eosinophilic Granulomatosis with Polyangiitis. Archives of Clinical and Medical Case Reports 6 (2022): 420-427.
View / Download Pdf Share at FacebookAbstract
Eosinophilic Granulomatosis with Polyangiitis (EGPA), commonly known in the past as Churg-Strauss syndrome, is a necrotizing systemic vasculitis accompanied by remarkable eosinophilia. Heart involvement occurs in approximately 15-60% of EGPA patients, especially those who are ANCA negative. Any cardiac structure can be involved. Heart involvement carries a poor prognosis and causes 50% of the deaths of these patients. It is often insidious and underestimated. Early diagnosis of cardiac involvement and subsequent therapy may prevent progression of cardiac disease. To our knowledge, so far there are only few cases reported in the literature describing pericarditis as a presenting feature of EGPA. Here we report a case of a 54- year- old male with history of repeated sinusitis and mild asthma admitted to our department for worsening dyspnea and chest pain with consequent diagnosis of acute pericarditis due to unknown EGPA. This case suggests that acute pericarditis can be the initial clinical feature of onset EGPA, even in patients with mild asthma history. Early diagnosis of cardiac involvement prevents progression of cardiac disease.
Keywords
Acute pericarditis; Dyspnea; Eosinophilia; Eosinophilic granulomatosis with polyangiitis; Cardiac involvement
Acute pericarditis articles; Dyspnea articles; Eosinophilia articles; Eosinophilic granulomatosis with polyangiitis articles; Cardiac involvement articles
Acute pericarditis articles Acute pericarditis Research articles Acute pericarditis review articles Acute pericarditis PubMed articles Acute pericarditis PubMed Central articles Acute pericarditis 2023 articles Acute pericarditis 2024 articles Acute pericarditis Scopus articles Acute pericarditis impact factor journals Acute pericarditis Scopus journals Acute pericarditis PubMed journals Acute pericarditis medical journals Acute pericarditis free journals Acute pericarditis best journals Acute pericarditis top journals Acute pericarditis free medical journals Acute pericarditis famous journals Acute pericarditis Google Scholar indexed journals Dyspnea articles Dyspnea Research articles Dyspnea review articles Dyspnea PubMed articles Dyspnea PubMed Central articles Dyspnea 2023 articles Dyspnea 2024 articles Dyspnea Scopus articles Dyspnea impact factor journals Dyspnea Scopus journals Dyspnea PubMed journals Dyspnea medical journals Dyspnea free journals Dyspnea best journals Dyspnea top journals Dyspnea free medical journals Dyspnea famous journals Dyspnea Google Scholar indexed journals Eosinophilia articles Eosinophilia Research articles Eosinophilia review articles Eosinophilia PubMed articles Eosinophilia PubMed Central articles Eosinophilia 2023 articles Eosinophilia 2024 articles Eosinophilia Scopus articles Eosinophilia impact factor journals Eosinophilia Scopus journals Eosinophilia PubMed journals Eosinophilia medical journals Eosinophilia free journals Eosinophilia best journals Eosinophilia top journals Eosinophilia free medical journals Eosinophilia famous journals Eosinophilia Google Scholar indexed journals polyangiitis articles polyangiitis Research articles polyangiitis review articles polyangiitis PubMed articles polyangiitis PubMed Central articles polyangiitis 2023 articles polyangiitis 2024 articles polyangiitis Scopus articles polyangiitis impact factor journals polyangiitis Scopus journals polyangiitis PubMed journals polyangiitis medical journals polyangiitis free journals polyangiitis best journals polyangiitis top journals polyangiitis free medical journals polyangiitis famous journals polyangiitis Google Scholar indexed journals Eosinophilic granulomatosis articles Eosinophilic granulomatosis Research articles Eosinophilic granulomatosis review articles Eosinophilic granulomatosis PubMed articles Eosinophilic granulomatosis PubMed Central articles Eosinophilic granulomatosis 2023 articles Eosinophilic granulomatosis 2024 articles Eosinophilic granulomatosis Scopus articles Eosinophilic granulomatosis impact factor journals Eosinophilic granulomatosis Scopus journals Eosinophilic granulomatosis PubMed journals Eosinophilic granulomatosis medical journals Eosinophilic granulomatosis free journals Eosinophilic granulomatosis best journals Eosinophilic granulomatosis top journals Eosinophilic granulomatosis free medical journals Eosinophilic granulomatosis famous journals Eosinophilic granulomatosis Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Xerostomia articles Xerostomia Research articles Xerostomia review articles Xerostomia PubMed articles Xerostomia PubMed Central articles Xerostomia 2023 articles Xerostomia 2024 articles Xerostomia Scopus articles Xerostomia impact factor journals Xerostomia Scopus journals Xerostomia PubMed journals Xerostomia medical journals Xerostomia free journals Xerostomia best journals Xerostomia top journals Xerostomia free medical journals Xerostomia famous journals Xerostomia Google Scholar indexed journals Cardiac involvement articles Cardiac involvement Research articles Cardiac involvement review articles Cardiac involvement PubMed articles Cardiac involvement PubMed Central articles Cardiac involvement 2023 articles Cardiac involvement 2024 articles Cardiac involvement Scopus articles Cardiac involvement impact factor journals Cardiac involvement Scopus journals Cardiac involvement PubMed journals Cardiac involvement medical journals Cardiac involvement free journals Cardiac involvement best journals Cardiac involvement top journals Cardiac involvement free medical journals Cardiac involvement famous journals Cardiac involvement Google Scholar indexed journals
Article Details
Abbreviations:
EGPA: Eosinophilic granulomatosis with polyangiitis; ANCA: Antineutrophil cytoplasm antibody; AAV: ANCA-associated vasculitis; MRI: Magnetic resonance imaging; CT: Computed Tomography Scan; EULAR: European League Against Rheumatism; ACR: American College of Rheumatology
1. Introduction
Eosinophilic Granulomatosis With Polyangiitis (EGPA) was first described by Churg and Strauss in 1951 [1]. The disease, newly recognised as part of Anti- Neutrophil Cytoplasm Antibody (ANCA)-Associated Vasculitis (AAV), is characterized by eosinophil-rich granulomatous inflammation and small to medium-size vessel vasculitis associated with asthma and eosinophilia [2]. ANCA are positive in 40-60% of cases, mainly anti-myeloperoxidase. Typical clinical features include asthma, sinusitis, transient pulmonary infiltrates and neuropathy. Blood eosinophils are often >1500/µl or more than 10% on the differential leukocyte count. Heart involvement occurs in approximately 15-60% of EGPA patients, especially those who are ANCA negative. Any cardiac structure can be involved, and patients present with myocarditis, heart failure, pericarditis, arrhythmia, coronary arteritis, valvulopathy, intracavitary cardiac thrombosis. All patients with EGPA should be studied not only with a detailed history of cardiac symptoms and ECG, but also with echocardiography; if abnormalities are detected, a cardiac magnetic resonance study should be performed. Coronary angiography and endomyocardial biopsy should be reserved to selected cases. Heart involvement causes 50% of the deaths of these patients. It is often insidious and underestimated. Early diagnosis of cardiac involvement and subsequent therapy may prevent progression of cardiac disease [3]. Here, we present a case of a 54-year-old male with asthma-like symptoms and sinus pathology refractory, as well as eosinophilia and unexplained cardiac disorder. This case highlights the need to collect a complete clinical history, involving a multidisciplinary team for prompt intervention in EGPA once the disease is identified. It also demonstrates the benefit of prompt intervention, through the rapid improvement seen in the patient thanks to high-dose corticosteroids plus immunosuppression.
2. Case Presentation
A 54-year-old male with general fatigue, fever and a 1-year history of asthma was admitted to our hospital for dyspnea and chest pain associated with first finding of peripheral eosinophilia. He suffered from grass pollen allergy, polyposis and chronic rhinosinusitis since several years. Treatment with inhaled fluticasone/formoterol was ineffective. Few months before he complained episodes of migrating pain in left shoulder, ankles, wrists and hands. On admission to the unit, the patient had severe dyspnea, pulse 75 beats/min, and blood pressure 94/60 mm Hg. Auscultation of the chest demonstrated pericardial friction rub. During the hospitalization, electrocardiogram, cardiac echocardiography, cardiac MRI, chest CT, abdominal echography, blood tests, electromyography and ophthalmology examination were performed.
2.1. Clinical evaluation
Levels of B-type natriuretic peptide (568 pg/mL; normal range <125 pg/mL), sequential troponin-T levels (79 ng/L; normal range <14 ng/L), myoglobin (95 μg/L, normal range <80 μg/L), creatine kinase-MB (5.8 μg/L, normal range <5 μg/L), lactate dehydrogenase (335 U/L, normal range <225 U/L) were elevated, such as eosinophilic count (3.05 10^9/L, normal range <0.45 10^9/L). Hypersensitive C-reactive protein level was 10 mg/dL (normal range <0.5). Serum total IgE level was 251 KUI/l (normally < 100 KUI/l). D-dimer level was 2.83 μg/mL (normal range <0.57). Serum tests were negative for antinuclear antibodies, antineutrophil cytoplasm antibodies (ANCA), rheumatoid factor, anti-citric-citrullinated peptide, double-strands DNA, extractable nuclear antigens, antiphospholipid antibodies, anti- transglutaminase, anti-thyroid peroxidase, anti-thyroglobulin and immunoglobulins. Serum levels of complement were within normal range. Serological markers for Human Herpes virus 6, 8, Parvovirus B19, Cytomegalovirus virus, Epstein-Barr virus, Hepatitis B virus, Hepatitis C virus, Chlamydia pneumoniae, Mycoplasma pneumoniae, Human immunodeficiency virus, Coxsackie virus were negative. Tuberculin skin test as well as quantiferon-TB Gold (QFT-G) were negative. Nasal swab for respiratory viruses, bacteria and SARS-CoV-2 was negative. Test for treponema pallidum was negative. Screening for parasites was negative. Blood and urine cultures were sterile. Electrocardiography revealed sinusal rhythm, slight right delay, non-specific anomalies of ventricular repolarization. Transthoracic echocardiography showed ubiquitous pericardial effusion mostly represented in the inferolateral site (21 mm in the parasternal long axis, 10 mm anterior to the right ventricle, 6 mm in proximity of diaphragmatic wall of right ventricle); biventricular systolic function within limits; no signs of obstruction to right ventricular filling. Treatment with ibuprofen and colchicine was started before doing magnetic resonance imaging (MRI) that revealed ubiquitous pericardial effusion of moderate-severe degree with a maximum detachment of 3 cm in the inferolateral wall without signs of haemodynamic compromise, with signs of organization and presence of voluminous fibrin aggregates. Pericardial leaflets were slightly thickened, edematous and with late enhancement. There were also signs of mild myocardial edema on T2 mapping and possible coexisting mild diffuse fibrosis. Mild myocardial enhancement with non-ischemic pattern was observed on late post-contrast images (Figure 1). Chest Computed Tomography (CT) scan revealed peri bronchial interstitial thickening associated with some bilateral bronchiolectasis, left pleural effusion with a maximum basal thickness of 2 cm, abundant pericardial effusion with a maximum thickness of 2.5 cm, lymphnodes of likely reactive significance. No signs of pulmonary embolism were seen on CT scan (Figure 2). Pulmonary aspergillosis and sarcoidosis were ruled out for absence of biochemical and instrumental diagnostic elements. Complete abdomen ultrasound and fecal occult blood tests were negative, allowing to exclude the paraneoplastic nature of eosinophilia. Electromyography showed no signs of neuropathy. Ophthalmologic examination showed an initial mild episcleritis. Early in admission, patient was carefully reviewed by our multidisciplinary team made up of internists, cardiologists, allergologists and rheumatologists. Persistent eosinophilia and acute pericarditis were the most important findings that leaded to EGPA diagnosis, along with his history of pollen grass allergy, chronic sinus infections and asthma. According to EULAR recommendations for the management of primary small and medium vessel vasculitis, we used high-dose glucocorticoids followed by a combination of parenteral methotrexate and glucocorticoids. Since the patient’s renal function was normal, this was a less toxic alternative to cyclophosphamide to induce clinical remission. Concomitant therapies included folic acid, colchicine, along with proton pump inhibitor, oral bisphosphonates, vitamin D and calcium supplementation for prophylaxis against steroid-induced side effects [4-6]. Almost immediately on commencing treatment, the patient reported less chest pain, resolution of dyspnea, increased energy levels and improved mood. Considering the possibility of an eventual cyclophosphamide treatment, semen cryopreservation was performed. Initial episcleritis was treated with tobramycin and dexamethasone eye drops. The patient’s pericardial effusion rapidly resolved once corticosteroids treatment was started, as well as left pleural effusion (Figure 3). He was successfully discharged 20 days after admission. A repeat echocardiogram at 2 months revealed absence of pericardial effusion and good left ventricular systolic function. Eosinophil count after two months kept being low (0.01 10^9/L after two months, 0.04 10^9/L after four months).
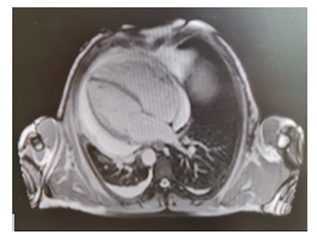
Figure 1: MRI revealed ubiquitous pericardial effusion of moderate-severe degree with a maximum detachment of 3 cm without signs of haemodynamic compromise.
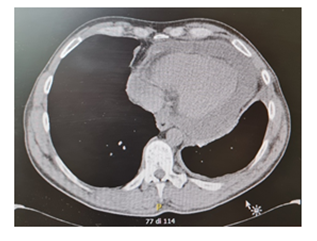
Figure 2: CT scan revealed left pleural effusion with a maximum basal thickness of 2 cm and abundant pericardial effusion.
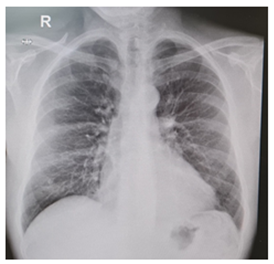
Figure 3: Pericardial and pleural effusion rapidly resolved once corticosteroids treatment was started.
3. Discussion and Conclusions
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare subtype of ANCA-associated vasculitis characterized by asthma, rhinosinusitis, peripheral eosinophilia and manifestations due to small-vessel vasculitis [7] (Table1). EGPA has been classically described to evolve through three different phases, starting with a prodromic allergic phase characterized by asthma and rhinosinusitis, followed by an increase in blood and tissue eosinophilia, and in the last phase patient show systemic symptoms and organ involvement due to small-vessel vasculitis [8]. Heart involvement occurs in approximately 15-60% of EGPA patients and is one of the most severe manifestations in EGPA, often leading to death. Interestingly, it has been shown that cardiac involvement is often found in ANCA-negative patients [9]. Typical manifestations include myocarditis, heart failure, arrhythmia and pericarditis, though it has been suggested that many patients with cardiac involvement may be asymptomatic [10]. Pericarditis, which is one of the major cardiac manifestations in EGPA, presents typically with slight pericardial effusion, even though few cases of cardiac tamponade have been reported [11]. To our knowledge, so far there are only few cases reported in the literature describing pericarditis as a presenting feature of EGPA [12-14]. Here we present the case of a 50-year-old male with a history of asthma, polyposis and chronic rhinosinusitis who was admitted to our hospital for dyspnea and chest pain. Laboratory test revealed an increased in eosinophilic count as well as inflammatory markers and radiological investigations showed sign of pericarditis and pleural effusion. Other causes of pericarditis were ruled out and the diagnosis of EGPA was made based on ACR/EULAR criteria [15] (Table 2). In order to avoid toxicity of cyclophosphamide and considering his clinical status, we decided to treat our patient with high dose of glucocorticoids followed by a combination of parenteral methotrexate and glucocorticoids. We would have added cyclophosphamide if systemic glucocorticoids alone had not achieved remission. Instead, the chosen treatment produced a progressive reduction in the size of the pericardial and pleural effusion, as well as the normalization of the eosinophil count and inflammatory markers. In summary, here we report a case of acute pericarditis leading to a new diagnosis of EGPA. This case highlights the need to consider EGPA as differential diagnosis in patients with pericarditis presenting with asthma-like symptoms and sinus pathology, as well as persistent eosinophilia. Blood eosinophils should always be tested in unexplained cardiac disorders and may normalize even after low doses of corticosteroids.
|
ANCA-associated vasculitis |
Necrotizing vasculitis, with few or no immune deposits, predominantly affecting small vessels, associated with MPO-ANCA or PR3-ANCA. Not all patients have ANCA. |
|
Microscopic polyangiitis |
Necrotizing vasculitis, with few or no immune deposits, predominantly affecting small vessels. Necrotizing arteritis involving small and medium-sized arteries may be present. Necrotizing glomerulonephritis is very common. Pulmonary capillaritis often occurs. Granulomatous inflammation is absent |
|
Granulomatosis with polyangiitis (Wegener’s) |
Necrotizing granulomatous inflammation usually involving the upper and lower respiratory tract, and necrotizing vasculitis affecting predominantly small to medium- sized vessels. Necrotizing glomerulonephritis is common. |
|
Eosinophilic granulomatosis with polyangiitis (EGPA) |
Eosinophil-rich and necrotizing granulomatous inflammation often involving the respiratory tract, and necrotizing vasculitis predominantly affecting small to medium-sized vessels, and associated with asthma and eosinophilia. ANCA is most frequent when glomerulonephritis is present |
Table 1: Definitions of AAV according to Chapel Hill Consensus Conference (CHCC) in 2012.
|
Clinical, laboratory and biopsy criteria |
SCORE |
|
Maximum eosinophil count ≥1×109/L |
5 |
|
Obstructive airway diseas |
3 |
|
Nasal polyps |
3 |
|
Cytoplasmic antineutrophil cytoplasmic antibody (ANCA) or anti-Proteinase 3–ANCA positivity |
-3 |
|
Extravascular eosinophilic predominant inflammation |
2 |
|
Mononeuritis multiplex/motor neuropathy not due to Radiculopathy |
1 |
|
Haematuria |
-1 |
|
After excluding mimics of vasculitis, a patient with a diagnosis of small- or medium-vessel vasculitis could be classified as having EGPA if the cumulative score was ≥6 points. |
Table 2: ACR/EULAR Classification Criteria for EGPA
References
- Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol 27 (1951): 277-301.
- Kitching AR, Anders HJ, Basu N, et al. ANCA-associated vasculitis. Nat Rev Dis Primers 6 (2020): 71.
- Brucato A, Maestroni S, Masciocco G, et al. Il coinvolgimento cardiaco nella sindrome di Churg-Strauss [Cardiac involvement in Churg-Strauss syndrome]. G Ital Cardiol (Rome) 16 (2015): 493-500.
- Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 68 (2009): 310-317.
- De Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 52 (2005): 2461-2469.
- Metzler C, Hellmich B, Gause A, et al. Churg Strauss syndrome--successful induction of remission with methotrexate and unexpected high cardiac and pulmonary relapse ratio during maintenance treatment. Clin Exp Rheumatol 22 (2004).
- Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy 68 (2013): 261-273.
- Gioffredi A, Maritati F, Oliva E, et al. Eosinophilic granulomatosis with polyangiitis: an overview. Front Immunol 5 (2014): 549.
- Almaani S, Fussner LA, Brodsky S, et al. ANCA-Associated Vasculitis: An Update. J Clin Med 10 (2021): 1446.
- Miszalski-Jamka T, Szczeklik W, Sokolowska B, et al. Standard and feature tracking magnetic resonance evidence of myocardial involvement in Churg-Strauss syndrome and granulomatosis with polyangiitis (Wegener's) in patients with normal electrocardiograms.
- Yano T, Ishimura S, Furukawa T, et al. Cardiac tamponade leading to the diagnosis of eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a case report and review of the literature. Heart Vessels 30 (2015): 841-844.
- Dey M, Nair J, Sankaranarayanan R, et al. Myopericarditis as a presentation of eosinophilic granulomatosus with polyangiitis (EGPA). BMJ Case Rep 12 (2019): 230593.
- Yano T, Ishimura S, Furukawa T, et al. Cardiac tamponade leading to the diagnosis of eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a case report and review of the literature. Heart Vessels 30 (2015): 841-844.
- Sharma A, De Varennes B, Sniderman AD. Churg-Strauss syndrome presenting with marked eosinophilia and pericardial effusion. Can J Cardiol 9 (1993): 329-330.
- Grayson PC, Ponte C, Suppiah R, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann Rheum Dis 81 (2022): 309-314.
