Acute Antegrade TAVI Migration Successfully Treated with Snare Utilization and Near Valve-in-Valve (Viv) Implantation
Article Information
Nikolaos Tsanaxidis1*, Benjamin Wrigley1, James Cotton1, Deepu Balakrishnan1
1New Cross Hospital, Heart and Lung Centre, The Royal Wolverhampton NHS Trust, UK
*Corresponding author: Nikolaos Tsanaxidis, New Cross Hospital, Heart and Lung Centre, The Royal Wolverhampton NHS Trust, UK.
Received: 22 November 2020; Accepted: 04 December 2020; Published: 23 September 2022
Citation: Nikolaos Tsanaxidis, Benjamin Wrigley, James Cotton, Deepu Balakrishnan. Acute Antegrade TAVI Migration Successfully Treated with Snare Utilization and Near Valve-In-Valve (Viv) Implantation. Cardiology and Cardiovascular Medicine 6 (2022): 499-501.
View / Download Pdf Share at FacebookAbstract
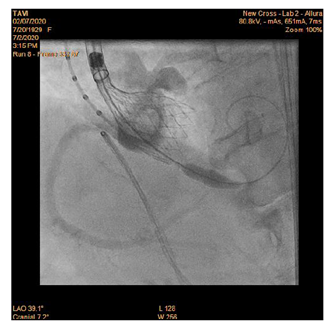
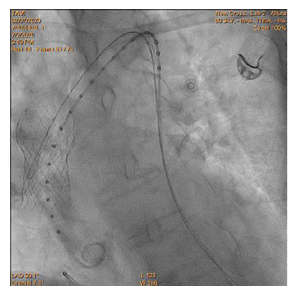
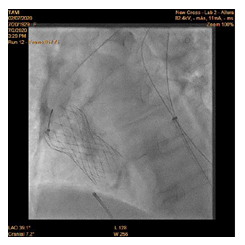
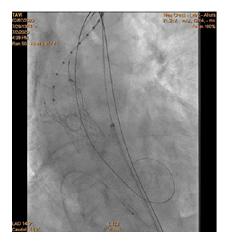
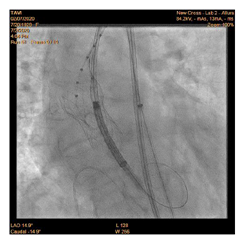
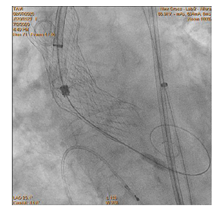
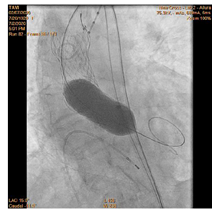
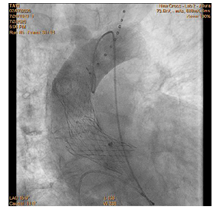
90-year-old woman admitted urgently in Cardiology ward at New Cross with acute heart failure symptoms on background of severe aortic stenosis. Also reported previous syncopal episodes. On admission echocardiogram performed which revealed aortic valve area of 0.48 cm2 and mean pressure gradient of 41mmHg. Her Left Ventricular Ejection Fraction was 35%. After stabilizing her with diuresis, she underwent computed tomography TAVI workup.This revealed reasonable ilio-femoral access and estimation of valvular annular size undertaken. Trans-catheter aortic valve implantation eventually took place. Ultrasound guidance used to gain access.6Fr sheath inserted into left femoral artery (graduated pigtail for aortogram),7Fr into left femoral vein (temporary pacing wire) and 6Fr into right femoral artery (RFA) which was pre-closed with 2 Proglides and upgraded to 9Fr sheath. Immediately,the aortic valve crossed in conventional manner and a 26mm Medtronic Evolut pro was implanted [1]. Unfortunately post release the valve migrated into ascending aorta,with pendular movements in an aneurysmal aorta [2]. As the patient was haemodynamically stable we implanted a larger valve after snaring the 26 Pro.A 9Fr long sheath inserted into RFA and an EN Snare (Merit Medical) used successfully to snare the migrated valve [3,4]. A 6Fr sheath introduced into the left radial artery (LRA) and graduated pigtail was inserted through there for landmarks. The aortic valve was re-crossed sequentially and a 29mm Evolut R manoeuvred carefully through first valve and implanted whilst pacing[5,6]. Further postdilatation with 26mm VACS 2 balloon (Osypka) at the annular and supra-annular level to expand the valve to optimal size [7]. Final result was satisfactory with at most mild AR [8].
Keywords
TAVI Migration; Aorta
Article Details
Supplementary Material (Discussion – Learning Points)
- CT measurements of the annular size are of utmost importance to decide on the size and choice of valves. In our case the CT was of very poor quality and had breathing artefacts at the level of the annulus making measurements virtually impossible. The patients herself was of very small stature making the large annular measurements obtained from the faulty CT appear disproportionate. Although in hindsight we realise that external physical appearance of tiny physical frame may have no bearing on their internal structures. A repeat CT, echocardiographic or angiographic assessments using pigtail or balloon sizing measurements of the annulus may have helped although none of them have been found to be as useful as CT guidance.
- A large sinus of Valsalva or aneurysmal Aorta should alert the operator to the possibility of Bicuspid Aortic valves which are usually larger in size. In addition one may encounter anchoring difficulties at the Aortic end after release as the valve is found floating in the aneurysmal Aorta not expressed to the walls. Here any upward movement caused upon release of valve in the absence of resistance from the Aortic walls may cause the valve to easily migrate especially if not optimally sized. A larger valve in any of these cases may be preferred if pre- release Aortogram shows mild to moderate AR.
- If the patient is haemodynamically stable, a larger valve can be inserted through the first valve after it has been stabilised with a snare. In our case we used an Ensnare to capture the valve although goose snares may be another option. Femoral access was used for snaring as we felt it may provide more coaxial anchoring and to avoid navigating a tortuous left arterial route to save time. In addition passage of the new valve will cause movement and tugging on the snared valve which will have to be pushed or pulled accordingly to enable re-entry through the valve and re-crossing of the Aortic valve. Care should be taken here as excessive movement of the valves in the ascending Aorta will risk thromboembolic phenomenon. Depending on the unfolding and ectasia of the Aorta radial route may be carefully chosen to align the snare along the axis of deployment. Long Radial artery sheaths may help prevent spam.
- While implanting the second valve, we tried to anchor the first valve against the Aortic wall using the crowns of the second valve, to prevent any further movement or migration. In our case although the crowns of the second valve was released inside the bottom edge of the first valve, it soon expanded further slipping out of the frame. We did not attempt to post-dilate the smaller valve to oppose to the Aortic wall to prevent any further disruption to the valve ring or the leaflets so as not to increase any further risk of thromboembolic phenomenon.
- Decision on optimal antiplatelet /anticoagulation regimen in these cases is difficult in the absence of strong evidence base. In our patient as she developed atrial fibrillation, we were happy for her to continue on NOAC for life. It is important to keep ACT above 300sec during the procedure and load with antiplatelet therapy if there are no bleeding complications immediately on completion of procedure to protect against thromboembolic phenomenon.