Accessory Breast Cancer: A Case Series
Article Information
Nicole Remmert1, Nawal Moin1, Karla Daniele2, Rakhsanda Layeequr Rahman1,2*
1Department of Surgery, Texas Tech University Health Sciences Center, Lubbock, TX, 79430 USA.
2Breast Center of Excellence School of Medicine, Texas Tech University Health Sciences Center, Lubbock, TX, 79430 USA.
*Corresponding Author: Rakhshanda Layeequr Rahman, Department of Surgery, Texas Tech University Health Sciences Center, Lubbock, TX, 79430 USA.
Received: 13 February 2023; Accepted: 23 February 2022; Published: 26 May 2023
Citation: Nicole Remmert, Nawal Moin, Karla Daniele, Rakhsanda Layeequr Rahman. Accessory Breast Cancer: A Case Series. Journal of Surgery and Research. 6 (2023): 217-220.
View / Download Pdf Share at FacebookAbstract
Accessory breast tissue (ABT) is ectopic breast tissue that occurs due to the failure of the embryonic mammary ridge to resolve during fetal breast development. This can occur anywhere along the milk line, with the axilla being the most common area of occurrence. Due to its pathophysiologic similarity with normal breast tissue, it is also susceptible to developing malignancies [1]. Primary accessory breast cancer accounts for 0.3-0.6% of all breast cancers [1]. The axillary area is the most commonly reported area of accessory breast carcinoma, accounting for 58% of reported ectopic cases [2]. Other reported areas include the parasternal line (18.5%), the subclavicular area (8.6%), the submammary region (8.6%), and the vulvar region (4%) [2]. Given the rarity of primary accessory axillary breast carcinoma, presentation and diagnosis can be challenging. Clinicians ought to be aware of this variance to ensure prompt management. Imaging plays an essential role in diagnosis. Treatment involves a multidisciplinary approach, including surgery, systemic therapy and radiation therapy as dictated by tumor biology and the stage of the disease. Here we report two cases of invasive ductal carcinomas arising in axillary accessory breast tissue.
Keywords
Accessory breast cancer; Axilla; Kajava classification; Invasive ductal carcinoma
Accessory breast cancer articles; Axilla; Kajava classification articles; Invasive ductal carcinoma articles
Accessory breast cancer articles Accessory breast cancer Research articles Accessory breast cancer review articles Accessory breast cancer PubMed articles Accessory breast cancer PubMed Central articles Accessory breast cancer 2023 articles Accessory breast cancer 2024 articles Accessory breast cancer Scopus articles Accessory breast cancer impact factor journals Accessory breast cancer Scopus journals Accessory breast cancer PubMed journals Accessory breast cancer medical journals Accessory breast cancer free journals Accessory breast cancer best journals Accessory breast cancer top journals Accessory breast cancer free medical journals Accessory breast cancer famous journals Accessory breast cancer Google Scholar indexed journals Axilla articles Axilla Research articles Axilla review articles Axilla PubMed articles Axilla PubMed Central articles Axilla 2023 articles Axilla 2024 articles Axilla Scopus articles Axilla impact factor journals Axilla Scopus journals Axilla PubMed journals Axilla medical journals Axilla free journals Axilla best journals Axilla top journals Axilla free medical journals Axilla famous journals Axilla Google Scholar indexed journals Kajava classification articles Kajava classification Research articles Kajava classification review articles Kajava classification PubMed articles Kajava classification PubMed Central articles Kajava classification 2023 articles Kajava classification 2024 articles Kajava classification Scopus articles Kajava classification impact factor journals Kajava classification Scopus journals Kajava classification PubMed journals Kajava classification medical journals Kajava classification free journals Kajava classification best journals Kajava classification top journals Kajava classification free medical journals Kajava classification famous journals Kajava classification Google Scholar indexed journals Invasive ductal carcinoma articles Invasive ductal carcinoma Research articles Invasive ductal carcinoma review articles Invasive ductal carcinoma PubMed articles Invasive ductal carcinoma PubMed Central articles Invasive ductal carcinoma 2023 articles Invasive ductal carcinoma 2024 articles Invasive ductal carcinoma Scopus articles Invasive ductal carcinoma impact factor journals Invasive ductal carcinoma Scopus journals Invasive ductal carcinoma PubMed journals Invasive ductal carcinoma medical journals Invasive ductal carcinoma free journals Invasive ductal carcinoma best journals Invasive ductal carcinoma top journals Invasive ductal carcinoma free medical journals Invasive ductal carcinoma famous journals Invasive ductal carcinoma Google Scholar indexed journals supraclavicular lymph nodes articles supraclavicular lymph nodes Research articles supraclavicular lymph nodes review articles supraclavicular lymph nodes PubMed articles supraclavicular lymph nodes PubMed Central articles supraclavicular lymph nodes 2023 articles supraclavicular lymph nodes 2024 articles supraclavicular lymph nodes Scopus articles supraclavicular lymph nodes impact factor journals supraclavicular lymph nodes Scopus journals supraclavicular lymph nodes PubMed journals supraclavicular lymph nodes medical journals supraclavicular lymph nodes free journals supraclavicular lymph nodes best journals supraclavicular lymph nodes top journals supraclavicular lymph nodes free medical journals supraclavicular lymph nodes famous journals supraclavicular lymph nodes Google Scholar indexed journals contralateral axillary breast tissue. articles contralateral axillary breast tissue. Research articles contralateral axillary breast tissue. review articles contralateral axillary breast tissue. PubMed articles contralateral axillary breast tissue. PubMed Central articles contralateral axillary breast tissue. 2023 articles contralateral axillary breast tissue. 2024 articles contralateral axillary breast tissue. Scopus articles contralateral axillary breast tissue. impact factor journals contralateral axillary breast tissue. Scopus journals contralateral axillary breast tissue. PubMed journals contralateral axillary breast tissue. medical journals contralateral axillary breast tissue. free journals contralateral axillary breast tissue. best journals contralateral axillary breast tissue. top journals contralateral axillary breast tissue. free medical journals contralateral axillary breast tissue. famous journals contralateral axillary breast tissue. Google Scholar indexed journals Periareolar injection articles Periareolar injection Research articles Periareolar injection review articles Periareolar injection PubMed articles Periareolar injection PubMed Central articles Periareolar injection 2023 articles Periareolar injection 2024 articles Periareolar injection Scopus articles Periareolar injection impact factor journals Periareolar injection Scopus journals Periareolar injection PubMed journals Periareolar injection medical journals Periareolar injection free journals Periareolar injection best journals Periareolar injection top journals Periareolar injection free medical journals Periareolar injection famous journals Periareolar injection Google Scholar indexed journals post-chemotherapy articles post-chemotherapy Research articles post-chemotherapy review articles post-chemotherapy PubMed articles post-chemotherapy PubMed Central articles post-chemotherapy 2023 articles post-chemotherapy 2024 articles post-chemotherapy Scopus articles post-chemotherapy impact factor journals post-chemotherapy Scopus journals post-chemotherapy PubMed journals post-chemotherapy medical journals post-chemotherapy free journals post-chemotherapy best journals post-chemotherapy top journals post-chemotherapy free medical journals post-chemotherapy famous journals post-chemotherapy Google Scholar indexed journals breast tissue develops articles breast tissue develops Research articles breast tissue develops review articles breast tissue develops PubMed articles breast tissue develops PubMed Central articles breast tissue develops 2023 articles breast tissue develops 2024 articles breast tissue develops Scopus articles breast tissue develops impact factor journals breast tissue develops Scopus journals breast tissue develops PubMed journals breast tissue develops medical journals breast tissue develops free journals breast tissue develops best journals breast tissue develops top journals breast tissue develops free medical journals breast tissue develops famous journals breast tissue develops Google Scholar indexed journals puberty articles puberty Research articles puberty review articles puberty PubMed articles puberty PubMed Central articles puberty 2023 articles puberty 2024 articles puberty Scopus articles puberty impact factor journals puberty Scopus journals puberty PubMed journals puberty medical journals puberty free journals puberty best journals puberty top journals puberty free medical journals puberty famous journals puberty Google Scholar indexed journals
Article Details
Case Series
Case 1:
A 49-year-old female presented for evaluation of a right axillary mass present for 1 month. On examination, a 2 cm mass was palpated in the right axilla, which correlated with mammographic and sonographic findings (Figure 1). A biopsy of the mass revealed a grade 2, ER/PR positive, HER2 negative invasive ductal carcinoma (IDC). The patient underwent ultrasound-guided, margin-negative resection of the right accessory breast with sentinel lymph node biopsy (SLNB). Final pathology confirmed a 1.5 cm IDC and 1 of 3 lymph nodes (LNs) positive for metastatic carcinoma (pT1cN1a). The patient was treated with adjuvant chemotherapy (docetaxel and cyclophosphamide) followed by tamoxifen and radiation with 5000 cGY (delivered in 25 fractions) to the right breast and right axilla covering the regional lymph nodes, including supraclavicular lymph nodes but not the internal mammary lymph nodes followed by boost to the right breast cavity in the right axilla of 100 cGy (delivered in 5 fractions) (Figure 2).
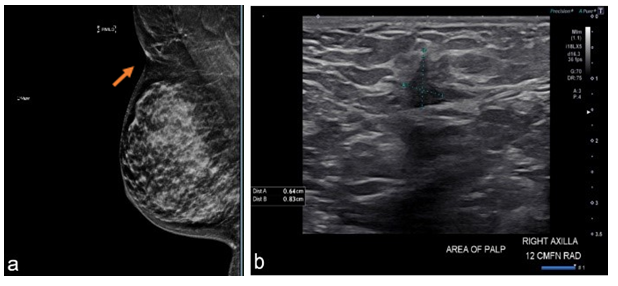
Figure 1-Case1:(a)MLOmammogramwithpresence ofectopic breast tissue in axilla(site of palpable abnormality is marked with a triangle; red arrow points to cancer).(b) Ultrasoundof right axilla showing irregular hypoechoic massmeasuring 0.6 x 0.8 x 0.5 cm.
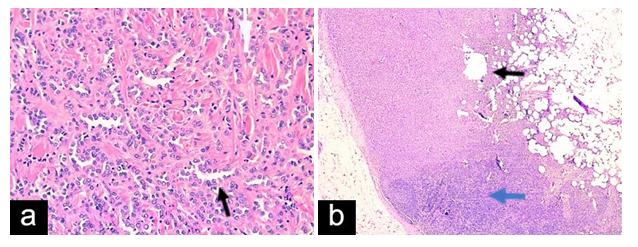
Figure 2- Case 1:(a)Breast tissue withfocus ofinvasive ductal carcinoma with lobular features,(H&E x 20). (b) Lymph nodewith metastatic carcinoma(H&E x 10).Tumorcells marked byblack arrow. Blue arrow shows lymphoid cells.
Case 2:
A 47-year-old female presented for evaluation of a palpable and enlarging mass to her right axilla, which had been present for 12 months. On physical examination, the patient was found to have bilateral accessory axillary breast tissue type 4 on Kajava classification (Figure 3), along with a palpable 3 cm mass in the right axilla with overlying skin thickening. Axillary lymph nodes were challenging to palpate due to the presence of accessory breast tissue. The patient’s diagnostic workup included sonographic and mammographic evaluation, which revealed a suspicious right axillary mass (Figure 4), denoted as BI-RADS 4. Ultrasound-guided core needle biopsy of the mass revealed IDC with lobular features, grade 2, ER/PR positive, HER2 negative. The patient underwent margin-negative resection of the involved side with SLNB and excision of the contralateral axillary breast tissue. Periareolar injection of technetium Tc 99m tilmanocept and peritumoral injection of isosulfan blue dye were performed for sentinel lymph node identification. Two lymph nodes were identified as sentinel nodes, one with a high radioactive count but no blue dye and one blue node without a radioactive count. Histologic evaluation of the surgical specimen demonstrated a 2.5 cm IDC with lobular features on the right side and one of the two lymph nodes, the blue node, positive for metastatic carcinoma (pT2pN1a) (Figure 5). At the time of this writing, the patient was undergoing adjuvant systemic therapy (adriamycin and cyclophosphamide) and had plans to receive radiotherapy and endocrine treatment post-chemotherapy.
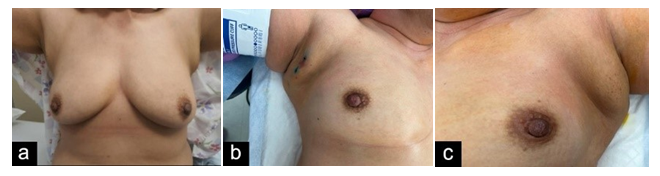
Figure 3-Case2:(a)Clinical photograph of(a)bilateral accessory breast tissuein axillary region(b)rightectopic breast tissuewith blue peritumoral dye injected(c)left normal ectopic breast.
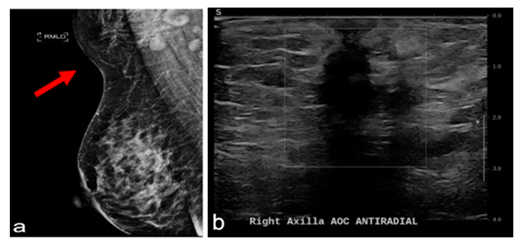
Figure 4- Case 2(a)MLO mammogramwith arrow pointing to accessory breast tissuewithspiculatedmass.(b) Ultrasoundof right axillashowsanill-definedheterogeneouslesionmeasuring 3.4 x 1.2 x 1.4 cm.
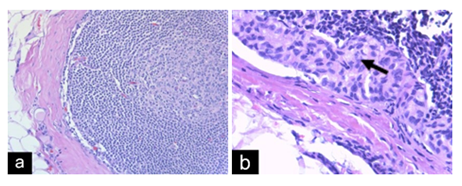
Figure 5- Case 2:(a)Breast tissue with focus of invasiveductal carcinoma with lobular features(H&E x20).(b) Lymph nodewith metastatic carcinoma(H&E x40).Arrow markstumorcells.
Discussion
During the fourth week of embryogenesis, breast tissue derived from ectoderm emerges from the mammary ridge. The ridges form bilaterally along the ventral surface of the body and run from the anterior axillary folds to the medial part of the inguinal folds. During normal development, all ridges, except those located in the pectoral area, regress. When the standard embryological regression fails, ectopic residual breast tissue develops under the hormonal influence at puberty.
A classification system developed by Kajava in 1915 is used to describe the spectrum of presentations of ectopic breast tissue (EBT) [4]. Kajava’s classification system differentiates supernumerary breast tissues into 8 different categories, as demonstrated in table 1 [1]. Both cases presented here fall into Class 4 of the Kajava system.
|
Kajava Classification |
Supernumerary breast components |
|
Class 1 |
Nipple, areola, and glandular tissue |
|
Class 2 |
Glandular tissue and a nipple; no areola |
|
Class 3 |
Areola and glandular tissue |
|
Class 4 |
Glandular tissue only – Most common type |
|
Class 5 |
Nipple and areola |
|
Class 6 |
Nipple only |
|
Class 7 |
Areola only |
|
Class 8 |
Patch of hair |
Table 1: Kajava’s classification of supernumerary breast tissue
Accessory breast tissue is rare, with a reported incidence between 0.4-6% [2], and pathologies of the tissue are even rarer. Diagnosis of accessory breast tissue in the axillary region can be challenging due to the broad range of pathologies that can manifest in the axilla, including but not limited to lymphoma, lipoma, adenocarcinoma of sweat glands, or hidradenitis suppurativa [1,9]. On average, women presenting with ectopic breast cancer are younger, with a reported median age of 53 years [2]. According to the National Cancer Institute, the median age of women diagnosed with breast cancer is 63 years [10]. In the cases presented here, both women were premenopausal, were symptomatic at presentation and had pathologic lymph node involvement. Screening for axillary breast tissue is similar to normal breast tissue. However, due to its location, axillary accessory breast tissue is usually not fully visualized on a standard mammogram; therefore, other screening modalities must be used [1]. Ultrasound and MRI are useful tools when assessing ectopic breast tissue and can help differentiate ectopic breast tissues from other pathologies [1,5,7]. In case 2, the patient’s axillary masses were initially thought to be axillary metastasis from pectoral breast cancer. MRI helped in identifying the axillary mass as the primary breast cancer.
Similar case series of accessory breast carcinoma (ABC) have reported lymph node involvement in as high as 46% of cases at the time of diagnosis [2,6]. This observed advanced stage at presentation could be attributed to a delay in diagnosis, propensity of malignant degeneration of ectopic breast tissue, and proximity of the axillary ABC to axillary lymph nodes resulting in higher rates of node-positive disease. The difficulty in assessing axillary lymph nodes clinically in the presence of axillary ABT and reported high rates of node-positive disease has led some surgeons to carry out axillary lymph node dissection (ALND) on a routine basis. In a study by Maki et al. accessory breast carcinoma was most commonly treated with radical resection and axillary lymph node dissection [11]. This results in overtreatment in many cases where lymph nodes were found to be negative [8].
There are no National Comprehensive Cancer Network (NCCN) guidelines for managing ABC. Literature on ABC shows the management of ABC to be similar to pectoral breast cancer using a multidisciplinary approach, including surgery, systemic therapy, and radiation depending on the staging and tumor biology [12].
Performing SNLB in a clinical node-negative ABC is somewhat controversial due to the inability to accurately palpate axillary lymph nodes in the presence of an axillary mass, making it difficult to discern from lymphadenopathy and the potential altered lymphatic drainage of aberrant tissue. Lymph node mapping may help to accurately identify sentinel lymph nodes and guide the surgeon in performing SNLB vs. ALND. No current guidelines from the American Society of Breast Surgeons or similar entities exist on whether a tracer should be injected periareolar or peritumorally in the axilla when localizing the sentinel node in the setting of ABC. SNLB success may be affected in cases where lymphatics have been disrupted by prior surgery in the axillary region [9,11]. In the cases presented here, tracers were injected peritumorally and periareolarly, blue dye was injected peritumorally, and a radioactive tracer was injected in periareolar space, which aided in localization and performing of SNLB successfully.
The role and extent of radiotherapy for ABC have yet to be clearly defined. It is unclear if radiation is needed after the complete removal of ABC and if the pectoral breasts should be included in the radiation field. Zhang et al. share the belief that lymph node metastasis is more likely to occur early in in ABC near the axillary region and therefore radiotherapy at the tumor site has been recommended with or without axillary lymph node metastasis to increase local control and reduce the risk of local recurrence [13,14]. In our first case, the patient received radiation to her right axillary region, which housed the primary tumor, the ipsilateral disease-free right breast, and the supraclavicular region. At the two-year follow-up, the patient had no evidence of locoregional recurrence. Radiotherapy to the ipsilateral disease free pectoral breast remains an area of controversy.
Conclusion
Patients with accessory breast tissue should be educated by their healthcare provider about the risk of cancer in the area and advised to report any abnormalities promptly. Physical examination of accessory breast tissue should be included in yearly breast examinations to rule out abnormalities. In the presence of accessory breast tissue, any suspicious masses in the axillary region identified by clinicians or reported by the patients should be subjected to a diagnostic workup akin to breast masses.
Awareness of this condition and the potential for malignancy should be on the clinician’s radar when patients present for evaluation of “swelling” in areas prone to hosting accessory breast tissue. Diagnosing ABC should include detailed clinical history, physical examination, imaging, and biopsy with histopathological studies.
Conflicts of interest
We have no conflicts of interest to disclose.
References
- DeFilippis E, Arleo E.The ABCs of Accessory Breast Tissue: Basic Information Every Radiologist Should Know 202 (2014): 1157-1162
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol 3 (1994): 295-304.
- Khatib, Yasmeen. Varied presentations of ectopic breast - polymastia, fibroadenoma, and carcinoma arising from ectopic breast tissue. Clinical Cancer Investigation Journal 4 (2015): 539-542.
- Kajava Y. The proportions of supernumerary nipples in the Finnish population. Duodecim 31 (1915): 143-170.
- Addae JK, Genuit T, Colletta J, Schilling K. Case of second primary breast cancer in ectopic breast tissue and review of the literature. BMJ Case Rep 14 (2021): e241361.
- Zhang S, Yu YH, Qu W, et al. Diagnosis and treatment of accessory breast cancer in 11 patients. Oncol Lett. 10 (2015): 1783-1788.
- MRI Appearance of Accessory Breast Tissue: A Diagnostic Consideration for an Axillary Mass in a Peripubertal or Pubertal Girl Tal Laor, Margaret H. Collins, Kathleen H. Emery, Lane F. Donnelly, Kevin E. Bove, and Edgar T. Ballard American Journal of Roentgenology 183 (2004): 1779-1781.
- Friedman-Eldar O, Melnikau S, Tjendra Y, et al. Axillary Reverse Lymphatic Mapping in the Treatment of Axillary Accessory Breast Cancer: A Case Report and Review of Management. European Journal of Breast Health 18 (2022): 1-5.
- Thasanabanchong P, Vongsaisuwon M. Unexpected presentation of accessory breast cancer presenting as a subcutaneous mass at costal ridge: A case report. Journal of Medical Case Reports 14 (2019): 23-66.
- Female Breast Cancer (2023).
- Takahashi E, Terata K, Nanjo H, et al. A male with primary accessory breast carcinoma in an axilla is strongly suspected of having hereditary breast cancer. Int Cancer Conf J 10 (2021): 107-111.
- Patel BK, Jafarian N, Abbott AM, et al. Imaging Findings and Management of Primary Breast Cancer in Accessory Axillary Breast Tissue. Clin Breast Cancer 15 (2015): e223-229.
- Zhang J, Zhang W, Min M, et al. Axillary accessory breast cancer with persistent leftsuperior vena cava: A case report and treatment controversy. International Journal of Surgery Case Reports 73 (2020): 71-74.
- Youn HJ, Jung SH. Accessory Breast Carcinoma. Breast Care 4 (2009): 104-106.
