Abatement of Forskolin-Induced Intestinal Fluid Secretion with Penicillin
Article Information
Earnest P Chen1#*, Jason L Own2,3#, John P Geibel 2,3,4*
1Icahn School of Medicine at Mount Sinai, New York, New York, USA
2Department of Surgery, Yale University, School of Medicine, New Haven, Connecticut, USA
3Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, Connecticut, USA
4The John B. Pierce Laboratory, New Haven, Connecticut, USA
#These authors contributed equally to this work
*Corresponding authors: John P Geibel, Department of Surgery Department of Cellular and Molecular Physiology Yale University School of Medicine, USA.
Earnest P Chen, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Received: 01 February 2024; Accepted: 06 February 2024; Published: 01 March 2024
Citation: Earnest P Chen, Jason L Own, John P Geibel. Abatement of Forskolin-Induced Intestinal Fluid Secretion with Penicillin. Journal of Biotechnology and Biomedicine. 7 (2024): 153-159.
View / Download Pdf Share at FacebookAbstract
Background: Antibiotics have been classically used as a method to treat diarrhea-related pathologies by modulating the gut microbiome. We recently reported that penicillin may protect against disease- induced excessive fluid and electrolyte secretion via a genetics-independent, microbiome- independent mechanism in individual colonic crypt cells. Therefore, we examined whether penicillin can be utilized as a microbiome-independent therapeutic for diarrhea, a disease that takes millions of lives every year, especially in developing countries.
Aim: To investigate whether penicillin has microbiome-independent protective effects against fluid secretion in rat small intestine at the whole-tissue level.
Methods: Small intestine segments were harvested from male Sprague Dawley rats, and mounted into an ex-vivo intestinal perfusion machine, which sustains extraluminal (serosa/basolateral) and intraluminal (mucosal/luminal) perfusion. The segments were perfused extraluminally with Ringer-HEPES and with varying doses (0.25mM, 0.50mM, 1.0mM, 2.5mM and 5.0mM) of Penicillin G (Pen-G) sodium salt intraluminally. Experiments were performed with a known secretagogue forskolin (FSK) to induce rapid intraluminal fluid secretion. For all experimental conditions, fluid secretion was observed by changes in fluorescent signal from an intraluminal perfusate fluorescein isothiocyanate (FITC)-inulin, a nonabsorbable fluorescent marker, over a period of 80 minutes with luminal perfusate sampling every 20 minutes. All samples were collected in triplicate and the mean value is recorded. Results: A statistically significant decrease in fluid secretion occurred in the presence of Pen-G at all concentrations tested. As Pen-G dosage increased from 0.25 mM to 5.0 mM, the extent of fluid secretion decrease was proportional to the dose of Pen-G applied in the luminal perfusate.
Conclusion: Penicillin has a significant dose-dependent protective effect against FSK cAMP fluid secretion in induced models of diarrhea in the microbiome deficient rat small intestine. Penicillin can rapidly bring fluid secretion down to levels comparable to healthy controls.
Keywords
Diarrhea; Intestinal secretion; Penicillin; Antibiotic; Fluid loss
Article Details
Introduction
Even though there are currently several medications to prevent and treat diarrheal diseases, diarrhea is still a leading cause of death amongst the infant and elderly populations in many developing countries [1, 2]. These deaths could be easily avoided given necessary means; therefore, there is a demand for a direct and low-cost treatment.
Diarrhea can be caused by severe intestinal fluid and electrolyte secretion and can lead to death when paired with the inability to replenish the lost fluids and nutrients. The most common cause of diarrhea comes from pathogenic microbes that release of toxins (of the estimated 2.5 million deaths caused by diarrhea-related diseases, about 60% of which are caused by enterotoxin- producing bacteria [3]) leading to cyclic-adenosine monophosphate (cAMP) activation of Cl- secretion, and inhibition of electrolyte absorption, which in turn stimulates release of water in the intestine [4, 5]. Activation of cAMP targets the protein kinase A (PKA) pathway and activates membrane transporters such as the cystic fibrosis transmembrane regulator chloride channel (CFTR) leading to downstream secretion of intracellular Cl- [6, 7]. In addition, the increase in intracellular cAMP upregulates the influx of intracellular calcium ions at numerous levels ranging from cAMP-induced activation of cyclic nucleotide-gated (CNG) channels and hyperpolarization- activated cyclic nucleotide-gated (HCN) channels, to exchange protein directly activated by cAMP (EPAC) and PKA-induced activation of voltage-gated calcium channels [8]. The influx of calcium ions activates calcium-activated chloride channels (CACC) which further increases the downstream secretion of intracellular Cl-. The complication with diarrheal diseases is that ion transport influenced by stimulation of Cl- secretion results in excessive secretion of water [5, 9].
Recent studies show that antibiotics are a promising avenue for such a remedy. Current research reports that azithromycin, fidaxomicin, levofloxacin and ciprofloxacin are viable antibiotic therapies for acute watery diarrhea and dysentery [10]. Treatments employing the use of antibiotics and loperamide, an adjunct therapeutic, have shown to shorten the duration of the symptoms associated with traveler’s diarrhea when compared to any of the antibiotics on their own [10]. Because of previous antibiotic usage as treatment for diarrheal diseases, we investigated penicillin’s ability to abate intestinal fluid secretion to prevent acute diarrhea.
As an antimicrobial, penicillin is a beta-lactam antibiotic containing a four-membered beta- lactam ring which blocks the activity of transpeptidases which are enzymes that form peptide cross-linkages in bacteria [11]. Previous studies demonstrated that Penicillin can increase intracellular cyclic nucleotide levels in mammals [12]. We recently reported that members of the Penicillin family function in a microbiome-independent manner to activate H/K ATPase in isolated rat colonic crypt cells by possibly modulating the cAMP pathway [13, 14]. Due to the ability of H/K ATPase to uptake potassium, Penicillin may provide a potassium rescue mechanism that prevents life-threatening electrolyte and fluid losses. Although antibiotics are associated with diarrhea by altering the host microbiome, Penicillin may trigger a compensatory response to reabsorb electrolytes in the colon [14]. However, whether Penicillin offers similar protective effects in the mammalian small intestine is still unknown. Previous reports elucidated that forskolin (FSK), the first main labdane diterpenoid isolated from the Indian plant Plectranthus barbatus Andrews, increases intracellular cAMP and can in turn affect transmembrane ion transport [4, 15]. FSK directly activates adenylyl cyclase (AC) which increases intracellular cAMP levels in a variety of cell types including those found in the gastrointestinal tract [16]. In one particular study, Ao et al. demonstrated that lithocholic acid attenuates FSK-induced cAMP-dependent Cl- secretion within human colonic epithelial cells [36]. Thus, FSK-induced cellular fluid secretion has been repeatedly used as a model for studying secretory diarrhea [17, 18, 19, 20]. In this present study, we used FSK to mimic diarrheal conditions in the rat small intestine.
Here, we report penicillin’s ability to abate FSK-induced intestinal fluid secretion in the rat small intestine. Thus, there is use for penicillin, given acutely, to treat diarrheal disorders as a fast-response short-term alternative at the beginning of the therapeutic window to quickly modulate excessive intestinal fluid secretion.
Materials and Methods
Animal Model
Male Sprague-Dawley rats (Charles River, Wilmington, MA) with weights between 275g and 410g were housed in rooms with controlled climate, humidity, and 24-hour light cycles. Protocols for animal handling, euthanasia, and tissue harvesting were approved by Yale University’s Animal Care and Use Committee (Protocol #2018-10253). The animal protocol was designed to minimize pain or discomfort to the rats. A total of 35 rats were used with 5 rats per experimental group. This sample size was determined in one of our previously published studies [21].
Tissue Harvesting
Rats were euthanized via the overdose of the inhaled anesthetic Isoflurane (IsoThesia TM, 99%/mL) (Dublin, OH) in an enclosed chamber. Midline laparotomy was performed on rats and the distal small intestine, defined as 10 cm segments of the ileum closest to the cecum, was isolated [21]. Cold (4°C) HEPES-Ringer buffer solution (HRBS) (115 mM NaCl, 5 mM KCl, 1.2 MgSO4, 1 mM CaCl2, 10 mM glucose, 2 mM NaH2SO4, and 32.2 mM HEPES at pH 7.4 and an osmolality of 300 mOsm) was used to wash the lumen of each segment removing any residual intraluminal debris and to maintain tissue viability [22].
Intestinal Perfusion
An ex-vivo perfusion device originally described by Munoz-Abraham et al in 2015 was used to perfuse intraluminal and extraluminal fluids at a rate of 6 mL/min (mimicking biological fluid flow rates) at 37°C [23]. In all experiments, 37°C HRBS was perfused both intraluminally and extraluminally to prolong the viability of tissues by mimicking the biological extracellular conditions of the gut environment. Powdered fluorescein isothiocyanate coupled with inulin (FITC-inulin) (Sigma Aldrich, St. Louis, MO) added to the intraluminal HRBS solution obtaining a concentration of 100 µM and was used as a fluorescent indicator for the extent of fluid secretion. Penicillin G at various dosages were also added intraluminally to different experimental groups to observe the protective effects against FSK induced fluid secretion. FSK was used to increase levels of cAMP within the cell and as seen in diarrheal diseases. Extraluminal perfusion contained warm HRBS alone. The pH and osmolality (mOsm) of all perfused solutions were adjusted to 7.4 and 300 ± 5 respectively at 37°C.
Experimental Conditions
In this study, we define an experiment as the harvest of the distal small intestine segment for use on our isolated perfusion apparatus [24]. Three separate series of experiments were performed: Control, FSK-induced, and FSK + penicillin G (PenG). Five separate experiments were performed under control conditions with standard perfusate in the absence of both FSK and penicillin G. as negative control. The standard perfusate contained HRBS and Inulin-FITC intraluminally, and HRBS only extraluminally. Five separate experiments were conducted under diarrheal conditions (enhanced fluid secretion compared to control) with 10 μM FSK added to the intraluminal control perfusate. As mentioned previously, FSK induces fluid secretion potentially via the upregulation of cAMP [15]. Twenty-five experiments were performed under diarrheal conditions (10 µM FSK) with various concentrations of penicillin G (0.25 mM, 0.50 mM, 1.0 mM, 2.5 mM, and 5.0 mM) introduced into the lumen of the small intestine (five experiments per dose, 5 samples taken at each time point for each tissue). The doses chosen are based on the previous reports showing the appropriate levels of penicillin needed to modulate fluid movement in the rat intestine [17]. The data from the FSK-induced and FSK + penicillin G groups were then compared to the control data.
Data Collection
The Nano fluorospectrometer (Nanodrop 3300, Thermo Fisher Scientific Inc., Wilmington, DE) recorded instantaneous fluorescence intensity, measured in arbitrary fluorescence units, of intraluminal FITC-Inulin. An increase or decrease in fluorescence intensity indicates an absorption or secretion by the small intestine, respectively. Starting from time zero, replicates of five intraluminal fluid samples were collected every 20 minutes for a total duration of 80 minutes. A standard curve correlating known concentrations of FITC-Inulin in the standard perfusate (made that day for the experiment) with corresponding fluorescence intensities was used to determine intraluminal FITC-Inulin concentrations. Basolateral perfusate samples were also taken and measured periodically to ensure that there was no leakage or perforation of the intestinal segment. If there was a fluorescent signal detected in the basolateral perfusate, the experiment was discarded as this was indicative of a leak in the intestine [25].
Data and Statistical Analysis
We used the software package GraphPad Prism 8.0.1 (GraphPad Software, La Jolla, CA) to calculate the linear regression of change in FITC-Inulin concentration over time to determine the slope of each experimental condition. The average of each slope and standard error was then recorded and graphed as previously described [26]. The percent change in FITC-Inulin concentration of each experiment was determined by dividing the change in FITC-Inulin concentration over the 80-minute period by the initial FITC-Inulin concentration. The average percent change with its standard error was graphed for each experimental condition. Student t-test analyses were used to determine statistical significance, comparing negative control with treatment groups and positive control. P-values < 0.05 were considered statistically significant.
Results
Modeling Diarrheal Conditions with Forskolin in the Small Intestine
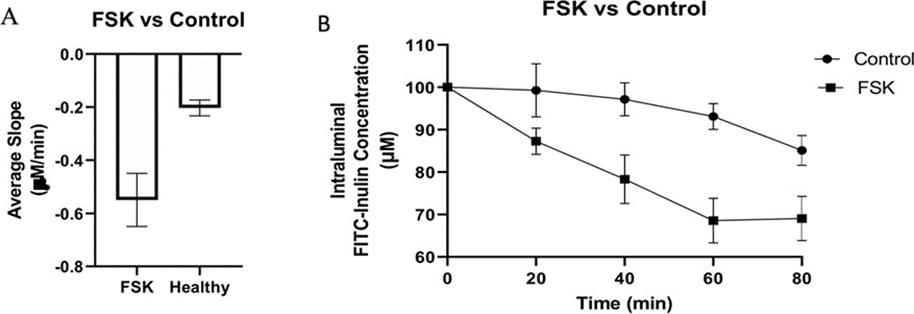
To determine the levels of intraluminal fluid secretion under control conditions in the intestinal segments we examined the change in FITC-Inulin concentration (as an increase or decrease in fluorescence in the collected fluid) in the lumen in the absence of FSK or penicillin G (PenG). Under these conditions there was a 17.67% decrease in FITC-Inulin concentration over the period of 80 min (Table 1, Figure 1). The fitted slope of this decrease was -0.2032 µM FITC-Inulin/min. This reflects the resting or basal level of fluid secretion (control). The fluorescence decreases as it is diluted with fluid secreted into the lumen. We next examined the effect of adding FSK to the intraluminal perfusate. The addition of FSK alone resulted in a ~35% decrease in FITC-Inulin concentration at 80 min with a slope of -0.5495 µM/min (Fig 2A, Table 1). This reflects the increased fluid secretion elicited by FSK. FSK stimulated fluid secretion was significantly greater with almost a 2-fold decrease in FITC-Inulin concentration compared to control conditions (34.92% vs 17.67%) (table 1 and Fig. 1b).
|
Treatment Group |
Average Percent Decrease in FITC-Inulin Concentration (%) |
SEM of Percent Decrease in FITC-Inulin Concentration |
Average slope of Change in FITC- Inulin Concentration (μM/min) |
SEM of Slope of Decrease in FITC-Inulin Concentration |
|
FSK |
34.92* |
±5.168 |
-0.5495* |
±0.0999 |
|
0.25 mM Pen G. |
24.18 |
±7.298 |
-0.3783 |
±0.1338 |
|
0.50 mM Pen G. |
19.3 |
±6.110 |
-0.2267 |
±0.1003 |
|
1.0 mM Pen G. |
21.98 |
±2.649 |
-0.2622§ |
±0.0446 |
|
2.5 mM Pen G. |
19.36§ |
±2.101 |
-0.2723§ |
±0.0293 |
|
5.0 mM Pen G. |
16.95§ |
±3.238 |
-0.2133§ |
±0.0433 |
|
Control |
17.67 |
±1.874 |
-0.2032 |
±0.0299 |
|
*Values were significantly different compared to the Healthy group. P-value= 0.03 for average percent decrease and p-value= 0.02 for average slope. |
||||
Table 1: Table of average percent decrease, average slope of change in FITC-Inulin, and their standard errors from the mean over 80 min. Student t-tests were used to determine statistical significance between groups.
Figure 1: Comparing FITC-Inulin concentration changes between FSK and the Control. The decrease in FITC-Inulin concentration in 10 cm distal rat small intestine segments illustrated as the average slope (A) and time course (Β) with standard error bars over 80 min with and without FSK. Five small intestinal segments from different rats were used per experimental condition and at each time point per segment, three separate FITC-Inulin measurements were recorded.
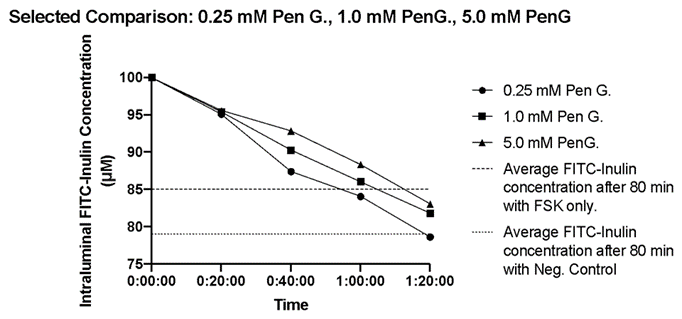
Figure 2: The time course of the change in intraluminal FITC-inulin concentrations in small intestinal segments in the presence of FSK and PenG concentrations of 0.25 mM, 1.0 mM and 5.0 mM. Five distal small intestinal segments were used per experimental condition and the average FITC-inulin concentrations were plotted every 20 minutes. The dashed line represents the average FITC-Inulin concentration after 80 min under control conditions (no FSK and no PenG). The dotted line represents the average FITC-Inulin concentration after 80 min under FSK only conditions.
Dosage Dependent Protective Effects of Penicillin G. Against Forskolin
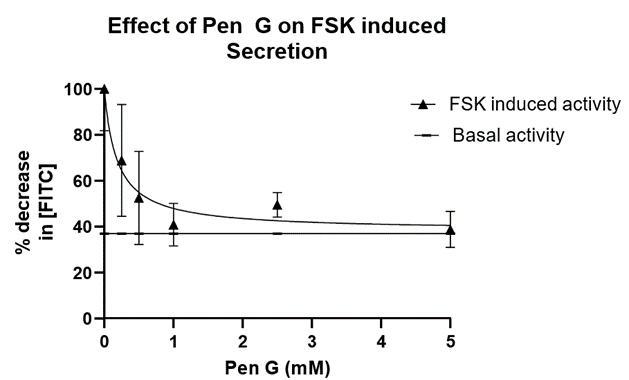
In a separate additional series, we studied the effects of PenG on the FSK stimulated fluid secretion. (Penicillin G: 0.25 mM, 0.50 mM, 1.0 mM, 2.5 mM, and 5.0 mM). HRBS containing a single dose of PenG were perfused intraluminally to each of the small intestinal segments treated with FSK (10 µM). There was a significant dose dependent decrease in fluid secretion as the concentration of Penicillin G was increased. The addition of 0.25 mM PenG resulted in a 24.18% decrease of FITC-Inulin concentration with a slope of -0.3783 µM/min (Table 1, Figure 3). In the presence of 5.0 mM PenG (our maximum dose) we observed a 16.95% decrease at -0.2133 µM/min in FITC-Inulin concentration (Table 1). This dose (5.0 mM) elicited the maximal effect on fluid secretion we obtained. With the addition of intermediate PenG concentrations (0.50 mM, 1.0 mM, and 2.5 mM PenG), there was a 19.30, 21.98, 19.36 percent decrease in FITC-Inulin concentrations, respectively, with slopes of -0.2267 µM/min, -0.2622 µM/min, -0.2723 µM/min, respectively. Although there was no significant difference between all treatment groups compared to basal conditions, shown in Figure 2 are the FSK induced rates of change in FITC-Inulin detection in the presence of PenG concentrations of 0.25 mM, 1.0 mM. When compared to 5.0 mM PenG the concentration of FITC-Inulin approaches that of control or “healthy intestinal” secretion across segments after 80 minutes. This can also be seen in Table 1. We also analyzed the decrease in fluorescence as a function of PenG concentration. This is shown in Figure 3 where we plotted the data in a nonlinear fit (inhibitor vs. response curve) using GraphPad. The IC50 of PenG is calculated from the Prism analysis fit to be 0.18 mM. The linear line in Figure 3 is drawn to demonstrate the control level in the decrease in FITC-inulin with application of FSK and no PenG. Thus, PenG is able to counteract the induced fluid secretion produced with FSK. This would indicate that PenG could be useful to reduce the excess fluid secretion observed in diarrheal diseases.
Figure 3: Effect of PenG on FSK induced secretion. The data from Table 1 (change in FITC- inulin concentration) was fit using a nonlinear least square fit (inhibitor vs response analysis, GraphPad). The IC50 of PenG is calculated to be 0.18 mM. The solid linear line was drawn to indicate the basal (control) activity in the absence of FSK and PenG.
Discussion
Throughout the history of medicine, many medications have been found to have alternative uses outside their originally intended function. In the field of Gastroenterology, current research has shown that the antibiotics azithromycin, fidaxomicin, levofloxacin, and ciprofloxacin all have been deployed as alternative therapies for cases of acute watery diarrhea and dysentery. There are many non-antibiotic drugs that target chloride channels which have the potential to modulate diarrhea-related fluid secretion [27, 28]. For example, NSAIDS such as diclofenac, ibuprofen, niflumic acid, and flufenamic acid have been shown to inhibit CFTR, a cAMP-activated apical Cl- channel, and can abate fluid secretion in secretory epithelia [27, 29, 30, 31]. Metformin, a AMP- activated protein kinase (AMPK) activator and a common drug to treat type 2 diabetes, also has inhibitory effects on CFTR and has been reported to decrease cholera toxin-induced chloride secretion in human and murine intestines [28]. In this study, we show that penicillin G, another commonly prescribed antibiotic, also has the potential to be repurposed and used as a therapeutic measure against cases of diarrhea and dysentery. A major consideration is also the cost savings using penicillin provides. A quick check of pharmacy prices shows azithromycin cost about $1.50 per tablet while penicillin is about $0.23 in the United States.
The breakthrough discovery of penicillin occurred in 1928 when it first became widely recognized as the original modern antibiotic [32]. Penicillin originally found its use as a cure for various infectious diseases, such as bacterial endocarditis, meningitis, pneumococcal pneumonia, and streptococcal septicemia [11]. However, since then, penicillin is no longer being used to treat these infections due to the rapid spread of bacterial resistance to penicillin. Although penicillin isn’t being widely utilized to treat bacterial infections, it has found new uses as a treatment for sexually transmitted diseases, and penicillin G currently remains the drug of choice for treating all stages of syphilis [33].
Our data suggests another off-label use for penicillin G for not only treating bacterial infections, but also as a treatment for diarrhea and dysentery. Our study using an ex-vivo perfusion device, demonstrates that the intraluminal application of penicillin G provides a protective effect on the distal small intestine against FSK induced fluid secretion (mimicking diarrhea or dysentery) in the rat model. The significant effects of penicillin G perfusion as a means to suppress secretagogues induced fluid secretion suggest that this antibiotic can be used both before the addition of FSK to prevent secretion and after the addition of FSK to abate the secretion once it has started and return it too normal. Therefore, Penicillin G has the potential to be a low-cost and easily accessible short-term prophylactic and therapeutic for diarrheal diseases intended for use in developing countries.
At the moment, Ciprofloxacin remains the most commonly used pharmaceutical in the prevention of diarrhea [32]. However, Ciprofloxacin has been shown to possess arrhythmogenic properties and is linked to the development of cardiac arrhythmias and an increased risk of tears of cardiac vasculature [34]. Therefore, penicillin G could be used as a treatment option for patients with pre-existing heart conditions, as it has no reported cardiac side effects, unlike present conventional antibiotic treatments. Penicillin G also has the reputation of being virtually non- toxic [37]. The absence of direct toxicity allows penicillin to be safely given in larger doses than any comparable biologically-active substance [38]. This makes the potential for penicillin G to be both safer and more effective than current antidiarrheals.
Our study demonstrated that intraluminal application of penicillin G. provides a dose dependent protective effect on rat distal small intestine against FSK induced fluid secretion. Penicillin doses down to 0.5 mM in the presence of FSK prevented fluid secretion with no statistical difference in fluid secretion compared to a healthy intestinal segment perfused with control solutions. The counteracting effects of penicillin G on FSK exposed tissue indicate the antibiotic’s ability to prevent excessive fluid loss that is associated with exposure to FSK and cellular fluid loss, which could be potentially due to cAMP upregulation[15]. Our results suggest a potential function for penicillin G as a cost-effective, rapid low-risk prophylactic and therapeutic measure against diseases with diarrhea related symptoms that acts through a microbiome- independent pathway in the small and large intestines.
One caveat is that antibiotics such as penicillin could upset the balance of the gut microbiome leading to opportunistic drug-resistant bacteria such as Clostridium difficile to infect the host intestinal tract[35, 36]. This secondary infection may induce additional diarrheal symptoms. Because we show that PenG acts through a microbiome-independent pathway modulating intestinal fluid secretion, Penicillin has potential to be an effective treatment for individuals lacking a functional gut microbiome. Future studies can also be pursued to test drug combinations that prevent secondary bacterial infections during Penicillin treatment of diarrhea-related fluid and electrolyte secretion.
Institutional Animal Care and Use Committee Statement
Animal protocols approved by Yale Institutional Animal Care and Use Committee. The Institutional Animal Care and Use Committee Approval Number is IACUC 2018-10253.
Funding
Charles Ohse Award from the Department of Surgery at the Yale University School of Medicine.
Conflict of Interest Statement
None of the authors have any conflicts of interest.
Data Sharing Statement
No additional data are available.
ARRIVE Guidelines Statement
The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
References
- Gore JI, Surawicz C. Severe acute diarrhea. Gastroenterol Clin North Am 32 (2003): 1249-
- Kotloff KL. The Burden and Etiology of Diarrheal Illness in Developing Countries. Pediatr Clin North Am 64 (2017): 799-814.
- Wongsamitkul N, Sirianant L, Muanprasat C, et al. A plant-derived hydrolysable tannin inhibits CFTR chloride channel: a potential treatment of diarrhea. Pharm Res 27 (2010): 490-497.
- Viswanathan VK, Hodges K, Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol 7 (2008): 110-119.
- Barrett KE. Epithelial transport in digestive diseases: mice, monolayers, and mechanisms. Am J Physiol Cell Physiol 318 (2020): C1136-C1143.
- Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med 2 (2012): a009563
- Thiagarajah JR, Verkman AS. Chloride channel-targeted therapy for secretory diarrheas. Curr Opin Pharmacol 13 (2013): 888-894.
- Rasmussen H, Goodman DB. Calcium and cAMP as interrelated intracellular messengers. Ann N Y Acad Sci 253 (1975): 789-796.
- Hoque KM, Woodward OM, van Rossum DB, et al. Epac1 mediates protein kinase A-independent mechanism of forskolin- activated intestinal chloride secretion. J Gen Physiol 135 (2010): 43–58.
- Lysyy T, Lalani AS, Olek EA, et al. The calcium-sensing receptor: A novel target for treatment and prophylaxis of neratinib-induced diarrhea. Pharmacol Res Perspect 7 (2019): e00521
- Munoz-Abraham AS, Judeeba S, Alkukhun A, et al. A new method to measure intestinal secretion using fluorescein isothiocyanate-inulin in small bowel of rats. J Surg Res 197 (2015): 225-230.
- Raabe W, Nicol S, Gumnit RJ, et al. Focal penicillin epilepsy increases cyclic GMP in cerebral cortex. Brain Res 144 (1978): 185-188.
- Egger A, Barahona M, Bauer S, et al. Penicillin Modulates Fluid Secretion through the Cyclic Nucleotide Pathway in Rat Colonic Crypts. Academic Surgical Congress (2020).
- Baratta VM, Norz V, Barahona MJ, et al. Penicillin G Induces H+, K+-ATPase via a Nitric Oxide-Dependent Mechanism in the Rat Colonic Cell Physiol Biochem 54 (2020): 1132-1142.
- Alasbahi RH, Melzig Forskolin and derivatives as tools for studying the role of cAMP Pharmazie 67(2012): 5-13.
- Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 23 (2003): 305-314.
- Barahona MJ, Maina RM, Lysyy T, et al. Activation of the Calcium Sensing Receptor Decreases Secretagogue- Induced Fluid Secretion in the Rat Small Intestine. Front Physiol 10 (2019): 439.
- Murugan S, Purusothaman D, Richard EJ, et al. Anti-diarrhoeal activity of a polyherbal formulation in rats and elucidation of its cellular mechanisms. Avicenna J Phytomed 10 (2020): 417-427.
- Wongsamitkul N, Sirianant L, Muanprasat C, et al. A plant-derived hydrolysable tannin inhibits CFTR chloride channel: a potential treatment of diarrhea. Pharm Res 27 (2010): 490-497.
- Feng Z, Carlson D, Poulsen HD. Zinc attenuates forskolin-stimulated electrolyte secretion without involvement of the enteric nervous system in small intestinal epithelium from weaned Comp Biochem Physiol A Mol Integr Physiol 145 (2006): 328-333.
- Maina RM, Barahona MJ, Geibel P, et al. Hydrogel-based 3D bioprints repair rat small intestine injuries and integrate into native intestinal tissue. J Tissue Eng Regen Med 15 (2021): 129-138.
- Rogers AC, Huetter L, Hoekstra N, et al. Activation of AMPK inhibits cholera toxin stimulated chloride secretion in human and murine intestine. PLoS One 8 (2013): e69050.
- Pongkorpsakol P, Pathomthongtaweechai N, Srimanote P, et al. Inhibition of cAMP-activated intestinal chloride secretion by diclofenac: cellular mechanism and potential application in cholera. PLoS Negl Trop Dis 8 (2014): e3119.
- Devor DC, Schultz BD. Ibuprofen inhibits cystic fibrosis transmembrane conductance regulator-mediated Cl- secretion. J Clin Invest 102 (1998): 679-687.
- Scott-Ward TS, Li H, Schmidt A, et al. Direct block of the cystic fibrosis transmembrane conductance regulator Cl(-) channel by niflumic acid. Mol Membr Biol 21 (2004): 27-38.
- Pongkorpsakol P, Yimnual C, Chatsudthipong V, et al. Cellular mechanisms underlying the inhibitory effect of flufenamic acid on chloride secretion in human intestinal epithelial cells. J Pharmacol Sci 134 (2017): 93-100.
- Gaynes R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Emerg Infect Dis 23 (2017): 849-853
- Diemert DJ. Prevention and Self-Treatment of Traveler's Diarrhea. Clin Microbiol Rev 19 (2006): 583-594.
- Haring B, Bauer W. Ciprofloxacin and the risk for cardiac arrhythmias: culprit delicti or watching bystander? Acta Cardiol 67 (2012): 351-354.
- Huttner A, Bielicki J, Clements MN, et al. Oral amoxicillin and amoxicillin-clavulanic acid: properties, indications and usage. Clin Microbiol Infect 26 (2020): 871-879.
- Smits WK, Lyras D, Lacy DB, et al. Clostridium difficile infection. Nat Rev Dis Primers 2 (2016):
- Lysyy T, Lalani AS, Olek EA, et al. The calcium-sensing receptor: A novel target for treatment and prophylaxis of neratinib-induced diarrhea. Pharmacol Res Perspect 7 (2019):
- Treatment of sexually transmitted Med Lett Drugs Ther 28 (1986): 23-28
- Munoz-Abraham AS, Judeeba S, Alkukhun A, et al. A new method to measure intestinal secretion using fluorescein isothiocyanate-inulin in small bowel of rats. J Surg Res 197 (2015): 225-230.
- Bertacco A, Dehner CA, Caturegli G, et al. Modulation of Intestinal Microbiome Prevents Intestinal Ischemic Front Physiol 8 (2017): 1064.
- Barahona MJ, Baratta V, Ollodart J, et al. Design and implementation of novel nutraceuticals and derivatives for treating intestinal disorders. Future Med Chem 11 (2019): 847-855.
- Stewart Gt. Toxicity Of The Penicillins. Postgrad Med J 40 (1964): 160-169.
- Ao M, Domingue JC, Khan N, et al. Lithocholic acid attenuates cAMP-dependent Cl- secretion in human colonic epithelial T84 cells. Am J Physiol Cell Physiol 310 (2016): C1010-C1023.