A Systems Pharmacology Approach, Using Molecular Docking and Dynamics Simulation, to Unlock Potential New Therapies for Alzheimer’s Disease: A Case Study of Cinnamon Species
Article Information
Wen Qiu1, #, Shouli Yuan 2, #, Robbie Kelleher3, 4, Lina Sun1, Wei Chen1, Helen Sheridan3, Junying Liu*, 3, Weiguang Lv*, 1
1Eco-environmental Protection Research Institute, Shanghai Academy of Agricultural Sciences,
Shanghai, China
2Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China
3NatPro Center, School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin, Dublin,
Ireland
4School of Medicine, Trinity College Dublin, Dublin, Ireland
#Contributes equally
*Corresponding author: Junying Liu. NatPro Center, School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin, Dublin, Ireland.Weiguang Lv, Eco-environmental Protection Research Institute, Shanghai Academy of Agricultural Sciences,Shanghai, China.
Received: 13 June 2023; Accepted: 22 June 2023; Published: 30 June 2023
Citation:
Wen Qiu, Shouli Yuan, Robbie Kelleher, Lina Sun, Wei Chen, Helen Sheridan, Junying Liu, Weiguang Lv. A Systems Pharmacology Approach, Using Molecular Docking and Dynamics Simulation, to Unlock Potential New Therapies for Alzheimer’s Disease: A Case Study of Cinnamon Species. Journal of Pharmacy and Pharmacology Research. 7 (2023): 97-132
View / Download Pdf Share at FacebookAbstract
Cinnamon (Cinnamomum spp.) is a time-honored species that has been used as a medicine, spice, and flavoring agent for millennia. Some forms of cinnamon are useful in treating neurological disorders including Alzheimer's disease (AD). However, the mechanisms of action are unclear. To elucidate the potential therapeutic value of mechanism, cinnamon ingredients and targets were determined by searching diverse herb databases. AD targets were searched on GeneCards and filtered by reprocessing scRNA-Seq data, which were filtered by STRING to construct the Herb-Ingredient-Target-Disease network. The intersection with AD targets (3445) gave a cinnamon - AD common target set (321). Metascape analysis revealed an enriched pathway/GO process and 123 genes enriched in the Alzheimer’s disease pathway were targeted for 78 cinnamon phytochemical components. Effective compounds including eugenol, cinnamaldehyde and coumarin, mainly function against hub targets, for which the binding was validated through molecular docking and dynamics simulation, e.g., cinnamaldehyde against ACHE, PTGS2. Data gathered here suggests that cinnamon may promote anti- Aß aggregation and clearance and modulate the calcium signaling pathway. It provides a therapeutic basis for (hybrid) drug development of cinnamon extracts. Ultimately this study contributes to understanding the mechanism of action of cinnamon constituent metabolites in the treatment of AD.
Keywords
Cinnamomum spp., Alzheimer’s disease, network analysis, molecular docking, molecular dynamics simulation
Cinnamomum spp articles, Alzheimer?s disease articles, network analysis articles, molecular docking articles, molecular dynamics simulation articles
Article Details
1. Introduction
Alzheimer’s Disease (AD) is a progressive and irreversible neurodegenerative disorder. Diverse mechanisms have been proposed for the pathogenesis and progression of AD, including deposition of β-amyloid (Aβ) peptide plaques, a decrease in the concentration of the neurotransmitter acetylcholine (ACh), homeostatic dysregulation of redox metal ions, and prolonged neuronal oxidative stress [1]. A significant understanding of the pathogenesis of AD has been gained, but a limited selection of medicines has been successfully developed to alleviate symptoms and slow down the disease progression of AD, and no therapy can cure AD outright. This is due to the complexity of AD pathology, the simultaneous progress of diverse pathogenetic pathways and the clinical-biological continuum [2].
Cinnamon (Cinnamomum. spp) is a time-honored species, that has been used as a medicine and spice for millennia. There are two main species Cinnamomum cassia (L.) J.Presl and Cinnamomum zeylanicum Blume (Lauraceae family). C. cassia Presl, also known as Cinnamomum arromaticum (Roxb.) Kuntze. Cinnamon is the raw material incorporated into traditional medicines in Asian countries such as China, India, Indonesia and Vietnam [3]. Cinnamon is also widely used as flavoring agent and a food supplement in America [4] and Europe [5]. The medical usage of cinnamon has been reported against dyspepsia, peptic ulcer disease, ischemic brain injury, and diverse cancers for its anti- inflammatory [6], anti-microbial [7], anti-cancer, anti-oxidant, anti-melanin and anti-diabetic effects [3, 8]. In addition, it exerts a neuroprotective effect against neurological disorders, including AD. Researchers have established that oral administration of an aqueous C. cassia decoction inhibits the formation of Aβ plaques and corrects cognitive impairment in AD animal models (fly and mice) [9].
Furthermore, Kang et al. [10] have demonstrated that aqueous C. cassia extracts significantly inhibit the formation of trademark pathological Aβ polypeptide oligomers in vitro in rat neuronal PC12 cells and in vivo in fly, and murine models, thus demonstrating a neuroprotective effect for C. cassia. Moreover, oral administration of powdered Cinnamonum verum, and its metabolite sodium benzoate (NaB), exerted a positive role in AD mice models, including protection of spatial learning and memory, attenuation of oxidative stress, suppression of neuronal degeneration and tau phosphorylation, and a reduction in amyloid polypeptide charge [11]. Others have reported that cinnamon feeding mitigates the negative effects of a high fat/high fructose diet on signaling and insulin in the brain and alleviates the induced increased expression of AD-associated mRNA, including PTEN, tau and amyloid precursor protein (APP) in vivo in rat models [12]. Peterson and co-authors found that the aqueous extract of C. zeylanicum inhibited the aggregation of human tau in vitro and filament formation, critical processes in AD [13].
George et al. [14] have established that cinnamon extracts play a protective role by preventing oxidation to tau-protein cysteine residues that can lead to dysfunction characteristic of AD. In addition, the extract of Indonesian cassia (Cinnamomum burmannil C. Nees & T. Ness) reduces the production of nitric oxide (NO) and prostaglandin E2 (PGE2), as well as inhibits interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in vitro in LPS-induced RAW264.7 cell line [15]. Metabolites with anti-AD activity provide an advantage over synthetic compounds because they can be consumed as a daily diet. On the other hand, a single compound with therapeutic activity at multiple biological targets might be promising since plant products usually contain phytochemicals that show a synergistic effect.
Therefore, there is heightened awareness of the therapeutic potential of cinnamon in the treatment of AD, an area of unmet clinical need [16, 17]. Recently, more than 160 components were identified within C. cassia [3]. However, all candidate molecular targets of cinnamon, and potential therapeutic mechanisms remain poorly understood. This study explores cinnamon's pharmacological mechanisms of action on AD using the strong combination of network analysis, molecular docking, and molecular dynamics simulation. This approach provides a new, lean strategy to focus the development of cinnamon-based therapeutics.
It will identify key targets and further inform scientists of potential experimental avenues for the therapeutic development of cinnamon extracts or metabolites. The current study confirms the potential therapeutic effects of cinnamon species, in particular C. cassia. The possible mechanisms of action of the cinnamon metabolome and its metabolites were acquired by employing network and molecular computational analyses, which were supported by scRNA-Seq data and experimental reports from the literature. Results here offer perspective options in treating AD, with cinnamon metabolites to promote anti-Aß aggregation and clearance and modulate the calcium signaling pathway. Furthermore, it provides a foundation for novel drug development of cinnamon ingredients. Finally, it supports potential hybrid drug development based on key cinnamon metabolites such as coumarin and cinnamate in the therapeutic treating of AD.
2. Methods and Materials
2.1 Compound Screening
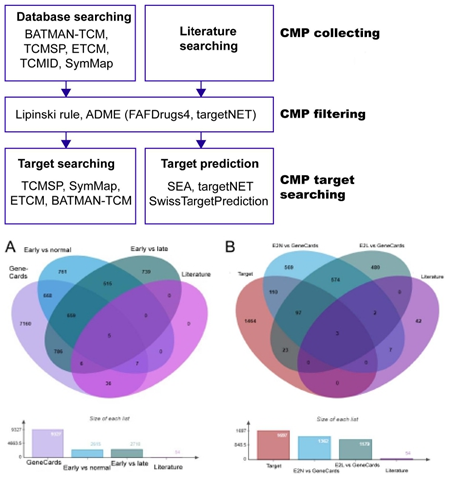
- cassia was screened to identify its metabolites and their targets. The metabolites of C. cassia prediction were investigated using the Encyclopaedia of Traditional Chinese Medicine (ETCM, http://www.nrc.ac.cn:9090/ETCM/) [18], the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://sm.nwsuaf.edu.cn/lsp/tcmsp.php) [19], the TCM Integrated Database (TCMID) [20], the Bioinformatics Analysis Tool for Molecular Mechanism of TCM (BATMAN-TCM, http://bionet.ncpsb.org/batman-tcm/) [21], and SymMap (http://www.symmap.org/) [22]. Absorption, distribution, metabolism, and excretion (ADME) was employed as a computational evaluation model in pharmacokinetic research to filter drug-like compounds, for which drug-likeness (DL) > 0.1 and oral bioavailability (OB) > 20% were applied [23].
2.2 Target Prediction and Collection
Compounds function by interacting with targets. Canonical SMILES were acquired from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Cinnamon targets were obtained either via searching the database: TCMSP, ETCM, BATMAN-TCM, and SymMap, or predicting through a search against TargetNet (http://targetnet.scbdd.com) [24], Swiss Target Prediction (http://www.swisstargetprediction.ch/) [25] and SEA (Similarity ensemble approach, https://sea.bkslab.org/) [26] with the target species setting as Homo sapiens, and duplications were removed [18].
2.3 Target Screening of Cinnamon (Rougui)
Of the 100 metabolites collected in TCMSP by searching the keywords “Rougui” only 11 were filtered with the criteria of an OB≥20% and a DL≥0.1. Other metabolites were obtained by searching the ETCM, BATMAN-TCM, TCMID, and SymMap databases. And 182 metabolites were established as the most effective components of cinnamon throughout the relevant literature and were included regardless of whether they met the OB and DL criteria. As a result, a total of 265 metabolites were acquired. In addition, canonical SMILES were collected from PubChem for 172 metabolites.
Accordingly, 85 metabolites were filtered based on Lipinski’s rules [27]. They met the criteria of drug- likeness (molecular weight, logP, tPSA, RotableB, HBD, HBA, Lipinski violation, oral bioavailability, solubility) for FAFDrugs4 and Target NET, from which the Open Babel logP computation program, drug-like soft in house and published Physchem filters were selected for FAF-Drugs4. Compounds which passed the filtering process were further analyzed. Targets of each active ingredient were identified by target fishing and by integrating the data acquired from the above database and predicted using SEA, Swiss Target Prediction, and TargetNET. The flowchart is described in Fig. 1.
2.4 Functional Classification of Cinnamon-AD common Targets
We collected AD-associated genes from the GeneCards database (https://www.genecards.org/) and other references (Table S2). Additionally, genes related to AD were collected and screened from SC2 diseases using single-cell RNA-sequencing (scRNA-seq) technologies [28], differentially expressed genes (DEGs) selection criteria is |logFC| > 0.58 and was summarized using Microsoft Excel software (version 2019, USA). Cinnamon targets interacting with AD-associated targets were considered cinnamon-AD common targets. The functional annotation, classification, and enrichment analysis of common targets were performed using Metascape analysis [29], and the organism was limited to Homo sapiens.
2.5 Construction of PPI Network and Herb-Metabolite-Target-Disease network
The cinnamon-AD common targets genes were subjected to the STRING platform (version 11.5) with species limited to Homo sapiens and the highest confidence score > 0.9. Besides STRING-filtered target genes, cinnamon compounds (herb metabolites) corresponding to AD genes were integrated and subjected to Cytoscape to construct the herb (cinnamon) - metabolite (cinnamon compound) – target (common disease genes)- Alzheimer disease network.
2.6 Molecular Docking
The molecular 2D structures for the molecular ligands and active ingredients were downloaded from PubChem to validate the binding of hub genes to their corresponding cinnamon ingredients. The crystal structures of key target proteins were downloaded from RCSB PDB (https://www.wwpdb.org/), respectively. Docking was performed by Autodock Vina 1.1.2, and the molecules with the lowest binding energy in the docking conformation were chosen to observe the binding effect by matching with the original ligands and intermolecular interactions (such as hydrophobic interaction, cation-π, hydrogen bond, anion-π, π-π stacking, salt bridge, metal complexation) [23]. The molecular docking patterns were finally visualized via PyMOL 3.0.
2.7 Molecular dynamics simulation
The molecular docking results were further validated by a molecular dynamics simulation strategy using Gromacs 2020.1 [30]. The ligand-receptor complex with the highest binding affinities was taken as an example. The system performed energy minimized calculations by using the steepest descent algorithm with a maximum force of 1000 kJ/mol/nm. Then, it was equilibrated in a constrained NVT (number of particles, volume, temperature) and NPT (number of particles, pressure, temperature) running for 100 ps. The Verlet cut-off scheme and a Leap-frog integrator with a step size of 2 fs were applied. The final analysis of the root-mean-square-deviation (RMSD) and the interaction energy was calculated by Gromacs 2020.1.
3. Results
3.1 Ingredient-target prediction and screening
As a result, 100 ingredients were found in the TCMSP by searching the keywords “Rou Gui,” and only 11 were collected with OB ≥ 20% and DL ≥ 0.1. Other compounds were obtained by searching BATMAN-TCM (score > 10, P < 0.05), ETCM, TCMID, and SymMap (OB > 30), as well as literature searching reported by Zhang et al. [3] and Momtaz et al. [31]. Canonical SMILES for 172 of the 265 compounds were identified from PubChem, which were then subjected to FAFDrugs4 and TargetNET for screening, respectively. The filtered 85 compounds were applied to search potential targets against SwissTargetPrediction (probability > 0.8), SEA (P < 0.01), and the TargetNET database. Together, 1697 targets related to 85 cinnamon metabolites were collected (Table 1), for which 50 were experimentally verified and 35 were identified from literature searching.
Table 1: Core compounds of cinnamon
3.2 Differentially expressed disease gene analysis
By searching keywords “Alzheimer’s Disease” in GeneCards and mapping in STRING limited to Homo sapiens, 9327 AD-related genes were acquired. While 2615 DEGs were collected from SC2diseases for AD vs. normal (E2N), 1531 unique DEGs for early-onset vs. late-onset phase (E2L only, and the other 1179 DEGs overlapped with E2N), 59 literature searched AD genes (Fig. 2a and Table S1-S2).
3.3 Herb-compound-target-disease network construction
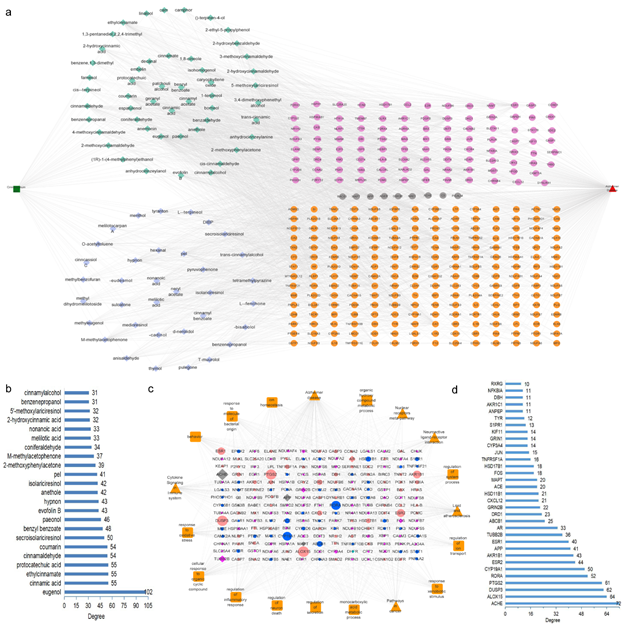
The interaction between cinnamon ingredient targets (1697) and AD-associated disease genes (4146) resulted in 321 cinnamon-AD common target sets: 210 genes for E2N, 202 genes for E2L only, and 12 AD-related genes from literature searching (Fig. 2b and detailed genes in Table S3). These genes maybe critical targets for AD treatment using cinnamon. This common target gene set was applied to Cytoscape v3.6.0 to construct an herb-compound-target-disease network (Fig.3). The cinnamon-AD common genes were imported into STRING (version 11.5), generating a total of 321 nodes and 582 edges with an average node degree 3.63. According to Fig. 3a, most of the individual compounds (82/85) were determined to interact with multiple AD-associated targets (≥ 3 targets) and 76 compounds were found to interact with more than 10 or equal to 10 AD targets. Especially, eugenol has the largest number of targets (102 targets), followed by cinnamic acid, ethylcinnamate and protocatechuic acid (55 targets), cinnamaldehyde and coumarin (54 targets), the other 9 compounds have ≥ 40 targets, indicating that these ingredients are highly likely to become key phytochemicals in AD treatment (Fig. 3b). Then, disconnected nodes were removed. A confidence score higher than 0.9 was applied, resulting in a PPI network in Fig. 3c. Screening of key ingredients and targets results in 33 genes were designed as hub targets with degree > 10, for which degree indicates the number of connections linked to other genes and ClosenessCentrality > 0.45 (Table 2). The expression of 30/33 genes was significant except ACHE, APP, and MAPT according to scRNA-seq data (Fig. 3c, Table S1). Among these, ACHE, ALOX15, DUSP3, PTGS2, RORA, CYP19A1, ESR2, AKR1BA, APP, and ESR1 were the top 10 hub targets for E2N comparison, suggesting that these were the main targets for cinnamon treat against early-onset AD (Fig. 3d). ACHE showed the highest degree (72), followed by ALOX15 (degree 64), DUSP3 (degree 62), PTGS2 (degree 61), RORA (degree 52), CYP19A1 (degree 50), ESR2 (degree 44), AKR1BA (degree 43), APP (degree 41) and ESR1 (degree 40). We further analyzed the number of hub targets and corresponding core targets of cinnamon ingredients. Cinnamic acid, eugenol, coumarin, pel, paeonol, protocatechuic acid, etc. have the largest number of 10 targets, followed by others.
Figure 3: The Herb-Compound-Targets-Disease network of cinnamon (a). The green square represents the herb cinnamon; the diamond represents the active ingredients (colored in light green means determined from cinnamon, in blue purple is searched from literature); the circles are common targets as the result of the intersection of the herb targets and disease targets (colored in cherry red are for E2N, in orange are for E2L only, and in grey are from literature searching); the red triangle is a disease. (b) Key compounds in the PPI network and correlated degree. The Y-axis is the cinnamon compounds of the common targets, the x-axis is the degree of the compounds. (c) Protein-Protein interaction network (PPI) of common targets. The size of the nodes represents the target degree. The diamond means in both comparisons (E2N and E2L), and circles represent in either comparison. Nodes colored in red/blue represent up/down-regulation, grey means the regulation is not significant in SC2disease data, cherry red is downregulated in E2N but upregulated in E2L, and turquoise blue is upregulated in E2N but downregulated in E2L. (d) Core genes in the PPI network and the X-axis indicates the degree.
Table 2: Top 33 hub genes with high targets connectivity
|
Name |
Annotation |
Degree |
ClosenessC entrality |
|
ACHE |
Acetylcholinesterase (cartwright blood group) |
72 |
0.553 |
|
ALOX15 |
Arachidonate 15-lipoxygenase |
64 |
0.538 |
|
DUSP3 |
Dual specificity protein phosphatase 3 |
62 |
0.534 |
|
PTGS2 |
Prostaglandin G/H synthase 2 |
61 |
0.532 |
|
RORA |
Rar-related orphan receptor alpha |
52 |
0.515 |
|
CYP19A1 |
Aromatase |
50 |
0.512 |
|
ESR2 |
Estrogen receptor beta |
44 |
0.502 |
|
AKR1B1 |
Aldose reductase |
43 |
0.5 |
|
APP |
Amyloid-beta A4 protein |
41 |
0.497 |
|
ESR1 |
Estrogen receptor |
40 |
0.495 |
|
TUBB2B |
Tubulin beta-2B chain |
36 |
0.489 |
|
AR |
Androgen receptor |
33 |
0.484 |
|
ABCB1 |
Multidrug resistance protein 1 |
25 |
0.472 |
|
DRD1 |
D(1A) dopamine receptor |
23 |
0.469 |
|
GRIN2B |
Glutamate receptor ionotropic, NMDA 2B |
22 |
0.467 |
|
CXCL12 |
Stromal cell-derived factor 1 |
21 |
0.466 |
|
HSD11B1 |
Corticosteroid 11-beta-dehydrogenase isozyme 1 |
21 |
0.466 |
|
ACE |
Angiotensin-converting enzyme |
20 |
0.465 |
|
MAPT |
Microtubule-associated protein tau |
20 |
0.465 |
|
FOS |
Proto-oncogene c-Fos |
18 |
0.462 |
|
HSD17B1 |
Estradiol 17-beta-dehydrogenase 1 |
18 |
0.462 |
|
TNFRSF 1A |
Tumor necrosis factor receptor superfamily member 1A |
16 |
0.459 |
|
JUN |
Transcription factor AP-1 |
15 |
0.457 |
|
CYP3A4 |
Cytochrome p450 family 3 subfamily a polypeptide 4 |
14 |
0.456 |
|
GRIN1 |
Glutamate receptor ionotropic, NMDA 1 |
14 |
0.456 |
|
KIF11 |
Kinesin-like protein KIF11 |
14 |
0.456 |
|
S1PR1 |
Sphingosine 1-phosphate receptor 1 |
13 |
0.455 |
|
TYR |
Tyrosinase |
12 |
0.453 |
|
ANPEP |
Alanyl aminopeptidase, membrane |
11 |
0.452 |
|
AKR1C1 |
20alpha/3alpha-hydroxysteroid dehydrogenase / dihydrodiol dehydrogenase |
11 |
0.452 |
|
DBH |
Dopamine beta-monooxygenase |
11 |
0.452 |
|
NFKBIA |
NF-kappa-B inhibitor alpha |
11 |
0.452 |
|
RXRG |
Retinoic acid receptor RXR-gamma |
10 |
0.451 |
3.4 Enrichment analysis of cinnamon-AD common genes
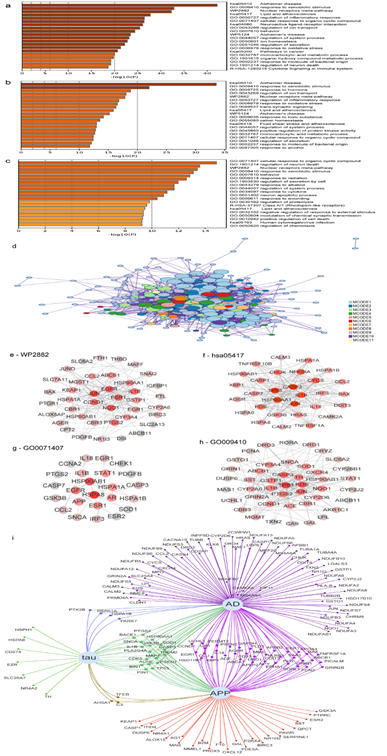
According to the results of Metascape analysis, cinnamon-AD common targets were remarkably enriched in 11 MCODE clusters, which were distributed in multiple biological processes and pathways (Fig. 4a, 4d and table S4). For early-onset vs. normal conditions, the top 20 enriched terms ranked by p-value were (Fig. 4b): Alzheimer's disease pathway of KEGG (hsa05010), response to xenobiotic stimulus (GO0009410), response to hormone (GO0009725), regulation of ion transport (GO0043269), nuclear receptors meta-pathway (WP2882), regulation of inflammatory response (GO0050727), response to oxidative stress (GO0006979), trans-synaptic signaling (GO0099537), Alzheimer’s Disease (WP5124), and so forth. The Alzheimer's disease pathway is the most significantly enriched (hsa05010, 2.81E-34, 40 targets).
In contrast, pathway/GO functional categories for early-onset Alzheimer vs. late-onset Alzheimer (Fig. 4c) were enriched in the cellular response to organic cyclic compound (GO0071407), regulation of neuron death (GO1901214), nuclear receptors meta-pathway (WP2882), response to xenobiotic stimulus (GO0009410), behavior (GO0007610), regulation of secretion by cell (1903530), lipid and atherosclerosis (hsa05417), and so forth. Nuclear receptors meta-pathway (WP2882), lipid and atherosclerosis (hsa05417), response to xenobiotic stimulus (GO0009410), and cellular response to organic cyclic compound (GO0071407) were enriched in both comparisons.
A PPI network of 42 cinnamon targets was involved in the nuclear receptors meta-pathway (WP2882) and contained 42 nodes and159 edges, in which JUN, EGFR, NQO1, and ESR1 play an important role (Fig. 4e). Lipid and atherosclerosis pathway (hsa05417), which has been linked to AD, is also significantly enriched. The cinnamon targets involved in lipid and atherosclerosis (hsa05417) form a PPI network with 33 nodes and 212 edges. At the same time, TP53, CASP3, JUN, HSP90AA1 and IL1B were core targets (Fig. 4f).
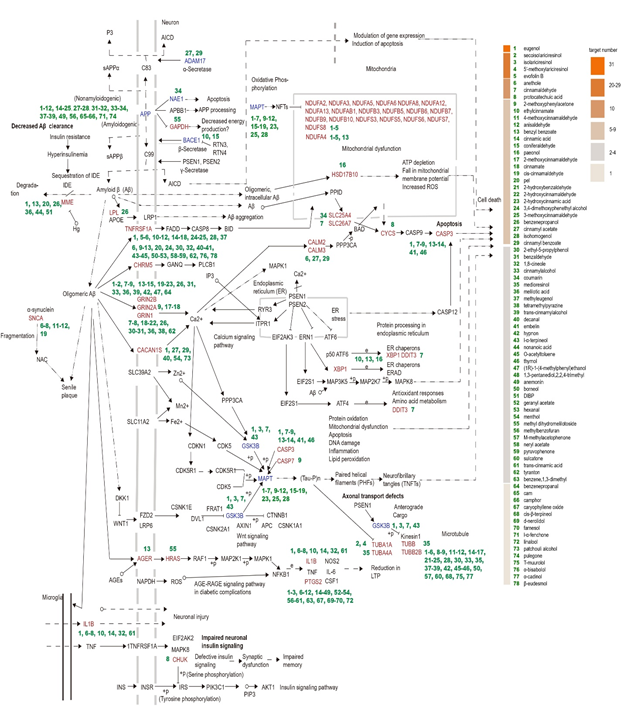
Cellular response to organic cyclic compound (GO0071407) was significantly enriched. As a result, a PPI network containing 44 nodes and 32 edges was formed, for which HSPA8, HSPA90A1 and EGFR were core targets (Fig. 4g). In addition, response to xenobiotic stimulus (GO009410) formed a PPI network with 46 nodes and 192 edges, with TP53, FOS, CASP3, JUN and PTGS2 play a key rule (Fig. 4h). To identify key cinnamon ingredients against AD, target genes involved in the Alzheimer's disease pathway (hsa05010, 7.57E-43, 54 targets, combine early-onset vs. normal and early-onset vs. late-onset Alzheimer) were visualized using KEGG mapper and corresponding ingredients was mapped in Fig. 5. The red font targets indicate the cinnamon targets. The green numbers 1 to 78 represent metabolites of cinnamon. Senile plaque deposition is a result of imbalanced Aβ production and degradation. Eugenol, benzyl benzoate, pel, benzenepropanol, melilotic acid (3-(2-hydroxyphenyl) propanoic acid), nonanoic acid and diisobutyl phthalate (DIBP) could act on membrane metalloendopeptidase and participate in the degradation of Aβ. The generation of Aβ depends on the cleavage of APP, catalyzed by α-secretase (ADAM17) and β-secretase (BACE1). According to Fig. 5, eugenol, ethylcinnamate, coniferaldehyde, cinnamyl acetate and cinnamyl benzoate are key compounds that modulate the production of Aβ.
Moreover, CSK3B, CASP3 and CASP7 mainly regulate tau protein phosphorylation, another important AD pathological process. Correspondingly, eugenol, isolariciresinol (8-(4-hydroxy- 3-methoxyphenyl)-6,7-bis(hydroxymethyl)-3-methoxy-5,6,7,8-tetrahydronaphthalen-2-ol), cinnamaldehyde, 2-methoxyphenylacetone and other compounds are determined to be key cinnamon ingredients that regulate tau protein phosphorylation. Eugenol, isolariciresinol, cinnamaldehyde and l- alpha-terpineol can interact with GSK3B; eugenol, cinnamaldehyde, protocatechuic acid and the other 5 compounds can interact with CASP3, and 2-methoxyphenylacetone can interact with CASP7.
Furthermore, multiple cinnamon metabolites interact with GRIN1, GRIN2A, GRIN2B, or CACAN1S, which affects the downstream calcium signaling pathway and cell death. In detail, eugenol, secroisolariciresinol, cinnamaldehyde, protocatechuic acid, and the other 17 compounds interact with GRIN2B, cinnamaldehyde, protocatechuic acid, cinnamate, and the other 10 compounds interact with GRIN1, while eugenol, cinnamyl acetate, cinnamyl benzoate and the other 3 compounds interact with CACAN1S. In addition, oligomeric forms of Aβ known to be an important inflammatory stimulus of neuronal cells. For example, benzyl-benzoate can interact with AGER, methyl dihydromelilotoside (2-(beta-D- glucopyranosyloxy)benzenepropionic acid methyl ester) can interact with HRAS, eugenol, anethole, cinnamaldehyde, and the other 5 compounds interact with IL1, eugenol, secroisolariciresinol, isolariciresinol, anethole, cinnamaldehyde, protocatechuic acid and the other 53 compounds interact with PTGS2 to improve the inflammation induced by oligomeric Aβ.
Eugenol can interact with 31 AD pathway targets, secroisolariciresinol can interact with 26 targets, isolariciresinol can interact with 25 targets, and 63 of 78 compounds have more than 2 targets suggesting that cinnamon compounds can act on different targets of the AD pathway; thus cinnamon may play a key role in the treatment of AD.
Figure 4: Metascape analyses of the Alzheimer's disease-associated genes target by cinnamon. (a-c) Bar graph of enriched terms colored by p-values to visualize the top 20 clusters for all common genes, early vs. normal conditions and early Alzheimer vs. late Alzheimer conditions, respectively. (d) Network of enriched terms: colored by cluster-ID and ranked by p-values for the top 20 clusters; (e-h) PPI network construction for nuclear receptors meta-pathway (WP2882), lipid and atherosclerosis (hsa05417), targets involved in response to xenobiotic stimulus (GO009410), and regulation of inflammatory.
Figure 5: The cinnamon targets associated with the Alzheimer's disease pathway (hsa05010) were constructed using KEGG mapping. A). Red/blue targets represent the cinnamon targets involved in the AD pathway. Regulation is significant for the red font and not the font in blue based on data from the SC2disease database. The green numbers 1 to 78 represent the main cinnamon ingredients. The gradient color on the left represents its related target number. Cinnamon compounds target the number next to the gene.
3.5 Molecular validation of compound-target interactions
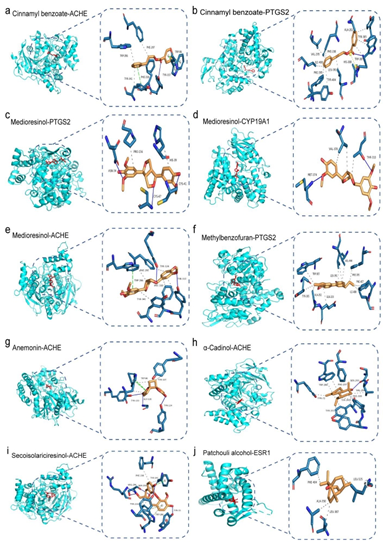
Molecular docking analysis was carried out to validate the binding of core targets and the corresponding cinnamon metabolites. The 85 active ingredients (Table 1) include cinnamyl benzoate (PubChem CID 5705112), coumarin (CAS no. 91-64-5), DIBP (CAS no. 84-69-5), the para- benzoquinone embelin (CAS no. 550-24-3), melilotocarpan A (PubChem CID 442792), etc. The protein structures of key targets were obtained from RCSB PDB, including ACHE, DUSP3, PTGS2, CYP19A1, APP, ESR1, MAPT, CASP3, HSP90AA1, FOS, TP53 and GAPDH based on the STRING interaction analysis and importance reference. Results here indicated that all the core targets were well combined with the corresponding cinnamon metabolites (Table 3, Table S5).
Table 3: The lowest 10 binding affinities (kcal/mol) and binding residues of the cinnamon compound and Alzheimer's disease gene.
|
Compound-tARGet |
affinity (kcal/mol) |
Binding residues |
|
Cinnamyl benzoate-ACHE |
-9.6 |
TRP86, TRP286, PHE297, TYR337, PHE338, TYR341 |
|
Medioresinol-PTGS2 |
-9.1 |
ASN34, HIS39, CYS41, CYS47, PRO156 |
|
Alpha-cadinol-ACHE |
-9.1 |
TYR72, TRP286, SER293, VAL294, PHE297, TYR337, PHE338, TYR341 |
|
Methylbenzofuran-PTGS2 |
-8.8 |
ALA202, GLN203, TYR385, TRP387, LEU391, PHE395, PHE407, ILE408, VAL444 |
|
Cinnamyl benzoate-PTGS2 |
-8.6 |
PHE200, ALA202, VAL295, TYR385, TRP387, HIS388, LEU391, PHE395, TYR404, ILE408 |
|
Anemonin-ACHE |
-8.2 |
TRP86, GLY120, TYR124, TYR133, TYR337 |
|
Medioresinol-ACHE |
-8.2 |
TYR124, TRP286, PHE297, TYR337, PHE338, TYR341 |
|
Medioresinol-CYP19A1 |
-8.1 |
THR310, VAL370, MET374 |
|
Patchouli alcohol-ESR1 |
-8.1 |
ALA350, LEU387, PHE404, LEU525 |
|
Secroisolariciresinol-ACHE |
-8.1 |
TYR72, TYR124, TRP286, SER293, ARG296, PHE338, TYR341 |
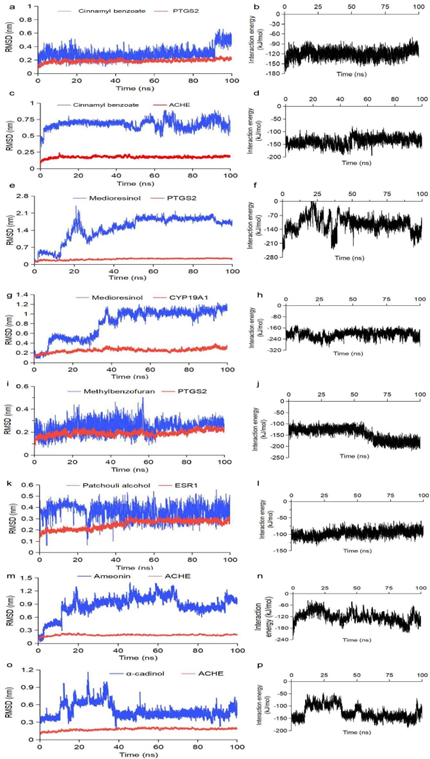
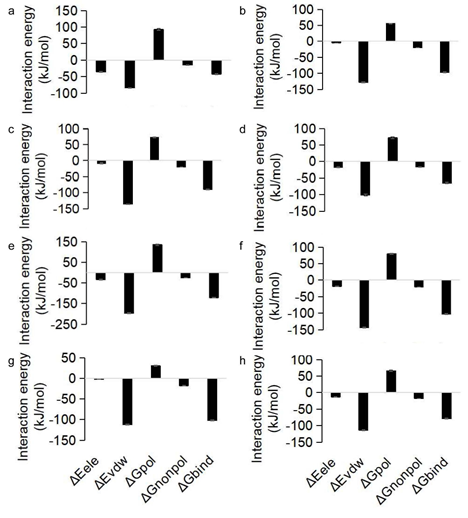
Furthermore, the binding affinities of all docking patterns were higher than 6 kcal/mol, suggesting stable binding in between. Docking affinities and binding residues for the lowest 10 compound-target pairs were listed in Fig. 6, Table 3 and Table S5. Eugenol showed the highest binding score with ACHE and PTGS2, with score values of -6.6 and -6.2 kcal/mol, respectively; cinnamaldehyde showed the highest binding score with ACHE, CYP19A1, TP53 and PTGS2, with score values of -6.5, -6.2, -6.2, and -6.1 kcal/mol, respectively; coumarin showed the highest binding score with ACHE, PTGS2, CYP19A1, ESR1 and APP, with score values of -7.3, -7.2, -6.7, -6.6 and -6.0 kcal/mol, respectively. It suggests that cinnamon metabolites are associated with changes in AD pathology, which may play a key role in treating AD. Molecular dynamic simulations were carried out to evaluate the stability of the interaction between core targets and the cinnamon ingredient predicted by the docking experiments. The RMSD values reflect the fluctuations for the protein-ligand complex. It can be seen that cinnamyl benzoate - PTGS2 (Fig. 7a), methybenzofuran - PTGS2 (Fig. 7c) and cinnamyl benzoate - ACHE (Fig. 7i) systems indicate strong stability. Patchouli alcohol - ESR1 system had a sharp drop within 30 ns and then tended to balance out the last 70 ns (Fig. 7k). The medioresinol (lignan) - PTGS2 (Fig. 7e), medioresinol - CYP19A1 (Fig. 7g) and α - cadinol - ACHE (Fig. 7o) systems had a sharp rise within 40 ns and balanced out the last 60 ns. MM/PBSA (molecular mechanics energies combined with the Poisson- Boltzmann and surface area continuum solvation) calculated the binding free energy and energy components, for which 0 ns to 100 ns RMSD phase trajectory was applied. Binding free energy analysis assessed protein and molecular binding affinity; here, all the complexes and relevant leading molecules’ structures are very stable, as shown in Fig. 8 (and detailed in Table 4). It is observed that the van der Waals interaction, SASA energy, and electronic energy promote the binding of the cinnamon component to the target protein. In contrast, the polar solvation energy has the opposite effect. Taken together, all these results confirm the structure stability of ligand-receptor complexes.
Figure 6: Docking patterns of selected targets according to the lowest binding affinities with corresponding active metabolite a-b) cinnamyl benzoate, c-e) medioresinol, f) methylbenzofuran, g) anemonin, h) α-cadinol, f) secroisolariciresinol, g) patchouli alcohol. The binding affinities (kcal/mol) and binding residues are listed in Table 3. For other docking patterns, see the supplementary Table S4.
Figure 8: Binding free energy decomposition of cinnamon ingredient and target. ΔEele: Electrostatic interaction energy. ΔEvdw: van der Waals interaction energy. ΔGpolc: Polar contributions to the solvation-free energy. ΔGnonpold: Nonpolar contributions to the solvation-free energy. ΔGbinde: Binding energy.
Table 4: Binding energies for the complex of protein and molecule using MM/PBSA during 0-100 ns of MD simulation trajectory
|
Protein - molecule |
Δeelea (kJ/mol) |
Δevdwb (kJ/mol) |
Δgpolc (kJ/mol) |
Δgnonpold (kJ/mol) |
Δgbinde (kJ/mol) |
|
ACHE - Ameonin |
-33.75 |
-83.23 |
92.54 +2.63 |
-15.08 |
-41.19 |
|
PTGS2 - Cinnamyl benzoate |
-4.44 |
-127.15 |
55.42 +0.78 |
-19.17 |
-96.02 |
|
ACHE - Cinnamyl benzoate |
-7.08 |
-134.45 |
72.85 +1.28 |
-19.49 |
-88.62 |
|
PTGS2 -Medioresinol |
-16.58 |
-98.3 |
71.15 +3.47 |
-16.74 |
-63.7 |
|
CYP19A1 - Medioresinol |
-31.03 |
-195.56 |
134.93 + 2.75 |
-25.66 |
-119.66 |
|
PTGS2 - Methylbenzofuran |
-18.1 |
-141.81 |
80.00 + 1.46 |
-20.16 |
-101.31 |
|
ESR1 - Patchouli alcohol |
-1.88 |
-110.9 |
29.87 + 1.19 |
-17.65 |
-100.95 |
|
ACHE - α-cadinol |
-12.2 |
-112.33 |
66.17 + 1.52 |
-18.09 |
-77.68 |
aElectrostatic interaction energy.
bvan der Waals interaction energy.
cPolar contributions to the solvation free energy.
dNonpolar contributions to the solvation free energy. e Binding energy.
Table 5: Literature validation of predicted nature products
|
CMP |
Origin |
Result |
Ref |
|
Medioresinol, cryptamygin A |
Cinnamomum cassia Blume (Lauraceae) |
Show anti-amyloidogenic effects (inhibits Aβ40 production) through decrease the amount of β- secretase in APP-Chinese hamster ovarian cells |
[1] |
|
Cinnamaldehyde |
Cinnamomum zeylanicum |
Aqueous extract of cinnamon inhibits aggregation of tau. Cinnamaldehyde displays tau aggregation inhibitory activity |
[2] |
|
Coumarin, Indonesian cassia extract (ICE) |
Cinnamomum burmannil C. Nees & T. Ness |
Both possess anti-inflammatory properties through reducing the production of NO and PGE2, inhibits IL-6, IL-1β and TNF-α in LPS-induced macrophages cell line RAW264.7 |
[3] |
|
Coumarin |
Coumarin-based hybrid drug |
Coumarin based conjugates have been described as potential AChE, BuChE, MAO and β- amyloid inhibitors |
[4,5] |
|
Aqueous cinnamon extract |
Cinnamomum spp. |
Markedly inhibits the formation of toxic Aβ oligomers and prevents the toxicity of Aβ on neuronal PC12 cells. When administered to an AD Drosophila melanogaster (fly) model, CEppt rectified their reduced longevity, fully recovered their locomotion defects and totally abolished tetrameric species of Aβ in their brain. Oral administration of CEppt to an aggressive AD transgenic mice model led to marked decrease in 56 kDa Aβ oligomers, reduction of plaques and improvement in cognitive behavior |
[6] |
|
Cinnamon powder and its metabolite sodium benzoate (NaB) |
Cinnamonum verum |
Oral administration of cinnamon powder to 5XFAD transgenic mice produce NaB in the hippocampus. NaB decreased the production of reactive oxygen species in activated microglia via suppressing the activation of p21rac, a member of the NADPH oxidase complex. Oral administration of both cinnamon and NaB inhibited the activation of p21rac and reduced oxidative stress, protects spatial learning and memory, suppresses neurons degeneration and Tau phosphorylation, reduces Aβ charge |
[7] |
|
Cinnamaldehyde |
C. cassia |
Inhibited NO production, inhibited mRNA expression of pro-inflammatory mediators (cytokines and chemokines) includes TNF-α, IL-1β, IL-6, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1α in LPS-activated cells. Decreased expression of inducible enzymes inducible nitric oxide synthase, cyclooxygenase-2, microsomal prostaglandin-E synthase-1. Increased mRNA expression and the production of antiinflammatory cytokines IL-10 and transforming growth factor-β |
[8] |
|
Phenylethyl alcohol |
C. cassia Presl (Lauraceae) |
Essential oil from the twigs significantly reduced oxytocin-induced writhing responses with a maximal inhibition of 66.5%. Decreased the expression levels of PGF2α, COX-2, p-MLC20 in oxytocin-induced mice uterine tissue |
[9] |
|
Methylbenzofuran |
Hybrid incorporate benzofuran and chalcone |
Decreased Aβ aggregation and increased ACh levels along with the overall availability of Ach at the synaptic junction. decrease AChE levels, reduce oxidative stress in Caenorhabditis elegans, lower lipid content, and to provide protection against chemically induced cholinergic neurodegeneration |
[10] |
|
Secoisolariciresinol |
Folk medicine Haplophyllum sahinii O. Tugay & D. Ulukus, H. vulcanicum Boiss. & Heldr |
Secoisolariciresinol and secoisolariciresinol dimethyl ether were the main lignans in the stems and flowers. The highest inhibition was caused by the stem extract of H. sahinii against BChE (IC50 =64.93±1.38 μg/mL). All of the extracts were found to exert a selective inhibition towards BChE to some extent. root extract of H. vulcanicum that could inhibit AChE at low level (IC50 =203.18±5.33 μg/mL). |
[11] |
|
Patchouli alcohol (PTA) |
C. cassia |
Antifungal activity against Aspergillus species (suppression colony growth) |
[12] |
|
Patchouli alcohol (PTA) |
Pogostemon cablin |
Immunomodulatory, anti-inflammatory, antioxidative, antitumor, antimicrobial, insecticidal, antiatherogenic, antiemetic, whitening, and sedative activity |
[13] |
|
β-eudesmol |
Folk medicine Atractylode macrocephala Koidz (Qi-Zhu) |
Show AChE inhibitory activity |
[14] |
|
Embelin |
Folk medicine Embelia ribes |
Stopping the formation of Aβ oligomers (via inhibition of BACE-1), improves cholinergic-transmission (via inhibition of AChE/BChE) and increases Aβ clearance (via P-gp induction). |
[15] |
|
Ethylcinnamate |
Melatonin– cinnamate hybrids |
Showed antioxidant properties and high neuroprotective effect in the rot/olig model. Cinnamate esters were previously reported as moderate Nrf2 inducers, Nrf2 activation in animal models of AD and other neurodegenerative diseases (NDDs) extended survival and reversed cognitive decline. implement the scavenger and neuroprotective effect of melatonin with the tuneable |
[16] |
|
Isolariciresinol |
Folk medicine Crataegus spp. |
Showed stronger inhibition of Aβ1-42 aggregation |
[17] |
|
Isolariciresinol |
Taxus baccata L. |
Exhibited a moderate inhibition against both BChE and LOX, whereas they were inactive towards AChE. displayed a great scavenging activity against DPPH especially at 500 and 1000 microg ml(-1). exert noteworthy reducing antioxidant power on ferric ions |
[18] |
|
α-Cadinol |
Folk medicine Alpinia oxyphylla Miq. |
Preventing Aβ(1-42)-induced neuronal apoptosis may be via inhibiting Aβ(1-42)- induced reactive oxygen species (ROS) production, attenuating Aβ(1-42)-induced caspase-3 activation and inhibiting caspase-3 activity in cultured rat hippocampal neurons |
[19] |
|
Espatulenol |
Folk medicine Filifolium sibiricum (L.) Kitam |
Significant antibacterial activities against Staphylococcus aureus |
[20] |
|
T-muurolol |
Calocedrus macrolepis var. formosana Florin |
Antifungal activity, strongly inhibited the growth of Rhizoctonia solani and Fusarium oxysporum |
[21, 22] |
|
Melilotocarpan A |
Folk medicine Red Mexican Propolis |
Pterocarpans constitute the second largest group of natural isoflavonoids, show antifungal and antibacterial activities |
[23, 25] |
|
Isohomogenol, eugenol |
C. cassia Presl |
Might affect the key targets of thermogenesis effect of mice model |
[26] |
|
Protocatechuic acid |
C. burmannii |
Addition of cinnamon extract to white chocolate result in an end-product rich in polyphenols, whose content and antioxidant activity in food are useful for a preliminary prediction |
[27] |
|
Ethyl cinnamate |
C. cassia Blume |
As judged by 24-hour LC50 values, two cassia oils (0.084–0.085 mg/ml) and four cinnamon oils (0.064–0.113 mg/ml) exhibited good nematicidal activity toward adult Bursaphelenchus xylophilus |
[28] |
|
Benzenepropanol/3- Phenyl-1-propanol |
Liquidamabar orientalis |
Essential oils showed 87.5% mortality against female spotted wing drosophila at 5.0 µg/fly concentration |
[29] |
|
Linalool (69.94%), camphor (10.90%), nerolidol (10.92%), safrole (8.24%) |
Cinnamomum camphora var. linaloofera Fujita |
Linalool had bactericidal activity against Escherichia coli |
[30] |
|
Cinnamaldehyde |
C. zeylanicum |
Antifungal activity against Aspergillus ochraceus and Penicillium verrucosum (inhibit growth and ochratoxin A production) |
[31] |
|
α-Terpineol, terpinen- 4-ol, δ-terpineol |
Cinnamomum longepaniculatum |
Antimicrobial activity towards Shigella flexneri, increase the membrane permeability, damage cell membranes. The minimum inhibition concentration (MIC) and minimum bactericide concentration (MBC) of α-terpineol and terpinen-4-ol were similar (0.766 mg/mL and 1.531 mg/mL, respectively), while the MIC and MBC values of δ-terpineol were 0.780 mg/mL and 3.125 mg/mL. |
[32] |
|
Paeonol |
Moutan Cortex of Guizhi Fuling capsule |
PGF2α level reduction, intracellular Ca2+ reduction, NO level elevation, suppression of COX-2 and OTR expression in primary dysmenorrhea treatment |
[33] |
|
Anethole/trans-anethole, trans - cinnamaldehyde |
C. cassia |
Trans-anethole is effective for fumigant activity, trans-cinnamaldehyde show contact toxicity against Drosophila suzukii |
[34] |
|
Farnesol |
Bee-hive propolis |
Antibacterial against Strptococcus mutans and Streptococcus sanguinis |
[35] |
|
Cinnamyl benzoate |
Synthesize from benzoyl chloride and cinnamyl alcohol |
Inhibited the growth of yeast-phase cells of the pathogenic fungus Sporothrix schenckii through inhibit sterol synthesis |
[36] |
|
Eugenol |
Ocimum sanctum L. |
Eugenol showed greater inhibitory effect against AChE and BChE activities, and demonstrated higher antioxidant potential compared to butylated hydroxylanisole (BHA) and butylated hydroxyl toluene (BHT) |
[37, 38] |
|
Protocatechuic acid (PA) |
Commercially purchased PA from Momordica charantia |
Oral administration of PA for 14 days at 200 mg/kg/day protected against cognitive impairment in Aβ-induced AD mouse model through attenuating Aβ25-35-induced neuroinflammation (downregulating inflammatory mediators, inducible nitric oxide synthase and cyclooxygenase-2 in the brain). |
[39] |
|
Eugenol |
Commercially purchased |
Eugenol bind to different AD targets (BACE1, MAO-B, cholinesterase's, Aβ1-42 fibrils), shows free radical scavenging activity and anti-aggregation activity against Aβ peptides. Pre- treatment with eugenol significant protected PC12 cells against H2O2-induced oxidative stress and Aβ-induced cytotoxicity |
[40] |
|
Cinnamic acid |
Commercially purchased |
As a potent ligand, cinnamic acid activates the nuclear hormone receptor PPARα and indues lysosomal biogenesis in mouse primary brain cells. Oral administration of cinnamic acid significantly attenuated cerebral Aβ plaque burden and improved memory and behavioral performance of male and female 5× Familial Alzheimer's disease mice. |
[41] |
|
Patchouli alcohol (PTA) |
Commercial purchased |
PTA as a selective agonist of ERβ efficiently ameliorated AD-like pathology of APP/PS1 model mice. Reduced amyloid plaque deposition in the hippocampus by promoting microglial phagocytosis. Alleviated o-Aβ25-35-induced oxidative stress in primary neurons through targeting ERβ and increasing catalase expression. PTA administration improved synaptic integrity through enhancing BDNF/TrkB/CREB signaling, ameliorated oxidative stress by Catalase level, and regulated Bcl-2 family proteins in the hippocampus. patchouli alcohol target ER. |
[42] |
|
Protocatechuic acid (PA) |
Commercial purchased |
PA increases PC12 cell viability and significantly decreases the levels of Aβ42, p-tau, β- secretase and Beclin-1, promotes the expression of p-Akt and MEF2D and suppresses the expression of p-GSK-3β. Thus, PA has potent as a drug candidate against AD |
[43] |
|
Cinnamaldehyde |
Commercial purchased |
significantly improved lifespan and healthspan of male AD flies (Drosophila melanogaster model) overexpressing the Tau proteins |
[44] |
|
Embelin |
Commercial purchased |
Inhibit AChE, docking prediction for interaction with AChE and amyloid beta (Aβ) binding sites |
[45] |
|
Embelin |
Commercial purchased |
nootropic and anti-amnesic effects on scopolamine-induced amnesia in rats. significantly exhibited a memory-enhancing effect in the absence of scopolamine, besides improving the recognition index when challenged with chronic scopolamine treatment, increase in inflection ratio in nootropic activity. contributed toward the elevated expression of BDNF, CREB1, and scavenger enzymes (SOD1 and CAT) mRNA levels. pretreatment of rats with embelin mitigated scopolamine-induced neurochemical and histological changes |
[46] |
|
Paeonol |
Commercial purchased |
Significantly relieved Aβ deposition and Aβ -mediated neuropathology in the brain of APP/PS1 mice |
[47] |
|
Farnesol |
Commercial purchased |
Neuroprotective role of geranylgeraniol / farnesol in the survival of neuronal cells |
[48] |
|
Anemonin Eugenol |
Commercial purchased |
Suppression of IL-1β/NF-κB pathway for inflammation related osteoarthritis |
[49] |
Note: nitric oxide (NO), prostaglandin E2 (PGE2), inhibits interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), acetylcholine (ACh)
References
- Kang, Y.J., D.G. Seo, and S.Y. Park, Phenylpropanoids from cinnamon bark reduced β-amyloid production by the inhibition of β-secretase in Chinese hamster ovarian cells stably expressing amyloid precursor protein. Nutr Res, 2016. 36(11): p. 1277-1284.
- Peterson DW, et al., Cinnamon extract inhibits tau aggregation associated with Alzheimer's disease in vitro. 2009. 17(3): p. 585-597.
- Sandhiutami, N.M., et al., In vitro assesment of anti-inflammatory activities of coumarin and Indonesian cassia extract in RAW264.7 murine macrophage cell line. Iran J Basic Med Sci, 2017. 20(1): p. 99-106.
- Yusufzai SK, et al., Molecular docking studies of coumarin hybrids as potential acetylcholinesterase, butyrylcholinesterase, monoamine oxidase A/B and β-amyloid inhibitors for Alzheimer's disease. Chem Cent J, 2018 12(1): p. 128.
- Bhatia R, et al., Multi-Target Directed Ligands (MTDLs): Promising Coumarin Hybrids for Alzheimer's Disease. Curr Alzheimer Res, 2021. 18(10): p. 802-830.
- Frydman-Marom A, et al., Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer's disease animal models. PLoS One, 2011. 6(1): p. e16564.
- Modi KK, et al., Cinnamon and its metabolite sodium benzoate attenuate the activation of p21rac and protect memory and learning in an animal model of alzheimer’s disease. PLoS One, 2015. 10(6): p. e0130398.
- Pannee C, Chandhanee I, and Wacharee L, Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide - stimulated J774A.1 cells. J Adv Pharm Technol Res, 2014. 5(4): p. 164-70.
- Sun, L., et al., The essential oil from the twigs of Cinnamomum cassia Presl inhibits oxytocin-induced uterine contraction in vitro and in vivo. J Ethnopharmacol, 2017. 206: p. 107-114.
- Sashidhara KV, et al., Benzofuran-chalcone hybrids as potential multifunctional agents against Alzheimer's disease: synthesis and in vivo studies with transgenic Caenorhabditis elegans. ChemMedChem, 2014 9(12): p. 2671-2684.
- Karahisar E, et al., Metabolite Profiling by Hyphenated Liquid Chromatographic Mass Spectrometric Technique (HPLC-DAD-ESI-Q-TOF-MS/MS) and Neurobiological Potential of Haplophyllum sahinii and H. vulcanicum Extracts. Chem Biodivers, 2019. 16(9)(9): p. e1900333.
- Kocevski, D., et al., Antifungal effect of Allium tuberosum, Cinnamomum cassia, and Pogostemon cablin essential oils and their components against population of Aspergillus species. J Food Sci, 2013. 78(5): p. M731-7.
- Hu G, et al., Availability, Pharmaceutics, Security, Pharmacokinetics, and Pharmacological Activities of Patchouli Alcohol. Evid Based Complement Alternat Med, 2017. 2017: p. 4850612.
- Zhu Q, et al., Chemical Constituents from the Wild Atractylodes macrocephala Koidz and Acetylcholinesterase Inhibitory Activity Evaluation as Well as Molecular Docking Study. Molecules, 2021. 26(23): p. 7299.
- Nuthakki VK, et al., Identification of embelin, a 3-undecyl-1,4-benzoquinone from Embelia ribes as a multitargeted anti-Alzheimer agent. Drug Dev Res, 2019. 80(5):(5): p. 655-665.
- Buendia I, et al., New melatonin-cinnamate hybrids as multi-target drugs for neurodegenerative diseases: Nrf2-induction, antioxidant effect and neuroprotection. Future Med Chem 2015. 7(15): p. 1961-1969.
- Huang XX, et al., Lignans from the seeds of Chinese hawthorn (Crataegus pinnatifida var. major N.E.Br.) against β-amyloid aggregation. Nat Prod Res, 2018. 32(14): p. 1706- 1713.
- Kucukboyaci N, et al., Assessment of enzyme inhibitory and antioxidant activities of lignans from Taxus baccata L. . Z Naturforsch C J Biosci, 2010. 65(3-4): p. 187-194.
- Ji ZH, et al., In-vitro neuroprotective effect and mechanism of 2β-hydroxy-δ-cadinol against amyloid β-induced neuronal apoptosis. Neuroreport, 2020 31(3): p. 245-250.
- Liang S, et al., Chemical composition and biological activities of essential oil from Filifolium sibiricum (L.) Kitam. Nat Prod Res, 2016 30(24): p. 2861-2863.
- Chang HT, et al., Antifungal activity of essential oil and its constituents from Calocedrus macrolepis var. formosana Florin leaf against plant pathogenic fungi. Bioresour Technol, 2008 99(14): p. 6266-6270.
- Cheng SS, et al., Phytochemicals from Cunninghamia konishii Hayata act as antifungal agents. J Agric Food Chem, 2012. 60(1): p. 124-128.
- Lotti C, et al., Chemical constituents of red Mexican propolis. J Agric Food Chem, 2010 58(4): p. 2209-2213.
- Chan SC, et al., Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Med, 1998 64(2): p. 153-158.
- Dao TT, et al., Pterocarpans with inhibitory effects on protein tyrosine phosphatase 1B from Erythrina lysistemon Hutch. Phytochemistry., 2009. 70(17-18): p. 2053-2057.
- Liu, X., et al., Study on the experimental verification and regulatory mechanism of Rougui-Ganjiang herb-pair for the actions of thermogenesis in brown adipose tissue based on network pharmacology. J Ethnopharmacol, 2021. 279: p. 114378.
- Muhammad DRA, et al., Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem, 2021. 340: p. 127983.
- Kong JO, et al., Nematicidal Activity of Cassia and Cinnamon Oil Compounds and Related Compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae). J Nematol, 2007 ;. 39(1): p. 31-36.
- Kim J, et al., Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Pestic Biochem Physiol, 2016. 133: p. 35-43.
- Wu K, et al., Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci Nutr, 2019.7(8): p. 2546-2555.
- Kalagatur NK, et al., Inhibitory effect of C. zeylanicum, C. longa, O. basilicum, Z. officinale, and C. martini essential oils on growth and ochratoxin A content of A. ochraceous and P. verrucosum in maize grains. Biotechnol Rep (Amst), 2020 27: p. e00490.
- Huang J, et al., Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol (Praha), 2021 66(1): p. 59-67.
- Sun L, L.L., Zong S, Wang Z, Zhou J, Xu Z, Ding G, Xiao W, Kou J,, Traditional Chinese medicine Guizhi Fuling capsule used for therapy of dysmenorrhea via attenuating uterus contraction. J Ethnopharmacol, 2016. 191: p. 273-279.
- Kim J, et al., Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Pestic Biochem Physiol, 2016 133: p. 35-43.
- Philip N, et al., Growth Inhibitory Effects of Antimicrobial Natural Products against Cariogenic and Health-Associated Oral Bacterial Species. Oral Health Prev Dent, 2020.18(1): p. 537-542.
- Teramoto Y, et al., Characterization of a novel antimycotic agent, cinnamyl benzoate, using yeast-phase Sporothrix schenckii. World J Microbiol Biotechnol, 1994. 10(4): p. 396-400.
- Adefegha, S.A., B.M. Okeke, and G. Oboh, Antioxidant properties of eugenol, butylated hydroxylanisole, and butylated hydroxyl toluene with key biomolecules relevant to Alzheimer's diseases-In vitro. J Food Biochem, 2021. 45(3): p. e13276.
- Thakur K and Pitre KS, Anti-inflammatory activity of extracted eugenol from Ocimum sanctum L. leaves. Rasayan J Chem, 2009. 2(2): p. 472–474.
- Choi, J.R., et al., Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer's disease mouse model. Food Chem Toxicol, 2020. 144: p. 111571.
- Chowdhury, S. and S. Kumar, Inhibition of BACE1, MAO-B, cholinesterase enzymes, and anti-amyloidogenic potential of selected natural phytoconstituents: Multi-target- directed ligand approach. J Food Biochem, 2021. 45(1): p. e13571.
- Chandra, S., et al., Cinnamic acid activates PPARα to stimulate Lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer's disease mouse model. Neurobiol Dis, 2019. 124: p. 379-395.
- Yan QY, et al., Patchouli alcohol as a selective estrogen receptor β agonist ameliorates AD-like pathology of APP/PS1 model mice. Acta Pharmacol Sin, 2022.
- Huang, L., et al., Protocatechuic acid attenuates β-secretase activity and okadaic acid-induced autophagy via the Akt/GSK-3β/MEF2D pathway in PC12 cells. Mol Med Rep, 2020. 21(3): p. 1328-1335.
- Pham HM, et al., Cinnamaldehyde Improves Lifespan and Healthspan in Drosophila melanogaster Models for Alzheimer's Disease. Biomed Res Int, 2018 p. 3570830.
- Bhuvanendran S, et al., Embelin, a Potent Molecule for Alzheimer's Disease: A Proof of Concept From Blood-Brain Barrier Permeability, Acetylcholinesterase Inhibition and Molecular Docking Studies. Front Neurosci, 2019. 13: p. 495.
- Bhuvanendran S, et al., Amelioration of Cognitive Deficit by Embelin in a Scopolamine-Induced Alzheimer's Disease-Like Condition in a Rat Model. Front Pharmacol, 2018 9: p. 665.
- Meng S, Wang B, and L. W, Paeonol administration alleviates cognitive deficits and attenuates neural pathological changes in APP/PS1 mice. J Integr Neurosci, 2021 20(4): p. 1001-1010.
- Pajak B, et al., Preliminary Study on Clusterin Protein (sCLU) Expression in PC-12 Cells Overexpressing Wild-Type and Mutated (Swedish) AβPP genes Affected by Non- Steroid Isoprenoids and Water-Soluble Cholesterol. Int J Mol Sci, 2019 20(6): p. 1481.
- Wang Z, et al., Anemonin attenuates osteoarthritis progression through inhibiting the activation of IL-1β/NF-κB pathway. J Cell Mol Med, 2017 21(12): p. 3231-3243.
4. Discussion
4.1 Background
Previous studies have shown that cinnamon may exert a neuroprotective role through the suppression of neuroinflammation and oxidative injury and thus have a potential therapeutic role due to its pro- cognitive effects in managing AD [16]. Systems-level screening of drug candidates for therapeutic AD treatment from cinnamon is attracting global research attention [1, 2] because cinnamon preparations, extracts, key metabolites [11], and hybridized molecules [32, 33] have made cinnamon an interesting and indispensable candidate for future drug research in the area of AD. Therefore, it is opportune to investigate the detailed mechanisms and apply the pharmacology of the cinnamon -AD network for predictive analysis.
4.2 Current study
In this study, we adopted network-pharmacological strategies to screen bioactive metabolites of cinnamon. The analysis incorporates 50 experimentally verified metabolites from cinnamon [3, 31] and 35 components screened using public databases. Targets of the above metabolites were collected by searching TCMSP, ETCM, Batman-TCM, SymMap, and TargetNET and predicting targets using SEA, and SwissTargetPrediction, resulting in 1697 targets related to the above 85 active metabolites. A previous study showed that 121 compounds were identified by HPLC-Q-TOF-MS in a cinnamon extract, including coumarin (1.96 mg/g), cinnamic acid (4.73 mg/g), cinnamaldehyde (8.04 mg/g) and 2-methoxycinnamaldehyde (3.66 mg/g) [34]. The disease targets were acquired by identifying AD- related genes and filtering by processing the expressing profiles of the high-throughput single-cell transcriptome SC2 diseases database. While 4246 DEGs (2615 for E2N and 1531 for E2L only) were obtained by bioinformatics analysis of scRNA-seq data, AD-associated genes from Genecards and DisGeNET resulted in 9327 targets mapped in the STRING database. Together, 321 cinnamon-AD common targets were matched. When we compared early-onset to normal and to. late-onset to normal Alzheimers genes, almost two-thirds (210/321) of the disease genes were unique for early-onset vs. normal conditions, which is attributed to the integration of single-cell technology [35].
4.3 General analysis of MOA of cinnamon metabolites
Metascape GO and KEGG enrichment analysis indicate that cinnamon metabolites may have therapeutic effects towards AD via the Alzheimer's pathways, regulation of inflammatory response, regulation of ion transport or ion homeostasis, as well as calcium signaling pathways. Aβ peptides are produced from the cleavage of APP, which is catalyzed by α-secretase, β-secretase (BACE1), and γ- secretase [36]. Prevention of Aβ aggregation to keep a homeostatic balance between Aβ production and clearance is critical to maintaining brain health [37], cinnamon metabolites interfere with Aβ involved in the Alzheimer's pathway and thus could be beneficial to the treatment of AD. For instance, oral administration of the phenolic compound paeonol (20 mg/kg) for three weeks significantly relieved the Aβ deposition burden, which contributes to the microglial inhibition and proinflammatory cytokine downregulation (TNF-α and IL-1β) in the hippocampus, and thus improved cognitive performance of AD model of APP/PS1 mice [38]. Methanol extract of cinnamon bark containing the lignan medioresinol efficiently reduced Aβ40 production (by 50%) via decreased BACE1 in Chinese hamster ovarian cells stably expressing APP [39]. The usage of embelin stops the formation of Aβ oligomers via inhibition of BACE1, improves cholinergic transmission through inhibition of AChE/BChE, and increases Aβ clearance via enhanced P-GP induction in a neuronal cell line [40]. Another study evaluated in vitro inhibitory activity of embelin toward AChE of the primary porcine brain endothelial cell model of the blood-brain barrier. It is evidenced that embelin has the potential to degrade mature fibrils of Aβ peptides through molecular binding with Aβ peptides [41]. Isolariciresinol at 20 μm shows inhibitory activity toward self-induced Aβ1–42 aggregation [42]. Treatment with patchouli alcohol (PTA) by administration of PTA (20, 40 mg/kg-1/d-1) for 90 days ameliorated AD- like pathology of APP/PS1 model mice through ameliorated o-Aβ25-35-induced reduction of synapse- related proteins via enhancing ERβ/BDNF/TrkB/CREB pathways and relieved oxidative stress in primary neurons through targeting Erβ, thus increasing catalase expression respectively [43]. Mechanistic evidence for activity of cinnamon derived metabolites in AD, has also been demonstrated through experiments using these same metabolites isolated from different sources. The commercial embelin can inhibit AChE and display nootropic as well as anti-amnesic effect in the model rat, while the embelin extract from Embelia ribes can stop the formation of Aβ oligomers by inhibiting BACE-1 and improve cholinergic-transmission. Secoisolariciresinol and secoisolariciresinol dimethyl ether are the main lignans in the stem extract of Haplophyllum sahinii and the flower extract of H. vulcanicum, which can inhibit BChE (IC50 = 65 μg/ml) and AChE (IC50 = 203 μg/ml), respectively. Isolariciresinol from Taxus baccata L. inhibited BChE and lipoxygenase with a noteworthy antioxidant activity [32], while those isolated from seeds of hawthorn (Crataegus spp.) inhibited Aβ1-42 aggregation [42]. Alpha- cadinol extracted from fruits of Alpinia oxyphylla Miquel prevents Aβ1-42-induced neuronal apoptosis [44].
Experimental studies strongly support that protocatechuic acid (PA) prevents the neurodegenerative processes including Alzheimer's diseases, due to its favorable influence on accumulation of the Aβ plaques in brain tissues, hyperphosphorylation of tau protein in neurons, excessive formation of ROS and neuroinflammation [45-47]. Aqueous extract of C. zeylanicum (containing cinnamaldehyde) show tau aggregation and filament formation inhibitory activity [13], and pure cinnamaldehyde has been shown to significantly improve lifespan and health-span of male AD flies overexpressing tau proteins in the model of Drosophila melanogaster [48]. In addition, cinnamic acid activates the nuclear hormone receptor PPARα and induces lysosomal biogenesis in mouse primary brain cells. Oral administration of cinnamic acid significantly attenuated cerebral Aβ plaque burden and improved memory and behavioral performance of male and female 5× Familial Alzheimer's disease mice [49]. Thus, cinnamon ingredients found in other species show similar effects on AD, which may open up other plant species' potential therapeutic use.
4.4 Major cinnamon metabolites with associated targets and pathways
The herb-compound-target network revealed that eugenol, cinnamic acid, cinnamaldehyde, and coumarin are the key cinnamon metabolites that counteract AD core targets, including ACHE, ALOX15, DUSP3, PTGS2, RORA, CYP19A1, ESR2, AKR1BA, APP, ESR1. Eugenol has the largest number of therapeutic targets for AD (degree = 78). Indeed, compared to synthetic phenolic compounds, eugenol is a natural antioxidant with no side effect, indicating a greater inhibitory effect against AChE, BChE, and monoamine oxidase (MAO) dose-dependently [50]. Cassia leaf oil (containing 78.35% of cinnamaldehyde and 4.52% of eugenol) shows a higher inhibition effect than cinnamaldehyde on NO production and iNOS expression cells in LPS-activated J774A.1 [6], since eugenol has been found to inhibit iNOS and NO production in LPS-stimulated human macrophages U937 [51]. Our results agree well with a recent report that eugenol could bind to different AD targets, including BACE1, MAO-B, cholinesterase's, and Aβ1-42 fibrils and shows free radical scavenging activity and anti-aggregation activity against Aβ peptides, while pre-treatment with eugenol significant protected PC12 cells against H2O2-induced oxidative stress and Aβ-induced cytotoxicity [52].
Cinnamaldehyde (3-phenyl-2-propenal) has the 3rd greatest number of AD targets (degree = 54). It displays significant neuroprotective effects in AD animal models by regulating neuroinflammation, suppressing oxidative stress, and improving the synaptic connection. The associated signaling pathways consist of TLR4/NF-κB, NLRP3, ERK1/2-MEK, NO, and Nrf2 pathways [53]. Using network pharmacology, Liu et al., speculated that cinnamaldehyde could play a role in the treatment of breast cancer via the inhibition of the peroxisome proliferator-activated receptor and P13K-Akt pathway [54]. The in vitro verification experiments showed that cinnamaldehyde can significantly inhibit cell proliferation, change cell morphology, cell migration, and invasion ability and promote cell apoptosis [23]. Surprisingly, our research group established the relationship between the estrogen receptor-α gene (ESR1) and neuroinflammation through the data mining of the high-throughput dataset of ESR1 knockdown breast cancer samples [55]. In this study, ESR1 is one of the targets of cinnamaldehyde. Therefore, cross-validation demonstrated that ESR1 dysfunction could trigger neuroinflammation or pyroptosis as a causative factor in the AD process and further applications of cinnamaldehyde in treating breast cancer. Pannee and colleagues compared the effects of cassia leaf oil from C. cassia and cinnamaldehyde on LPS-activated J774A.1 cells. Both saw remarkable down- regulation of TNF-α, IL-6, and IL-1β and decreased the mRNA expression of chemokines MCP-1 and MCP-1α [6] while stimulating the production of anti-inflammatory mediator cytokines TGF-β and IL- 10 [51], suggesting that the inhibitory effects mainly came from cinnamaldehyde [51]. Another aqueous extract of C. zeylanicum (containing cinnamaldehyde) was shown to inhibit the aggregation of human tau in vitro and filament formation [13]. On the other hand, cinnamaldehyde significantly improved the lifespan and health span of male AD flies overexpressing the tau proteins in model Drosophila melanogaster [48]. Another study showed that cinnamon extracts containing either cinnamaldehyde or epicatechin might play a protective role in preventing oxidation to tau-protein cysteine residues that can lead to dysfunction characteristic of AD [14]. Pre-incubating tau187 with cinnamaldehyde prevented the formation of tau fibers, critical structures necessary for driving the aggregation of tau tangles [14]. Incubation with C. verum extract containing cinnamaldehyde significantly inhibited the formation of fibrillar assemblies of hen egg white lysozyme by disrupting the pi-pi interactions [10]. AD rat model treated with 100 mg/kg of cinnamaldehyde (intraperitoneal) daily for 2 weeks, improved recognition/spatial memory deficits and anxiety-like behavior, negated the effects of streptozotocin on Aβ aggregation and caspase-3 cleavage in the hippocampus through decreasing phosphorylation and modulating the hippocampal IRS-1/AKT/GSK-3β signaling pathway [56]. Essential oil of C. verum (containing 68.23% of (E)-cinnamaldehyde) demonstrated potent activity toward AChE and BChE with IC50 values of 453.7 and 184.7 µg/mL, respectively [57]. However, the clinical application of cinnamaldehyde is limited due to possible allergic reactions [58], developments on cinnamaldehyde based derivatives or hybrid drugs with anti-Alzheimer's potent is promising but needs full exploration [59].
Among the targets revealed by network analysis, drugs targeted for ACHE, APP, 1L1B, ESR2, KDR and PLG have been proven to be successful, MAPT, SNCA, BACE1, DRD1, GRIN1, CHRNB4 and HSD11B1 are under clinical investigation through TTD or DrugBANK searching [60, 61], 9 of 13 genes were targeted by cinnamon metabolites, indicating our data searching strategy is of high quality and cinnamon-based drug development is promising. Molecular docking on 8 (ACHE, DUSP3, PTGS2, CYP19A1, ESR1, APP, MAPT, and FOS) of 33 key and another 4 targets (HSP90AA1, CASP3, GAPDH, TP53) and 77 metabolites were carried out to validate the result of network analysis. The docking within the docking pockets between the target protein and ingredients was visualized by AutoDockVina and PyMOL, resulting in binding affinities ranging from 6.0 to 9.6 kcal/mol, which suggests stable binding. Then, the molecular dynamics simulation was further applied to assess the stability of cinnamyl benzoate, methylbenzofuran, medioresinol and PTGS2 in the binding pocket at 100 ns. Dynamics simulation results consist of molecular docking results. PTGS2, also known as the cyclooxygenase 2 gene (COX2), showed the highest binding affinities with medioresinol (9.1), followed by the other 40 metabolites (range from 6.0 to 8.8). COX-2-induced AD development and COX-2 dysregulation significantly affect abnormal cleavage of Aß, aggregation and deposition of Aß in amyloid plaques and the inclusion of phosphorylated tau in neurofibrillary tangles [62]. Growing evidence suggests that treating patients with AD using a COX-2 selective inhibitor (celecoxib) improves cognitive performance and protects the learning ability losses for patients with AD [63-65]. Together, it indicates that cinnamon may play a protective effect on AD by inhibiting the response of COX-2 [62, 66, 67]. In addition, heat shock proteins as cellular chaperones have also been involved in the pathogenesis of AD [68]. Our results showed that six cinnamon metabolite target HSP90AA1, while two cinnamon compounds (2-hydroxycinnamic acid and methyl dihydromelilotoside) target HSPA8 (also known as HSP70). HSPA8 interacts with tau, and significant downregulation of HSPA8 was observed in AD samples [69]. HSP90 may function by maintaining oligomeric p-tau levels [70], HSPA8 and HSP90 may prevent tau aggregation and neurofibrillary tangles formation [70], and the HSP70/HSP90 balance may be a key factor in modulating p-tau stabilization [71]. These findings imply a therapeutic potential of HSPA8 or HSP90AA1 inhibitors in treating AD [72]. Indeed, HSP90 inhibitors reduced levels of tau phosphorylation [73], while daily administration of HSP90 inhibitor (17-AAG) over 7 d resulted in a significant up-expression of synaptic protein PSD95 in hippocampi [72], and PSD95 reduction degree (both mRNA and protein levels) is correlating with Aß oligomer levels and disease severity [74]. Therefore, the therapeutic effect of cinnamon against AD may be partly associated with Aß and tau phosphorylation. The interaction of cinnamon metabolites with HSPA8 and HSP90AA1 deserves more attentions.
Besides playing a role in APP or tau pathology, cinnamon metabolites may function in other AD pathology-related processes. Insoluble polymeric Aβ cleaved from APP aggregates into amyloid plaques, contributing majorly to AD pathogenesis. The damage to synapses caused by Aβ oligomers triggers microglial and astrocytic activation, leading to an inflammatory response and disrupting neuronal ionic homeostasis. It then causes oxidative injury that contributes to the formation of tau tangles and consequently causes synaptic dysfunction and neuronal loss [1, 2]. Five effective cinnamon metabolites have been widely investigated and confirmed, exerting beneficial effects on AD or AD- related inflammation and immunity. For instance, cinnamaldehyde [13, 14], epicatechin [14], eugenol [51], coumarin [15], have the potential to be used as an anti-inflammatory agent. Coumarin and Indonesian cassia extract exert anti-inflammatory activities by reducing NO and PGE2 production and inhibiting TNF-α, IL-6, and IL-1β production in macrophage cell line RAW264.7 [15]. In addition, cinnamon metabolites target the critical elements of calcium hypothesis of AD: calcium signaling pathways, and oxidative stress/inflammation pathway, offering an opportunity for the development of cinnamon-based AD therapeutic agents [75, 76].
4.5 Summary of findings, limitation and prospect
Findings from our network analysis studies have provided valuable information to support the use of cinnamon for developing therapeutic strategies against AD. According to the target distribution of cinnamon metabolites, multiple active ingredients display a synergistic effect in treating AD. The binding affinity results show that various ingredients can bind to the same protein. For instance, AChE is a target for 63 compounds, PTGS2 is the target for 41 compounds, CYP19A1 is the target for 28 metabolites, DUSP3 is the target for 7 compounds, and 8 compounds target APP and MAPT. This suggests systemic regulation of cinnamon for AD targets. On the one hand, it may pose a great challenge for new drug discovery [77]. On the other hand, besides the direct application of cinnamon extracts or purified commercially available compounds, the specific design and development of multi- target compounds incorporating active cinnamon ingredients, which target AD, are promising therapeutic candidates [32]. One good example is coumarin-based hybrid molecules, which have shown excellent potential for AChE inhibition, MAO-B inhibition, and anti-Aβ aggregation, and have been well discussed [32, 33]. Furthermore, melatonin-cinnamate hybrids show antioxidant properties and the highest neuroprotective effect in the rat/olig model through the implementation of tunable Nrf2 induction properties of cinnamates and the scavenger and neuroprotective effect of melatonin [78]. Another hybrid incorporates benzofuran and chalcone fragments which decrease Aβ aggregation and AChE levels, reduce oxidative stress, and provide protection against chemically induced cholinergic neurodegeneration in the worms Caenorhabditis elegans [32].
We propose the mechanism of cinnamon counteracting AD: interference with Aβ involved Alzheimer's pathway, reduces Aβ production, increases Aβ clearance, e.g., degrades mature fibrils of Aβ peptides through molecular binding with Aβ peptides, or relieves oxidative stress, targets critical elements of calcium signaling pathways. This study presents with several limitations, for example, although the molecular docking and molecular dynamics showed positive results, further experimental validation are needed to evaluate the predicted compound–target relationships.
5. Conclusion
In this case study, we see the significant contribution that a system analysis approach can make to understanding the therapeutic benefits of plant metabolites in AD. The current study using cinnamon as a case study confirms the potent therapeutic effects of cinnamon species, particularly C. cassia, which is used globally as raw materials in foods, nutraceuticals, and traditional medicinal use. The possible mechanisms of action of the cinnamon metabolome and its metabolites were acquired by employing network systems pharmacology and molecular computational analyses, which were supported by scRNA-Seq data and experimental reports from the literature. Results here offer perspective options in treating AD, with cinnamon metabolites to promote anti-Aß aggregation and clearance and modulate the calcium signaling pathway. To support these findings, studies will need to be carried out to identify an optimized standardized extract, as will studies to establish the maximum tolerated and optimal dosing for therapeutic effect and to further verify related pathways and targets.
Author Contributions
Wen Qiu: Software, Investigation, Writing - Original Draft, Visualization. Shouli Yuan: Software, Formal analysis. Junying Liu: Methodology, Resources, Formal analysis. Lina Sun, Wei Chen: Project administration. Robbie Kelleher: Writing - Review & Editing. Helen Sheridan: Conceptualization, Writing - Review & Editing. Weiguang Lv: Supervision.
Funding
This work was financially supported by Hu-Nong-Ke-Zhuo (2022) 008, and National Agricultural Experimental Station for Agricultural Environment Program (NAES035AE03).
Conflicting Interests
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sierksma A, et al. Translating genetic risk of Alzheimer's disease into mechanistic insight and drug targets. Science 370 (2020): 61-66.
- Hh A, et al. Omics sciences for systems biology in Alzheimer's disease: State-of-the-art of the evidence - ScienceDirect. Ageing Research Reviews 69 (2021).
- Zhang C, et al. Cinnamomum cassia Presl: A Review of Its Traditional Uses, Phytochemistry, Pharmacology and Toxicology. Molecules 24 (2019).
- Wang YH, et al. Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States. J Agric Food Chem 61 (2013): 4470-4476.
- Assessment report on Cinnamomum verum J. S. Presl, cortex and corticis aetheroleum (2011).
- Pannee C, et al. Anti inflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A.1 cells. J Adv Pharm Technol Res 5 (2014): 164-70.
- Gucwa K, et al. Investigation of the Antifungal Activity and Mode of Action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus Essential Oils. Molecules 23 (2018).
- Zaidi SF, et al. Review: Diverse pharmacological properties of Cinnamomum cassia: A review. Pak J Pharm Sci 28 (2015): 1433-8.
- Frydman-Marom A, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer's disease animal models. PLoS One 6 (2011): e16564.
- Ramshini H, et al. Effect of Cinnamomum Verum Extract on the Amyloid Formation of Hen Egg-white Lysozyme and Study of its Possible Role in Alzheimer's Disease. Basic Clin Neurosci 6 (2015): 29-37.
- Modi KK et al. Cinnamon and its metabolite sodium benzoate attenuate the activation of p21rac and protect memory and learning in an animal model of alzheimer’s disease. PLoS One 10 (2015): e0130398.
- Anderson RA, et al. Cinnamon counteracts the negative effects of a high fat/high fructose diet on behavior, brain insulin signaling and Alzheimer-associated changes. PLoS One 8 (2013): e83243.
- Peterson DW, et al. Cinnamon extract inhibits tau aggregation associated with Alzheimer's disease in vitro 17 (2009): 585-597.
- George RC, et al. Interaction of cinnamaldehyde and epicatechin with tau: implications of beneficial effects in modulating Alzheimer’s disease pathogenesis. J Alzheimers Dis 36 (2013): 21–40.
- Sandhiutami NM, et al. In vitro assesment of anti-inflammatory activities of coumarin and Indonesian cassia extract in RAW264.7 murine macrophage cell line. Iran J Basic Med Sci 20 (2017): 99-106.
- Yulug B and Cankaya S. Translational perspective: is cinnamon a suitable agent for cognitive impairment and Alzheimer's disease associated with brain trauma? Neural Regen Res 14 (2019): 1372-1373.
- Seibel R, et al. Effects of Spices (Saffron, Rosemary, Cinnamon, Turmeric and Ginger) in Alzheimer's Disease. Curr Alzheimer Res 18 (2021): 347-357.
- Xu HY, et al., ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res 47 (2019): D976-D982.
- Ru J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 6 (2014): 13.
- Huang L, et al., TCMID 2.0: a comprehensive resource for TCM. Nucleic Acids Res 46 (2018): D1117-d1120.
- Liu Z, et al. BATMAN-TCM: a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci Rep 6 (2016): 21146.
- Wu Y, et al. SymMap: an integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res 47 (2019): D1110-d1117.
- Liu JY, et al. Exploring the pharmacological mechanism of Houttuynia cordata on pneumonia caused by SARS-CoV-2 with network pharmacology and molecular docking. Front Pharmacol (2021).
- Yao ZJ, et al. TargetNet: a web service for predicting potential drug-target interaction profiling via multi-target SAR models. J Comput Aided Mol Des 30 (2016): 413-24.
- Daina A, et al. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res 47 (2019): W357-w364.
- Gu S and Lai LH. Associating 197 Chinese herbal medicine with drug targets and diseases using the similarity ensemble approach. Acta Pharmacol Sin 41 (2020): 432-438.
- Lipinski CA, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46 (2001): 3-26.
- Zhao T, et al. SC2disease: a manually curated database of single-cell transcriptome for human diseases. Nucleic Acids Research 49 (2020): D1413-D1419.
- Zhou Y, et al. Metascape provides a biologist-oriented resource for the analysis of systems- level datasets. Nat Commun 10 (2019): 1523.
- Trott O. and Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31 (2010): 455-61.
- Momtaz S, et al., Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol Res 130 (2018): 241-258.
- Du YK, et al. Study on the Mechanism of Acori Graminei Rhizoma in the Treatment of Alzheimer's Disease Based on Network Pharmacology and Molecular Docking. BioMed research international (2021): 5418142.
- Yusufzai SK, et al., Molecular docking studies of coumarin hybrids as potential acetylcholinesterase, butyrylcholinesterase, monoamine oxidase A/B and β-amyloid inhibitors for Alzheimer's disease. Chem Cent J 12 (2018): 128.
- Li X, et al. Network pharmacology-based research uncovers cold resistance and thermogenesis mechanism of Cinnamomum cassia. Fitoterapia 149 (2021): 104824.
- Hampel H, et al. Omics sciences for systems biology in Alzheimer's disease: State-of-the-art of the evidence. Ageing Res Rev 69 (2021): 101346.
- Zeng P, et al. Therapeutic Mechanism and Key Alkaloids of Uncaria rhynchophylla in Alzheimer's Disease from the Perspective of Pathophysiological Processes. Front Pharmacol 12 (2021): 806984.
- Roher AE, et al., Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement 5 (2009): 18-29.
- Meng S, et al. Paeonol administration alleviates cognitive deficits and attenuates neural pathological changes in APP/PS1 mice. J Integr Neurosci 20 (2021): 1001-1010.
- Kang YJ, et al. Phenylpropanoids from cinnamon bark reduced β-amyloid production by the inhibition of β-secretase in Chinese hamster ovarian cells stably expressing amyloid precursor protein. Nutr Res 36 (2016): 1277-1284.
- Nuthakki VK, et al., Identification of embelin, a 3-undecyl-1,4-benzoquinone from Embelia ribes as a multitargeted anti-Alzheimer agent. Drug Dev Res 80 (2019): 655-665.
- Bhuvanendran S, et al. Embelin, a Potent Molecule for Alzheimer's Disease: A Proof of Concept from Blood-Brain Barrier Permeability, Acetylcholinesterase Inhibition and Molecular Docking Studies. Front Neurosci 13 (2019): 495.
- Huang XX, et al. Lignans from the seeds of Chinese hawthorn (Crataegus pinnatifida var. major N.E.Br.) against β-amyloid aggregation. Nat Prod Res 32 (2018): 1706-1713.
- Yan QY, et al. Patchouli alcohol as a selective estrogen receptor β agonist ameliorates AD- like pathology of APP/PS1 model mice. Acta Pharmacol Sin (2022).
- Ji ZH, et al. In-vitro neuroprotective effect and mechanism of 2β-hydroxy-δ-cadinol against amyloid β-induced neuronal apoptosis. Neuroreport 31 (2020): 245-250.
- Karahisar E, et al. Metabolite Profiling by Hyphenated Liquid Chromatographic Mass Spectrometric Technique (HPLC-DAD-ESI-Q-TOF-MS/MS) and Neurobiological Potential of Haplophyllum sahinii and H. vulcanicum Extracts. Chem Biodivers 16 (2019): e1900333.
- Choi JR, et al. Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer's disease mouse model. Food Chem Toxicol 144 (2020): 111571.
- Huang L, et al. Protocatechuic acid attenuates β-secretase activity and okadaic acid-induced autophagy via the Akt/GSK-3β/MEF2D pathway in PC12 cells. Mol Med Rep 21 (2020): 1328-1335.
- Pham HM, et al. Cinnamaldehyde Improves Lifespan and Healthspan in Drosophila melanogaster Models for Alzheimer's Disease. Biomed Res Int (2018): 3570830.
- Chandra S, et al. Cinnamic acid activates PPARα to stimulate Lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer's disease mouse model. Neurobiol Dis 124 (2019): 379-395.
- Adefegha SA, et al. Antioxidant properties of eugenol, butylated hydroxylanisole, and butylated hydroxyl toluene with key biomolecules relevant to Alzheimer's diseases-In vitro. J Food Biochem 45 (2021): e13276.
- Lee YY, et al., Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J Endod 33 (2007): 698-702.
- Chowdhury S, and Kumar S. Inhibition of BACE1, MAO-B, cholinesterase enzymes, and anti- amyloidogenic potential of selected natural phytoconstituents: Multi-target-directed ligand approach. J Food Biochem 45 (2021): e13571.
- Hajinejad M, et al. Natural Cinnamaldehyde and Its Derivatives Ameliorate Neuroinflammatory Pathways in Neurodegenerative Diseases. Biomed Res Int (2020): 1034325.
- Liu Y, et al. Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer. Front Pharmacol 11 (2020): 582719.
- Liu J, et al. ESR1 dysfunction triggers neuroinflammation as a critical upstream causative factor of the Alzheimer's disease process. Aging (Albany NY) 14 (2022): 8595-8614.
- Bagheri-Mohammadi S, et al. Cinnamaldehyde Regulates Insulin and Caspase-3 Signaling Pathways in the Sporadic Alzheimer's Disease Model: Involvement of Hippocampal Function via IRS-1, Akt, and GSK-3β Phosphorylation. J Mol Neurosci 72 (2022): 2273-2291.
- Saeedi M, et al. Cinnamomum verum J. Presl. Bark essential oil: in vitro investigation of anti-cholinesterase, anti-BACE1, and neuroprotective activity. BMC Complement Med Ther 22 (2022): 303.
- Shreaz S, et al. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 112 (2016): 116-31.
- Chun YL, et al. Novel Cinnamaldehyde Derivatives Inhibit Peripheral Nerve Degeneration by Targeting Schwann Cells. Antioxidants (Basel) 11 (2022).
- Zhou Y, et al. Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res 50 (2022): D1398- D1407.
- Wishart DS et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46 (2018): D1074-D1082.
- Guan PP and Wang P, Integrated communications between cyclooxygenase-2 and Alzheimer's disease. FASEB J 33 (2019): 13-33.
- Chen CY, et al. Maintenance therapy of celecoxib for major depression with mimicking neuropsychological dysfunction. Gen Hosp Psychiatry 32 (2010): 647.e7-9.
- Leoutsakos JM, et al. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: findings from the randomized controlled Alzheimer's Disease Anti-inflammatory Prevention Trial. Int J Geriatr Psychiatry 27 (2012): 364-374.
- Small GW, et al. Cognitive and cerebral metabolic effects of celecoxib versus placebo in people with age-related memory loss: randomized controlled study. Am J Geriatr Psychiatry 16 (2008): 999-1009.
- Chen Q, et al., Influence of four polymorphisms in ABCA1 and PTGS2 genes on risk of Alzheimer's disease: a meta-analysis. Neurol Sci 37 (2016): 1209-1220.
- Cudaback E, et al. Therapeutic implications of the prostaglandin pathway in Alzheimer's disease. Biochem Pharmacol 88 (2014): 565-572.
- Lackie RE, et al. The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases. Front Neurosci 11 (2017): 254.
- Silva PN, et al. Analysis of HSPA8 and HSPA9 mRNA expression and promoter methylation in the brain and blood of Alzheimer's disease patients. J Alzheimers Dis 38 (2014): 165- 170.
- Dou F, et al. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A 100 (2003): 721-726.
- Mao Y, et al. Protein-protein interactions underlying the behavioral and psychological symptoms of dementia (BPSD) and Alzheimer's disease. PLoS One 15 (2020): e0226021.
- Chen Y, et al. Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J Neurosci 34 (2014): 2464-2470.
- Dickey CA, et al. HSP induction mediates selective clearance of tau phosphorylated at proline- directed Ser/Thr sites but not KXGS (MARK) sites. FASEB J 20 (2006): 753-755.
- Proctor DT, et al. Reduction in post-synaptic scaffolding PSD-95 and SAP-102 protein levels in the Alzheimer inferior temporal cortex is correlated with disease pathology. J Alzheimers Dis 21 (2010): 795-811.
- Popugaeva E, et al. Dysregulation of neuronal calcium homeostasis in Alzheimer's disease - A therapeutic opportunity? Biochem Biophys Res Commun 483 (2017): 998-1004.
- Pall ML, Low Intensity Electromagnetic Fields Act via Voltage-Gated Calcium Channel (VGCC) Activation to Cause Very Early Onset Alzheimer's Disease: 18 Distinct Types of Evidence. Curr Alzheimer Res 19 (2022): 119-132.
- Yuan H, et al. How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? . Molecules 22 (2017): 1135.
- Buendia I, et al. New melatonin-cinnamate hybrids as multi-target drugs for neurodegenerative diseases: Nrf2-induction, antioxidant effect and neuroprotection. Future Med Chem 7 (2015): 1961-1969.
- Ashfaq M, et al. Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease. Neurosciences Neurobiology Rehabilitation Medicine Human Genetics, ed. Ghulam Md Ashraf and Athanasios Alexiou (2019): 82.