A Slow Growing Paratesticular Leiomyosarcoma: An Unusual Case Report
Article Information
Yasmine Laraqui Housseini1*, Fouad Zouaidia1, Souhail Regragui2, Sabrine Derqaoui1, Khouloud Raissouni1, Ahmed Jahid1, Kaoutar Znati1, Zakiya Bernoussi1, Hafsa Elouazzani1
1Department of Pathology, Ibn Sina University Hospital Center Rabat, Morocco
2Department of Urology, B Ibn Sina University Hospital Center Rabat, Morocco
*Corresponding Author: Yasmine Laraqui Housseini, Department of Pathology, Ibn Sina University Hospital Center, Rabat, Morocco
Received: 06 December 2019; Accepted: 28 December 2019; Published: 22 January 2020
Citation: Yasmine Laraqui Housseini, Fouad Zouaidia, Souhail Regragui, Sabrine Derqaoui, Khouloud Raissouni, Ahmed Jahid, Kaoutar Znati, Zakiya Bernoussi, Hafsa Elouazzani. A Slow Growing Paratesticular Leiomyosarcoma: An Unusual Case Report. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 065-070.
View / Download Pdf Share at FacebookAbstract
Paratesticular leiomyosarcoma is a rare tumor of the mature adult who develops habitually in the spermatic cord [1]. Preoperative diagnosis can be difficult because of nonspecific symptoms and non pathognomonic radiological features. In addition, its slow growing does not allow adequate differential diagnosis with respect to other scrotal tumors. We present a case of an uncommon paratesticular leiomyosarcoma: it’s about a 64-year-old man with a history of painless enlargement of the left testis for 03 years. The scrotal ultrasound and MRI showed a paratesticular mass of 4.5cm in contact with spermatic cord. Patient underwent left high inguinal orchidectomy. Histopathological examination with immunostaining made the final diagnosis of grade 1 paratesticular leiomyosarcoma of NFFCS, with hepatic metastases in the abdominal CT.
Keywords
Paratesticular; Leiomyosarcoma; Slow growing
Paratesticular articles, Leiomyosarcoma articles, Slow growing articles
Paratesticular articles Paratesticular Research articles Paratesticular review articles Paratesticular PubMed articles Paratesticular PubMed Central articles Paratesticular 2023 articles Paratesticular 2024 articles Paratesticular Scopus articles Paratesticular impact factor journals Paratesticular Scopus journals Paratesticular PubMed journals Paratesticular medical journals Paratesticular free journals Paratesticular best journals Paratesticular top journals Paratesticular free medical journals Paratesticular famous journals Paratesticular Google Scholar indexed journals Leiomyosarcoma articles Leiomyosarcoma Research articles Leiomyosarcoma review articles Leiomyosarcoma PubMed articles Leiomyosarcoma PubMed Central articles Leiomyosarcoma 2023 articles Leiomyosarcoma 2024 articles Leiomyosarcoma Scopus articles Leiomyosarcoma impact factor journals Leiomyosarcoma Scopus journals Leiomyosarcoma PubMed journals Leiomyosarcoma medical journals Leiomyosarcoma free journals Leiomyosarcoma best journals Leiomyosarcoma top journals Leiomyosarcoma free medical journals Leiomyosarcoma famous journals Leiomyosarcoma Google Scholar indexed journals Slow growing articles Slow growing Research articles Slow growing review articles Slow growing PubMed articles Slow growing PubMed Central articles Slow growing 2023 articles Slow growing 2024 articles Slow growing Scopus articles Slow growing impact factor journals Slow growing Scopus journals Slow growing PubMed journals Slow growing medical journals Slow growing free journals Slow growing best journals Slow growing top journals Slow growing free medical journals Slow growing famous journals Slow growing Google Scholar indexed journals Malignant tumors articles Malignant tumors Research articles Malignant tumors review articles Malignant tumors PubMed articles Malignant tumors PubMed Central articles Malignant tumors 2023 articles Malignant tumors 2024 articles Malignant tumors Scopus articles Malignant tumors impact factor journals Malignant tumors Scopus journals Malignant tumors PubMed journals Malignant tumors medical journals Malignant tumors free journals Malignant tumors best journals Malignant tumors top journals Malignant tumors free medical journals Malignant tumors famous journals Malignant tumors Google Scholar indexed journals genitourinary tract articles genitourinary tract Research articles genitourinary tract review articles genitourinary tract PubMed articles genitourinary tract PubMed Central articles genitourinary tract 2023 articles genitourinary tract 2024 articles genitourinary tract Scopus articles genitourinary tract impact factor journals genitourinary tract Scopus journals genitourinary tract PubMed journals genitourinary tract medical journals genitourinary tract free journals genitourinary tract best journals genitourinary tract top journals genitourinary tract free medical journals genitourinary tract famous journals genitourinary tract Google Scholar indexed journals spermatic cord articles spermatic cord Research articles spermatic cord review articles spermatic cord PubMed articles spermatic cord PubMed Central articles spermatic cord 2023 articles spermatic cord 2024 articles spermatic cord Scopus articles spermatic cord impact factor journals spermatic cord Scopus journals spermatic cord PubMed journals spermatic cord medical journals spermatic cord free journals spermatic cord best journals spermatic cord top journals spermatic cord free medical journals spermatic cord famous journals spermatic cord Google Scholar indexed journals hepatic metastases articles hepatic metastases Research articles hepatic metastases review articles hepatic metastases PubMed articles hepatic metastases PubMed Central articles hepatic metastases 2023 articles hepatic metastases 2024 articles hepatic metastases Scopus articles hepatic metastases impact factor journals hepatic metastases Scopus journals hepatic metastases PubMed journals hepatic metastases medical journals hepatic metastases free journals hepatic metastases best journals hepatic metastases top journals hepatic metastases free medical journals hepatic metastases famous journals hepatic metastases Google Scholar indexed journals Scrotal Ultrasonography articles Scrotal Ultrasonography Research articles Scrotal Ultrasonography review articles Scrotal Ultrasonography PubMed articles Scrotal Ultrasonography PubMed Central articles Scrotal Ultrasonography 2023 articles Scrotal Ultrasonography 2024 articles Scrotal Ultrasonography Scopus articles Scrotal Ultrasonography impact factor journals Scrotal Ultrasonography Scopus journals Scrotal Ultrasonography PubMed journals Scrotal Ultrasonography medical journals Scrotal Ultrasonography free journals Scrotal Ultrasonography best journals Scrotal Ultrasonography top journals Scrotal Ultrasonography free medical journals Scrotal Ultrasonography famous journals Scrotal Ultrasonography Google Scholar indexed journals echogenicity articles echogenicity Research articles echogenicity review articles echogenicity PubMed articles echogenicity PubMed Central articles echogenicity 2023 articles echogenicity 2024 articles echogenicity Scopus articles echogenicity impact factor journals echogenicity Scopus journals echogenicity PubMed journals echogenicity medical journals echogenicity free journals echogenicity best journals echogenicity top journals echogenicity free medical journals echogenicity famous journals echogenicity Google Scholar indexed journals Computerized Tomography articles Computerized Tomography Research articles Computerized Tomography review articles Computerized Tomography PubMed articles Computerized Tomography PubMed Central articles Computerized Tomography 2023 articles Computerized Tomography 2024 articles Computerized Tomography Scopus articles Computerized Tomography impact factor journals Computerized Tomography Scopus journals Computerized Tomography PubMed journals Computerized Tomography medical journals Computerized Tomography free journals Computerized Tomography best journals Computerized Tomography top journals Computerized Tomography free medical journals Computerized Tomography famous journals Computerized Tomography Google Scholar indexed journals
Article Details
1. Introduction
Malignant tumors of the mesenchymal line of the genitourinary tract represent approximately 5% of all sarcomas and less than 2% of all urological tumors [2]. Sarcomas of paratesticular location are a rare entity, with histological subtypes including rhabdomyosarcoma, leiomyosarcoma and liposarcoma. Leiomyosarcomas are malignant tumours that arise from smooth muscle-containing tissue, it represents 30% of paratesticular sarcomas [3]. They are presented as a nodular mass, mostly near the spermatic cord and less frequent in the epididymis, completely separated from the testis [4]. Radical inguinal orchidectomy is the standard treatment, with other approaches remaining controversial. Its behavior and prognosis is variable.
2. Case Report
A 64-year-old male presented with a gradually increasing left scrotal mass during the past 2 years. There was no significant past history. Scrotal Ultrasonography revealed a mixed echogenicity paratesticular mass in contact with the spermatic cord (Figure 1a). MRI showed a left paratesticular tumor process with hyposignal T1 (Figure 1b). Laboratory findings were normal. Thus, the patient underwent left high inguinal orchidectomy.Abdominal Computerized Tomography revealed the presence of hepatic metastasis. In our laboratory, we received an orchidectomy specimen measured 7 × 4 × 2cm with a fasciculated, grayish and white tumor (4,5 cm of diameter) (Figure 2).
The histological analysis of the tumor shows a fusiform cell proliferation of moderate to marked cell density reminiscent of a smooth muscle differentiation. Tumor cells has oval nuclei, heterogeneous and densified chromatin and nucleolated with images of nuclear pleomorphism. The cytoplasm was eosinophilic and the cytoplasmic boundaries are imprecise. The mitosis figures were numerous estimated at 12 mitoses / 10 cps at GX40. There was no tumor necrosis (Figure 3). On the periphery, the tumor was well limited but without capsule. Otherwise, the fatty tissue analyzed was without abnormality, testicular parenchyma, albuginea, vaginal and spermatic cord were free of tumor element. Immunohistochemically, tumor cells stained positively for H-caldesmone, smooth muscle actine (Figure 4), focally for desmin, and negatively for PS 100, CD34, estrogen and progesterone receptors. Proliferation index with Ki67 was increased (70%). After histopathologic examination and immunostaining, a diagnosis of grade 1 NFFCS primary paratesticular leiomyosarcoma was ascertained. Abdominal Computerized Tomography revealed the presence of hepatic metastasis so the patient has been referred to an oncology center for further management.
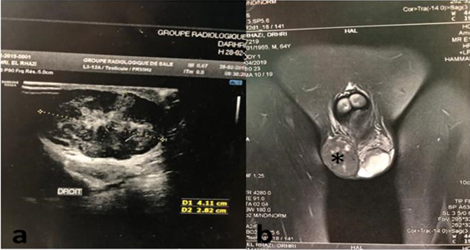
Figure 1: (a) Ultrasound image showing mixed echogenicity paratesticular mass in contact with the spermatic cord; (b) MRI showing a paratesticular tumor process with hyposignal T1 (*).
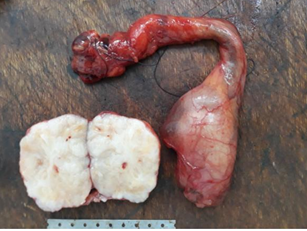
Figure 2: Grossly, the orchidectomy specimen measured 7 × 4 × 2 cm with lobulated tumor with a fasciculated, grayish and white tumor measuring 4,5 cm of diameter.
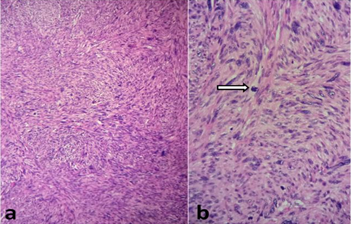
Figure 3: Microscopically, (a) fusiform cell proliferation suggesting smooth muscle origin (haematoxylin and eosin stain X10), (b) with nuclear pleomorphism, blunt edged nuclei and mitoses (arrow) (haematoxylin and eosin stain X20).
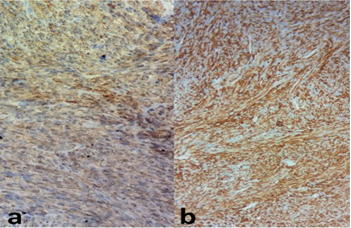
Figure 4: Positive staining with smooth muscle actine (a) and H-caldesmone (b).
3. Discussion
Leiomyosarcoma is a malignant mesenchymal tumor with smooth muscle differenciation. It represents the second most common malignant mesenchymal tumor of paratestis [4]. The paratesticular region involve testicular tunics, spermatic cord, epididymis, and inguinal canal; spermatic cord being the most common, accounting for 90% [5]. It is thought to arise from smooth muscle structures and may spread by lymphatic, hematogeneous and local extension [6, 7]. It is a rare tumor, arising frequently in older men, age at presentation being between sixth and seventh decade of life, with a median age around 64 years [2]. Usually, they present as a painless, firm, slow-growing mass, entirely separated from the testis. In the physical examination, it is a well defined, lobulated and mobile mass, which can be associated with a hydrocele. Thus, clinical findings does not distinguish between benign and malignant tumors but the age of presentation and the slow evolution can provide some clues [8].
The study of tumor markers is fundamental in its differential diagnosis. In fact, normal serum tumor markers such as alpha fetus protein (AFP), human chorionic gonadotrophin (HCG) and lactate deshydrogenase (LDH), permit to exclude the diagnosis of a germinal tumor [5]. The exam of choice in the initial investigation for any scrotal mass is ultrasound scanning, with a sensitivity of 95% for differentiating intratesticular from extratesticular lesions [4]. A solid mass, with mixed echogenicity is usually identified, with increased vascularization on color Doppler [1]. Extension studies include CT and magnetic resonance to delimitate the tumor extension and detect possible metastasis [5]. Paratesticular leiomyosarcoma is difficult to diagnose preoperatively and requires a histologic examination for a definitive diagnosis [6]. Grossly, the tumors are typically located outside tunica albuginea, centred on epididiymis or spermatic cord. They are usually well circumscribed, firm and grey mass, sometimes with necrotic and hemorrhagic areas, and measuring between 2 and 10 cm (median size around 4 cm).
Histologically, paratesticular leiomyosarcoma is characterized by classic features of soft tissue leiomyosarcomas. They are composed of spindle cells with “cigar-shaped” nuclei arranged into intersecting fascicles and containing eosinophilic cytoplasm with longitudinal fibrils (9). The nuclear pleomorphism and the number of mitoses, sometimes atypical, are variable. Some cases have prominent myxoid stroma, inflammatory component, or epithelioid foci. French Federation of Cancer Centre Sarcoma Grading System divided it in 3 grades based on the mitotic activity, percentage of necrosis and degree of nuclear pleomorphism. It has been shown to be reproducible among pathologists and correlates with the clinical outcome [5].
In the immunohistochemical study, tumor cells typically express smooth muscle actin, desmin and h-caldesmone and are negative for myogenin. The expression of CD34 and cytokeratin has also been reported in some cases [9]. Some leiomyosarcomas have numerous bizarre pleomorphic nuclei. They may resemble undifferentiated pleomorphic sarcomas. Usually, there are focal areas in the tumor with smooth muscle differentiation that indicate a correct diagnosis. Other differential diagnosis includes dedifferentiated liposarcoma with smooth muscle differentiation that shown more typical liposarcomatous areas with atypical spindle cells and multivacuolated lipoblasts embedded in a loose myxoid to dense fibrous stroma. Fibrous mesothelioma associated with hydrocele can be evoked, yet it associated with asbestos exposure, infiltrative margins and usually keratin positive and desmin negative.
In our case, because of the low growing presentation of the tumor, we discussed also cellular angiofibroma or angiomyofibroblastoma-like tumor. It consists of spindle cells proliferation in a myxoid stroma with collagen and small size blood vessels and can show an abrupt smooth muscle differentiation area. It’s habitually positive for CD34 and Hormonal Receptors. The standard primary surgical treatment is radical orchiectomy with high spermatic cord section, being for some authors sufficient. However, even after adequate surgery with negative margins, one third of patients have persistence of microscopic disease and recurrence with a frequency of approximately 30–50%. Other researchers consider adjuvant treatment with chemotherapy and / or radiotherapy, but it remains controversial; some authors recommend adjuvant therapy for nonoperable or metastatic tumors and others recommend adjuvant radiotherapy for all grades to reduce local recurrence [6, 7]. Currently, prophylactic retroperitoneal lymphnode have no clear indication for paratesticular leiomyosarcoma, so dissection recommended only if nodes are suspicious for involvement. As wide resection margins can be difficult to obtain in the paratesticular region, the tumor tends to show local recurrence [5]. That’s why all patient require a careful follow-up to prevent this risk of recurrence, which represent a major problem scrotal recurrence rate being between 25 and 37 [10].
The main prognostic factors of leiomyosarcoma are related to the site, size, histological grade, and presence of nodal or distant metastasis. Modes of spread include haematogenous (primarily pulmonary or hepatic) , lymphatic (external iliac, hypogastric, common iliac and retroperitoneal nodes ) and local extension to scrotum, inguinal canal or pelvis [9, 7].
4. Conclusion
Extratesticular malignancies are rare leiomyosarcomas which constitute a significant part of paratesticular masses and can present an aggressive clinical evolution. It should be considered as a differential diagnosis in elderly patients with scrotal mass. Due to the risk of local recurrence and distant metastases, long term follow up of paratesticular leiomyosarcomas after radical orchidectomy is essential.
References
- Kyratzi I, Lolis E, Antypa E, et al. Imaging features of a huge spermatic cord leiomyosarcoma: Review of the literature. World J Radiol 3 (2011): 114-119.
- Varzaneh FE, Verghese M, Shmookler BM. Paratesticular leiomyosarcoma in an elderly man. Urology 60 (2002): 1112.
- Juan C, Pérez A, Abad Licham M, et al. Sarcomas paratesticulares en el paciente adulto. Manejo y evolución de la enfermedad. Actas Urol Esp 33 (2009): 639-645.
- Rodríguez D, Barrisford GW, Sanchez A, et al. Primary spermatic cord tumors: disease characteristics, prognostic factors, and treatment outcomes. Urol Oncol 32 (2014): 52.e19-25.
- Alfarelos J, Gomes G, Campos F, et al. Paratesticular Leiomyosarcoma: A Case Report and Review of the Literature. Urol Case Rep 11 (2017): 30-32.
- Kolev NH, Dunev VR, Karaivanov MP, et al. Paratesticular leiomyosarcoma: A clinical case report. Urol Case Rep. 1 nov 27 (2019): 100913.
- Moloney J, Drumm J, Fanning DM. A rare case of paratesticular leiomyosarcoma. Clin Pract 2 (2012).
- Ko BS, Kim NY, Ryu AJ, Kim DS, et al. A Case of Paratesticular Leiomyosarcoma Successfully Treated with Orchiectomy and Chemotherapy. Cancer Res Treat 44 (2012): 210-214.
- Haran S, Balakrishan V, Neerhut G. A Rare Case of Paratesticular Leiomyosarcoma. Case Reports in Urology (2014).
- Rezvani S, Bolton J, Crump A. A rare case of paratesticular leiomyosarcoma. J Surg Case Rep 10 (2018).
