A Rare Fatal Case of Cardiac Perforation During Cardioverter-Defibrillator Implantation
Article Information
Enrica Rosato*, Martina Bonelli, Cristian D’Ovidio
Section of Legal Medicine, Department of Medicine and Aging Sciences, Center for Advanced Studies and Technology (C.A.S.T.), University ‘G. d’Annunzio’, Chieti, Italy
*Corresponding Author: Dr. Enrica Rosato, Section of Legal Medicine, Department of Medicine and Aging Sciences, Center for Advanced Studies and Technology (C.A.S.T.), University ‘G. d’Annunzio’, via L. Polacchi 11, 66100 Chieti, Italy
Received: 11 May 2020; Accepted: 28 May 2020; Published: 10 July 2020
Citation: Enrica Rosato, Martina Bonelli, Cristian D’Ovidio. A Rare Fatal Case of Cardiac Perforation During Cardioverter-Defibrillator Implantation. Archives of Clinical and Medical Case Reports 4 (2020): 638-644.
View / Download Pdf Share at FacebookAbstract
Right ventricular perforation is a rare but fatal complication during implantation and removal of cardiac devices. The causes of such an event are not well defined, although among the various factors involved, the thickness of the ventricular wall and the force exerted by the operator appear to be important. We report here a case of fatal cardiac resynchronization therapy with defibrillation in a patient without apparent predictive factors for perforation. This case highlights the importance of revisiting the diagnostic pathways before introduction of a cardiac device, and of further studies on the predictive factors, to avoid a complication being considered as a surgical error.
Keywords
Cardiac resynchronization therapy; Defibrillation; Cardiac perforation; Professional liability
Cardiac resynchronization therapy articles, Defibrillation articles, Cardiac perforation articles, Professional liability articles
Cardiac resynchronization therapy articles Cardiac resynchronization therapy Research articles Cardiac resynchronization therapy review articles Cardiac resynchronization therapy PubMed articles Cardiac resynchronization therapy PubMed Central articles Cardiac resynchronization therapy 2023 articles Cardiac resynchronization therapy 2024 articles Cardiac resynchronization therapy Scopus articles Cardiac resynchronization therapy impact factor journals Cardiac resynchronization therapy Scopus journals Cardiac resynchronization therapy PubMed journals Cardiac resynchronization therapy medical journals Cardiac resynchronization therapy free journals Cardiac resynchronization therapy best journals Cardiac resynchronization therapy top journals Cardiac resynchronization therapy free medical journals Cardiac resynchronization therapy famous journals Cardiac resynchronization therapy Google Scholar indexed journals resynchronization therapy articles resynchronization therapy Research articles resynchronization therapy review articles resynchronization therapy PubMed articles resynchronization therapy PubMed Central articles resynchronization therapy 2023 articles resynchronization therapy 2024 articles resynchronization therapy Scopus articles resynchronization therapy impact factor journals resynchronization therapy Scopus journals resynchronization therapy PubMed journals resynchronization therapy medical journals resynchronization therapy free journals resynchronization therapy best journals resynchronization therapy top journals resynchronization therapy free medical journals resynchronization therapy famous journals resynchronization therapy Google Scholar indexed journals Defibrillation articles Defibrillation Research articles Defibrillation review articles Defibrillation PubMed articles Defibrillation PubMed Central articles Defibrillation 2023 articles Defibrillation 2024 articles Defibrillation Scopus articles Defibrillation impact factor journals Defibrillation Scopus journals Defibrillation PubMed journals Defibrillation medical journals Defibrillation free journals Defibrillation best journals Defibrillation top journals Defibrillation free medical journals Defibrillation famous journals Defibrillation Google Scholar indexed journals Cardiac perforation articles Cardiac perforation Research articles Cardiac perforation review articles Cardiac perforation PubMed articles Cardiac perforation PubMed Central articles Cardiac perforation 2023 articles Cardiac perforation 2024 articles Cardiac perforation Scopus articles Cardiac perforation impact factor journals Cardiac perforation Scopus journals Cardiac perforation PubMed journals Cardiac perforation medical journals Cardiac perforation free journals Cardiac perforation best journals Cardiac perforation top journals Cardiac perforation free medical journals Cardiac perforation famous journals Cardiac perforation Google Scholar indexed journals Professional liability articles Professional liability Research articles Professional liability review articles Professional liability PubMed articles Professional liability PubMed Central articles Professional liability 2023 articles Professional liability 2024 articles Professional liability Scopus articles Professional liability impact factor journals Professional liability Scopus journals Professional liability PubMed journals Professional liability medical journals Professional liability free journals Professional liability best journals Professional liability top journals Professional liability free medical journals Professional liability famous journals Professional liability Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Cardio articles Cardio Research articles Cardio review articles Cardio PubMed articles Cardio PubMed Central articles Cardio 2023 articles Cardio 2024 articles Cardio Scopus articles Cardio impact factor journals Cardio Scopus journals Cardio PubMed journals Cardio medical journals Cardio free journals Cardio best journals Cardio top journals Cardio free medical journals Cardio famous journals Cardio Google Scholar indexed journals kidney articles kidney Research articles kidney review articles kidney PubMed articles kidney PubMed Central articles kidney 2023 articles kidney 2024 articles kidney Scopus articles kidney impact factor journals kidney Scopus journals kidney PubMed journals kidney medical journals kidney free journals kidney best journals kidney top journals kidney free medical journals kidney famous journals kidney Google Scholar indexed journals gastrojejunostomy articles gastrojejunostomy Research articles gastrojejunostomy review articles gastrojejunostomy PubMed articles gastrojejunostomy PubMed Central articles gastrojejunostomy 2023 articles gastrojejunostomy 2024 articles gastrojejunostomy Scopus articles gastrojejunostomy impact factor journals gastrojejunostomy Scopus journals gastrojejunostomy PubMed journals gastrojejunostomy medical journals gastrojejunostomy free journals gastrojejunostomy best journals gastrojejunostomy top journals gastrojejunostomy free medical journals gastrojejunostomy famous journals gastrojejunostomy Google Scholar indexed journals
Article Details
1. Introduction
Implantable cardiac devices are widely used in the management of many heart diseases, as they provide the benefits of reduced risk of sudden cardiac death, improved quality of life, and increased survival [1]. However, implantation requires invasive procedures to be carried out, and hence the knowledge of the potential fatal complications. Major complications can include cardiac perforation/cardiac tamponade, generator or lead malfunction (e.g., lead breakage, bad connection to lead generator), hematomas with clinical significance, infections, lead displacement, pneumothorax/hemothorax, pre-erosion or erosion of the pocket, and thromboembolic events (e.g., transient ischemic attack, stroke, pulmonary thromboembolism, thrombosis, deep venous thrombosis). Minor complications can include cellulitis, local and shoulder pain, peripheral nerve injury, superficial phlebitis, and uncomplicated hematomas.
A fatal case during implantation for cardiac resynchronization therapy with defibrillation (CRT-D) is described here. This was characterized by cardiac perforation with tamponade that occurred during the procedure.
2. Case Report
A man aged 77 with chronic ischemic heart disease and who had undergone multiple percutaneous coronary revascularization procedures was admitted to the Department of Cardiology for CRT-D. At admission, his arterial pressure was 125/70 mmHg, his heart rate was 70 bpm, as arrhythmic with frequent extrasystoles. His left ventricular ejection fraction was 34%, with New York Heart Association functional class III. He also reported worsening dyspnea with ankle edema. After pharmacological therapy, the patient underwent implantation of an implantable cardioverter defibrillator (ICD).
After the introduction and placement of the electrocatheter in the right ventricle and auricle, the patient suddenly showed hypotension, progressive bradycardia, and loss of consciousness, with evidence of pericardial effusion with hypo-akinesia of both ventricles. Despite resuscitation maneuvers and pericardiocentesis attempts, the patient died.
An autopsy was performed 24 h later. Upon opening of the pericardium was seen to contain about 400 mL of partially coagulated blood. On following the internal course of the ICD leads, these were seen to be well positioned in the left subclavian vein, the right atrium, and the right ventricle. At the end of the ventricular catheter, there was a full-thickness laceration of the posterolateral wall of the right ventricle at the apex. Measurement in this site showed a wall thickness of ~0.4 mm (Figure 1).
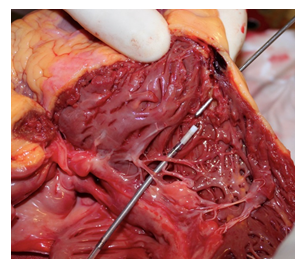
Figure 1: Site of cardiac perforation.
Multiple signs were also seen of previous myocardial infarction with double coronary stenting of the left and right coronary arteries. No other significant alterations were seen that were relevant to the case. For the perforation site, the histological findings showed the important replacement of myocardial muscle by adipose tissue.
3. Discussion
The use of ICDs is based on the introduction of transvenous leads that provide electrical impulses to defibrillate the heart, with the interruption of potentially fatal arrhythmias. Some models also provide biventricular stimulation to restore the synchrony of the ventricular contraction (i.e., CRT-D). Despite the effectiveness of the procedures, with a reported success rate of 94.4% [2], CRT-D is associated with complications secondary to the introduction and removal of the transvenous leads [3]. These complications can be divided into major and minor, according to their severity. Those of a mechanical nature (e.g., perforation, pericardial effusion or tamponade, pneumothorax, hemothorax) have been reported in 3.2% of cases, while problems with dislocation or repositioning of the leads account for 6.2%, and infections for 1.4% [4, 5]. Peri-procedural deaths have been reported to occur in 0.3% of cases [4], with the deaths considered secondary to the procedure if they are caused by mechanical complication or acute clinical deterioration during or after the procedure [6].
Among the major peri-procedural complications (i.e., those occurring within 24 h), our interest is directed toward the risk of cardiac perforation. This rare but potentially fatal complication can lead to hemopericardium, cardiac tamponade, and death [7]. Right ventricular perforation is an uncommon but life-threatening complication that can occur secondary to the positioning of a pacemaker or an ICD, with reported incidence of 0.1% to 0.8% and 0.6% to 5.2%, respectively [8 – 11]. Although the perforation rates reported have varied across different studies (e.g., 0.1-3% [7]; 0.7% [6, 12]; 0.32% [13]), the data can be considered as substantially uniform. In particular, for CRT-D the cardiac perforation rate reported is 0.24-0.48% [14]. Several predictive factors have been associated with the risk of cardiac puncture during placement of an ICD, including sex, age, body mass index, type of ICD, type of procedure, operator experience, procedure priority (i.e., emergency, elective) [2], use of steroids and concurrent anticoagulant therapy [8], worsened New York Heart Association Class and non-ischemic cardiomyopathy [15].
Female sex represents an independent risk factor for cardiac perforation [16]; is reported that women have a 30% higher risk of any complication, mainly due to cardiac perforation [2], and that about half (49%) patients who develop this cardiac event were female [14]. This gender difference could be due to hormonal differences, body composition and in particular, to the possibility that women may have cardiac anatomical features predisposing perforation as a thinner right ventricular wall [14, 15, 17]. In addition, the body mass index is a significant predictive factor for early complications related to the procedure in women [17], generally underweight is associated with an increased risk of overall complications like the use of antithrombotic [18] and chronic steroids [19].
The risk of right ventricular lead complications also depends on the type of procedure; in fact, CRT-D is associated with the highest risk of major complications in comparison with other types of interventions and is more prevalent in patients with cardiac perforation [2, 14, 15, 17]. Other independent risk factor for cardiac perforation is the New York Heart Association functional class, in fact, its worsening is associated with the greater risk of perforation and this may be related to generic more susceptibility to complications due to heart failure. For this factor the results are contrasting as even conditions associated with a normal left ventricular ejection fraction (LVEF), such as a more fragile right ventricle in arrhythmogenic right ventricular cardiomyopathy, may increase perforation risk; alternatively, a higher LVEF, may result in more forceful myocardial contractions against a lead tip, predisposing to cardiac perforation [15].
The thicknesses of the atrial and ventricular cardiac walls represent a further risk factor [20]. Indeed, perforations generally involve areas with thinner walls, such as the apex of the right ventricle, rather than the interventricular septum [8, 21], while perforations are less common with right ventricular hypertrophy [8, 10]. This demonstrates that the characteristics of the cardiac muscle can promote or protect from iatrogenic perforation. The mechanisms responsible for the occurrence of perforations are not well understood, although this is considered multifactorial and to be correlated to the size of the electrocatheter and the pressure from the force exerted on the thin conductors. Therefore, perforations might derive from an imbalance between the force exerted by the tip of the electrocatheter and the resistance of the ventricular wall [7]. A smaller lead diameter was associated with a greater risk of cardiac perforation; in fact, it is possible that a tip with a smaller diameter implies greater force on the myocardium predisposing to myocardial perforation [15] independently of the mechanical pressure exercised by the operator.
The present case confirms this characteristic. Indeed, during the autopsy, full-thickness perforation of the apex of the right ventricle was seen, which was caused by the electrocatheter. The measurements indicated a right parietal thickness of 0.4 mm at the perforation site. In this area, the histological findings revealed the important replacement of myocardial muscle by adipose tissue in the absence of previous post-ischemic tissue alterations. Retrospective evaluation of this uncommon complication allows us to highlight the importance of a further predictive factor that would appear to have been little investigated: the wall thickness. The main known protective factor for cardiac perforation is right ventricular systolic pressure >35 mm Hg [10]. To the best of our knowledge, no other protective factors have been reported in the literature other than parietal hypertrophy and right ventricular systolic pressure.
Our case highlights the importance of studying the characteristics of the right ventricular wall in patients with indication for treatment with implantable cardiac devices. The latest available update of the guidelines for echocardiographic evaluation (method more widely available than cardiac magnetic resonance imaging) and various authors [22] highlight the intrinsic difficulties of ultrasound evaluation together with the lack of criteria to define an abnormally right ventricle wall thin [23].
Our case highlights the importance of revisiting the diagnostic pathways before the introduction of a cardiac device. In patients with greater risk secondary to previous myocardial infarction, further studies of the cardiac wall thickness and composition would be desirable before implantation of cardiac devices. Therefore, new studies on known and further predictive parameters and/or indices are recommended, from both the clinical and forensic points of view, and also to protect the operator from charges of professional liability.
4. Conclusions
Although the use of ICDs shows low complication rates, this should not be underestimated from either a clinical or a legal point of view. Cardiac perforation during the introduction of electrocatheters is a very rare, but possible, event that can result in concerns as to the possible responsibility of the operator. Therefore, new studies on known and further predictive parameters and/or indices are recommended to avoid the selection of patients with increased risk of complications during these procedures and to protect the operator from charges of professional liability.
References
- Nowak B, Przibille O. Device implantation: the human factor. Europace 12 (2010): 15-16.
- Kirkfeldt RE, Johansen B, Nohr EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 35 (2014): 1186-1194.
- Botto GL, Forleo GB, Capucci A, Solimene F, Vado A, Bertero G, Palmisano P, Pisano E, Rapacciuolo A, Infusino T, Vicentini A, Viscusi M, et al. The Italian subcutaneous implantable cardioverter-defibrillator survey: S-ICD, why not? Europace 19 (2017): 1826-1832.
- European Society of Cardiology (ESC). European Heart Rhythm Association (EHRA), Brignole M, Auricchio A, Baron-Esquivias G, et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 15 (2013): 1070-1118.
- Al-Majed NS, McAlister FA, Bakal JA, et al. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med 154 (2011): 401-412.
- Poole JE, Gleva MJ, Mela T, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation 122 (2010): 1553-1561.
- Nichols J, Berger N, Joseph P, et al. Subacute right ventricle perforation by pacemaker lead presenting with left hemothorax and shock. Case Rep Cardiol (2015): 983930.
- Khalid M, Murtaza G, Ayub MT, et al. Right ventricle perforation post pacemaker insertion complicated with cardiac tamponade. Cureus 10 (2018): e2266.
- kbarzadeh MA, Mollazadeh R, Sefidbakht S, et al. Identification and management of right ventricular perforation using pacemaker and cardioverter-defibrillator leads: a case series and mini review. J Arrhythm 33 (2017): 1-5.
- Banaszewski M, Stepinska J. Right heart perforation by pacemaker leads. Arch Med Sci 8 (2012): 11-13.
- Lin YS, Hung SP, Chen PR, et al. Risk factors influencing complications of cardiac implantable electronic device implantation: infection, pneumothorax and heart perforation: a nationwide population-based cohort study. Medicine 93 (2014): e213.
- Pakarinen S, Oikarinen L, Toivonen L. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace 12 (2010): 103-108.
- Carrión-Camacho MR, Marín-León I, Molina-Doñoro JM, et al. Safety of permanent pacemaker implantation: a prospective study. J Clin Med 8 (2019): 35.
- Kawata H, Erande A, Lafi O, et al. Occurrence, mortality and predictors of complicated cardiac perforation in patients with CRT-D: Based on the National Inpatient Sample registry. International Journal of Cardiology 293 (2019): 109-114.
- Hsu JC, Varosy PD, Bao H, et al. Cardiac Perforation From Implantable Cardioverter-Defibrillator Lead Placement Insights From the National Cardiovascular Data Registry. Circ Cardiovasc Qual Outcomes 6 (2013): 582-590.
- Peterson PN, Daugherty SL, Wang Y, et al. Gender Differences in Procedure-Related Adverse Events in Patients Receiving Implantable Cardioverter-Defibrillator Therapy. Circulation 119 (2009): 1078-1084.
- Hosseini SM, Moazzami K, Rozen G, et al. Utilization and in-hospital complications of cardiac resynchronization therapy: trends in the United States from 2003 to 2013. European Heart Journal 38 (2017): 2122-2128.
- Shakya S, Matsui H, Fushimi K, et al. In-hospital complications after implantation of cardiac implantable electronic devices: Analysis of a national inpatient database in Japan. Journal of Cardiology 70 (2017): 405-410.
- Merkel M, Grotherr P, Radzewitz A, et al. Leadless Pacing: Current State and Future Direction. Cardiol Ther 6 (2017): 175-181.
- Prestipino F, Nenna A, Casacalenda A, et al. Ventricular perforation by pacemaker lead repaired with two hemostatic devices. Int J Surg Case Rep 5 (2014): 906-908.
- Oda T, Kono T, Akaiwa K, et al. Surgical repair of subacute right ventricular perforation after pacemaker implantation. Case Rep Cardiol (2017): 3242891.
- Smolarek D, Gruchala M, Sobiczewski W. Echocardiographic evaluation of right ventricular systolic function: The traditional and innovative approach. Cardiology Journal 24 (2017): 563-572.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28 (2015): 1-39.
