A Prospective Observational Study on the Role of Special Stains in Physiological and Histopathological Study of Liver
Article Information
Maheswari N1, Sanatan Behera2, Arupam Mahapatro3, Satyashree Ray*, 4
1Senior Resident, Department of Anatomy, Santhiram Medical College and Hospital, Nandyala, Andhra Pradesh, India
2Associate Professor, Department of Hepatology, SCB Medical College and Hospital, Cuttack, Odisha, India
3Junior Consultant, Department of Hepatology, SCB Medical College and Hospital, Cuttack, Odisha, India
4Associate Professor, Department of Anatomy, SCB Medical College and Hospital, Cuttack, Odisha, India
*Corresponding Author: Satyashree Ray, Associate Professor, Department of Anatomy, SCB Medical College and Hospital, Cuttack, Odisha, India.
Received: 05 March 2024; Accepted: 18 March 2024; Published: 07 April 2024
Citation: Maheswari N, Sanatan Behera, Arupam Mahapatro, Satyashree Ray. A Prospective Observational Study on the Role of Special Stains in Physiological and Histopathological Study of Liver. Fortune Journal of Health Sciences. 7 (2024): 192-196.
View / Download Pdf Share at FacebookAbstract
Aim and objectives: This study aims to assess the value of special stains, such as Masson's trichrome and Reticulin, in enhancing the analysis of non-neoplastic liver specimens. Additionally, it seeks to quantify glycogen levels using PAS-stained slides and image J software for potential therapeutic implications in clinical settings.
Material and methods: This prospective observational investigation was carried out on 25 liver tissues obtained from the cadavers inS.C.B Medical college from March 2021 to August 2022. Liver tissue from diverse sources underwent histological processing and staining techniques including H&E, Masson's trichrome, Reticulin stain, Perl's Iron stain, and P.A.S. stain. Tissue preservation involved sequential immersion in various solutions followed by staining, dehydration, and mounting. Stains were prepared and applied according to established protocols. Image analysis of P.A.S. slides was conducted using Image J software to estimate liver glycogen content.
Results: Out of 50 liver specimens, 23 exhibited preserved microarchitecture; 25 specimens underwent staining and histomorphometric analysis. PAS staining revealed a glycogen volume of 620.161g at 10X and 637.4g at 40X magnification, with no significant difference in area involvement between 10X and 40X fields (mean area: 100,750.18 at 10X, 7,041.77 at 40X).
Conclusion: The study demonstrates significant glycogen content variation in liver specimens, highlighting the importance of meticulous histomorphometric analysis in understanding hepatic pathology. Further investigations are warranted to elucidate underlying factors contributing to these variations.
Keywords
Liver Histomorphometry, Glycogen Content, Staining Techniques, Hepatic Pathology
Liver Histomorphometry articles, Glycogen Content articles, Staining Techniques articles, Hepatic Pathology articles
Article Details
1. Introduction
The human liver, weighing around 1.5 kg, has a prismatic structure, and is enclosed in a connective tissue capsule [1,2]. This organ is supported by the peritoneum by the help of various ligaments, such as the left and right triangular,coronary, and falciform, therebyensuring the structural stability of liver [3]. While it is composed of elastic, pinkish-brown tissues, the liver is primarily involved in digestion, nutrient storage, metabolism, and immunity. Internally, a hundred thousand hexagonal lobules are identified within the liver, with a central vein surrounding each lobule in addition to the portal veins, and hepatic arteries. Furthermore, anatomically, the portal veins and the arteries resulting in the central vein are connected via sinusoids, whilethe four lobes of the liverreceive blood from the digestive tract, and the spleen [3]. Additionally, it has been well established that the functioning of liver relies on its capacity to regenerate damaged tissues and maintain its standard size and function [4-7].
Histologically, the liver show cases hepatocytes as the parenchyma, Glissons capsule, sinusoids, and peri-sinusoidal spaces. The structural units of the liver or hepatic lobule, portal lobule, and liver acinus, possess intricate architecture, emphasizing the presence of distinct zones within the liver acinus [8, 9]. Over the years, it has been proven that special stains are essential for histopathological studies of the liver [10,11]. While standard H&E stains offer limited visibility, specific stains such as reticulin, trichrome, PAS (periodic acid Schiff), iron,and copper offer an edge in highlighting the cellular structures, pathological aspects, and fibrosis of the liver [10-13]. These stains have also found applicability in liver biopsies, tohelp in the diagnosis of liver ailments such as inflammation, cirrhosis, and infections [14,15]. Image analysis is an important component in staining studies, and frequently, this involves the use of Image J, a versatile software, which offers both qualitative and quantitative measurements for live tissues and microbes [16]. The application of this software in histomorphometry ascertains the precision and consistency of the data, especially in research pertaining to the efficacy of drugs, nutrients, and degree of tissue damage [17,18]. Moreover, integrating Image J in the hepatic histopathological and physiological studies enhance the reliability and standardization of these measurements, thereby addressing the concerns related to measurement subjectivity. The present study investigates the use of special stains, including Masson's trichrome and Reticulin, in enhancing the evaluation of non-neoplastic liver specimens beyond standard H&E staining. The primary aim of this investigation is to document the staining patterns for various elements, while the secondary objective involves quantification of glycogen through histomorphometric analysis using PAS-stained slides and image J software.
2. Material and Methods
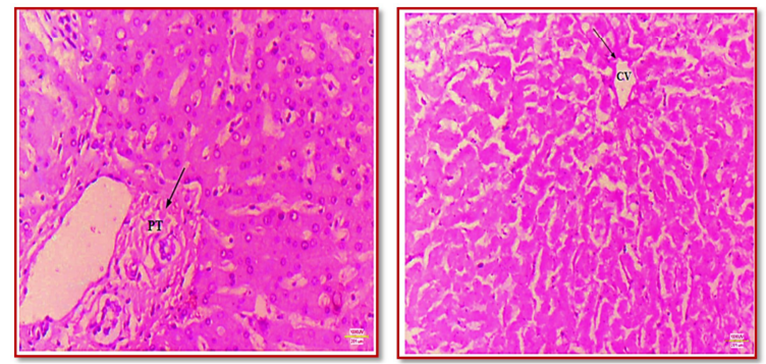
The research took place in the Histology lab of the Anatomy Department, in collaboration with the Pathology Department at S.C.B. Medical College, Cuttack, spanning from March 2021 to August 2022. Liver specimens were sourced from the autopsies conducted by the Department of Forensic Medicine, establishing an observational study with a diverse population of randomly selected liver samples from all age groups and liver biopsy samples from newly diagnosed chronic liver disease patients at the Department of Hepatology, S.C.B. Medical College and Hospital. The inclusion criteria for the study comprised liver tissues obtained from the mortuary at S.C.B. Medical College and liver biopsy samples taken from patients diagnosed with chronic liver disease at the Department of Hepatology. Patients with history of previous treatment for any liver disease or with neoplastic liver specimens, or livers displaying gross or microscopic features of autolysis, were excluded from this study. The study, involved a sample size of 25 liver samples obtained from cadavers. A 10 % buffered formalin solution was used to preserve the liver tissues for 2 to 4 days before the manual processing of tissues. The processed tissue blocks then underwent sectioning with the help of a rotary microtome, to create ribbons of approximately 3-4 µ in thickness. These ribbons were carefully placed on egg albumin-coated slides, dried using a hot plate, fixed, and stained with various special stains like H & E, Reticulin stain, Masson's trichrome, P.A.S. stain, and Perl's Iron stain. The resulting slide images were captured at various magnifications (10x and 40x), documented and analyzed to summarize the stain-specific outcomes. This includes collagen staining by Masson's trichrome, reticulin fiber staining by Reticulin stain, emphasis of hemosiderin and ferritin by Iron stain, and identification of various elements by Periodic Acid-Schiff stain.
Glycogen Estimation
Images of P.A.S. slides are analyzed using the Image J software. The glycogen area of the selected region of liver was calculated, and data was saved to Excel. The mean glycogen value was compared with the estimated liver weight. Image analysis involved steps such as adjustment of the hue, saturation, and brightness, converting the image to binary information, and measuring glycogen area. The calculated glycogen amounts in the liver were then converted to cc.
Ethical approval
The study prioritized ethical standards, obtaining written consent and institutional ethics committee approval (application No.756) to uphold participant autonomy and informed consent principles.
Statistical analysis
Individual liver data on glycogen content were organized in an Excel sheet, and results were presented as mean ± SD. Significant values (p-value < 0.05) obtained using SPSS (version 21.0) was utilized for data analysis, employing the Shapiro-Wilk test to assess normality, and parametric tests such as the independent t-test were applied for bivariate analyses, specifically for comparing two groups.
Results
A total of 50 liver specimens were obtained from human autopsies, but only 23 exhibited preserved microarchitecture; the remaining samples were either fully or partially autolysed. Additionally, two liver samples were sourced from the hepatology department. These 25 liver specimens were stained with haematoxylin and eosin (H&E), Masson's Trichrome, PAS and reticulin stains. The demographic variations of the cadavers involved for this study has been depicted in Table-1.
Table 1: Demographic variations of the cadavers in the study cohort
|
S.No. |
Age of the cadaver (years) |
Sex |
Livers (n) |
|
1 |
28 |
M |
1 |
|
2 |
30 |
M |
2 |
|
3 |
32 |
F |
2 |
|
4 |
38 |
M |
1 |
|
5 |
39 |
M |
1 |
|
6 |
42 |
F |
1 |
|
7 |
43 |
M |
1 |
|
8 |
45 |
F |
2 |
|
9 |
47 |
M |
1 |
|
10 |
48 |
M |
2 |
|
11 |
49 |
M |
2 |
|
12 |
52 |
F |
2 |
|
13 |
59 |
F |
1 |
|
14 |
62 |
M |
2 |
|
15 |
64 |
M |
2 |
|
16 |
65 |
F |
2 |
In PAS staining, various components such as polysaccharides (glycogen), neutral mucopolysaccharides, mucoproteins, glycoproteins, and glycolipids are highlighted in magenta, while nuclei appear as dark magenta. The calculated glycogen volume through histomorphometry in PAS-stained slides was 620.161g at 10X and 637.4g at 40X magnification (Figure 1).
Notably, significant variations were noted in the histomorphometrically assessed area on 10X and 40X fields, with a larger area observed in the 10X field. At 10X, the mean area is 100,750.18 with a standard deviation of 22,009.85, while at 40X, the mean area is 7,041.77 with a standard deviation of 1,272.31 (table 2).
Table 2: Comparison of the area involved by histomorphometric analysis of 10x and 40x field
|
Group |
N |
Mean |
Standard Deviation (S.D) |
Standard Error Mean (S.E.M) |
|
|
Area |
10 x |
100 |
100750.1817 |
22009.85077 |
2200.985077 |
|
40 x |
100 |
7041.76864 |
1272.305017 |
127.230502 |
|
|
p-value |
< 0.005 |
Comparison of the percentage of area involvement by histomorphometry between the 10X and 40X fields revealed differences but it did not attain statistical significance. At 10X, the mean area is 52.53 with a standard deviation of 10.18, while at 40X, the mean area is 54.57 with a standard deviation of 9.86 (table 3).
Table 3: Comparison of the % area involved by histomorphometric analysis of 10x and 40x
|
Group |
N |
Mean |
Standard Deviation (S.D) |
Standard Error Mean (S.E.M) |
|
|
Area |
10 x |
100 |
52.53391 |
10.182853 |
1.018285 |
|
40 x |
100 |
54.57388 |
9.860389 |
0.986039 |
|
|
p-value |
0.152 |
Discussion
The study encompassed 50 human liver autopsy specimens, of which only 23 retained intact microarchitecture, while the rest displayed varying degrees of autolysis. Two additional samples were obtained from the hepatology department. Stains such as Masson's Trichrome, H&E, reticulin, and PAS, were applied to the 23 liver specimens for detailed analysis. Analysis of the age distribution of the specimens showed a diverse representation, varying from 28 to 65 years. This highlighted the heterogeneity of the study cohort. Liver biopsy examinations, particularly those involving specialized histochemical stains, play a crucial role in clinical diagnostics [14,15]. The significance of specific stains varies depending on the origin of the biopsy material, whether from a tumor patient, a healthy individual, or a liver transplant recipient [19]. Iron accumulation in Kupffer cells, which is indicative of hemosiderosis, is associated with complications in sickle cell disease patients following blood transfusions. Accurate assessment of liver iron concentration (LIC) becomes pivotal for effective management, especially in conditions like sickle cell disease or beta-thalassemia requiring long-term transfusions [20].
The note worthy clinical implications such as hemochromatosis are linked to higher levels of stainable iron in liver tissue, which potentially act as a sole cause or contributing factor to liver diseases [21]. The present study is concordant with this notion and emphasizes the importance of conducting liver biopsies to identify iron accumulation, aiding in closer patient monitoring. This is also in line with recent studies which indicate that iron accumulation in the liver is associated with accelerated health deterioration and reduced life expectancy in cirrhosis patients, underlining the need for vigilant examination [22-24]. The discussion on liver biopsy protocols in this study underscores the absence of a universal standard in the medical fieldaligning with the outcomes of a study conducted by Neuberger et al [25]. While H&E staining remains a staple, the choice of initial stains often depends on individual pathologists' training or prevailing practices. The importance of specialized stains, such as trichrome, reticulin, and iron, has been highlighted for comprehensive liver biopsy evaluations, with special emphasis on glycogen estimation. Despite ImageJ software offering good staining analysis capabilities, limitations were encountered during glycogen estimation. These included challenges arising from inaccurate magenta staining of glycogen. Furthermore, inadequate contrast between cell elements hindered the software's ability to discern variations in hue, saturation, and brightness, thereby affecting measurement accuracy. These constraints highlighted the importance of improving staining techniques and fine-tuning software parameters. Future studies in this domain should aim at enhancing histomorphometric analysis accuracy and achieve results comparable to those of Skurat et al. and Schaart et al., to ultimately enhance precision in hepatic pathology assessment. The present investigation reveals that liver biopsies, coupled with specific histochemical stains, are invaluable for diagnosing a spectrum of liver conditions. The study's detailed analysis, considering stains like H&E, trichrome, reticulin, and PAS, contributes to enhancing diagnostic accuracy, especially in evaluating chronic hepatitis, cirrhosis, fatty liver disease, and other hepatic disorders.
Conclusion and Recommendation
This observational study highlights the diverse applications of staining techniques in liver histopathology. Trichrome stain proves valuable in evaluating fibrosis, while reticulin stain serves as a crucial tool for assessing liver architecture, hepatocyte necrosis, and hepatocyte regeneration. PAS staining emerges as a revealing method, unveiling alpha-1 antitrypsin globules in hepatocytes, identifying storage cells in conditions like Gaucher's and Niemann-Pick's disease, and detecting abnormalities in the bile duct basement membrane associated with biliary diseases. These staining methods collectively contribute to a comprehensive understanding of liver pathology, aiding in the diagnosis and characterization of various hepatic conditions. The study recommends further research to refine staining techniques and optimize software parameters, aiming to enhance histomorphometric analysis accuracy. Additionally, collaboration between histology and pathology departments is recommended to facilitate comprehensive investigations into hepatic pathology.
Limitations
The limitations of this study include its observational nature, which restricts establishing causal relationships, and the reliance on specific staining techniques, potentially overlooking nuances detectable through alternative methods.
Acknowledgement
We express our gratitude to the Histology and Pathology Departments at S.C.B. Medical College, Cuttack, for their invaluable support and collaboration throughout this research.
Source of funding: None.
Conflict of interest: None.
References
- Kan Z, Madoff DC. Liver anatomy: microcirculation of the liver. SeminInterventRadiol 25 (2008): 77-85.
- Tuch BE, Szymanska B, Yao M, Tabiin MT, Gross DJ, Holman S, et al. Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene therapy 10 (2003): 490-503.
- Hu J, Huang J, Liu X, Zuo Z. Clinical Anatomy of the Liver. In Atlas of Anatomic Hepatic Resection for Hepatocellular Carcinoma (2019): 1-6.
- Lau WY. Applied Anatomy of the Liver. In Applied Anatomy in Liver Resection and Liver Transplantation (2021): 1-6.
- Ibukuro K, Fukuda H, Tobe K, Akita K, Takeguchi T. The vascular anatomy of the ligaments of the liver: gross anatomy, imaging and clinical applications. The British Journal of Radiology 89 (2016): 20150925.
- Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology 49 (2006): 450-65.
- Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. Journal of Hepatology 50 (2009): 36-41.
- Burra P, Mioni D, Cecchetto A, Cillo U, Zanus G, Fagiuoli S, et al. Histological features after liver transplantation in alcoholic cirrhotics. Journal of hepatology 34 (2001): 716-22.
- Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. Journal of Hepatology 71 (2019): 616-30.
- Bordoloi B, Jaiswal R, Siddiqui S, Singh RB. A history of evolution of special stains. Int J Med Lab Res 2 (2017): 55-62.
- Alturkistani HA, Tashkandi FM, Mohammedsaleh ZM. Histological stains: a literature review and case study. Global journal of health science 8 (2016): 72.
- Gill GW. Special Stains. In Cytopreparation (2013): 227-243.
- Floyd AD. Evolution of Use of Special Stains. Connection (2010): 45.
- Lefkowitch JH. Special stains in diagnostic liver pathology. InSeminars in diagnostic pathology 23 (2006): 190-198.
- Gasmi B, Kleiner DE. Liver histology: diagnostic and prognostic features. Clinics in liver disease 24 (2020): 61-74.
- Collins TJ. ImageJ for microscopy. Biotechniques 43 (2007): S25-30.
- Esteves-Oliveira M, de Guglielmi CA, Ramalho KM, Arana-Chavez VE, de Eduardo CP. Comparison of dentin root canal permeability and morphology after irradiation with Nd: YAG, Er: YAG, and diode lasers. Lasers in medical science 25 (2010): 755-60.
- Zur G, Klement E. Use of ImageJ software for histomorphometric evaluation of normal and severely affected canine ear canals. CanadianJournal of Veterinary Research 79 (2015): 316-22.
- Stains CU. Special stains in interpretation of liver biopsies. Connection (2010): 92.
- Lachman N, Pawlina W. The liver and biliary apparatus: basic structural anatomy and variations. Practical Gastroenterology and Hepatology: Liver and Biliary Disease 5 (2010): 1.
- Bergmann, O., Mathahs, M., Broadhurst, K. et al. Altered expression of iron regulatory genes in cirrhotic human livers: clues to the cause of hemosiderosis? Lab Invest 88 (2008): 1349–1357.
- Kowdley KV. Iron Overload in Patients with Chronic Liver Disease. GastroenterolHepatol (NY) 12 (2016): 695-698.
- Gkamprela E, Deutsch M, Pectasides D. Iron deficiency anemia in chronic liver disease: etiopathogenesis, diagnosis and treatment. Ann Gastroenterol 30 (2017): 405-413.
- Büyükasik NS, Nadir I, Akin FE, Cakal B, Kav T, Ersoy O, et al. Serum iron parameters in cirrhosis and chronic hepatitis: detailed description. Turk J Gastroenterol 22 (2011): 606-11.
- Neuberger J, Patel J, Caldwell H, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 69 (2020): 1382-1403.
- Skurat AV, Segvich DM, DePaoli-Roach AA, Roach PJ. Novel method for detection of glycogen in cells. Glycobiology 27 (2017): 416-424.
- Schaart G, Hesselink RP, Keizer HA, et al. A modified PAS stain combined with immunofluorescence for quantitative analyses of glycogen in muscle sections. Histochem Cell Biol 122 (2004): 161–169.