A Pragmatic, Individually Randomised, Open-Label, Retrospective Study of The Efficacy of Topical Therapy with Framycetin in The Treatment of Exacerbation of Chronic Nasopharyngitis
Article Information
Vasyl I. Popovych*, [1], Ivana V. Koshel 2
1Department of Lviv Medical University, 76 V. Polishchuk St., Lviv, Lviv Region, 79018, Ukraine
2Department of Ivano-Frankivsk National Medical University, Halytska Street, 2, Ivano-Frankivsk, Ivano-Frankivsk Region 76000, Ukraine
*Corresponding author: Vasyl I. Popovych, Department of Lviv Medical University, 76 V. Polishchuk St., Lviv, Lviv Region, 79018, Ukraine
Received: 31 August 2023 Accepted: 14 September 2023 Published: 25 September 2023
Citation: Vasyl I. Popovych, Ivana V. Koshel. A Pragmatic, Individually Randomised, Open-Label, Retrospective Study of The Efficacy of Topical Therapy with Framycetin in The Treatment of Exacerbation of Chronic Nasopharyngitis. Archives of Microbiology and Immunology. 7 (2023): 213-221.
View / Download Pdf Share at FacebookAbstract
The treatment efficacy of chronic nasopharyngitis with systemic antibacterial agents is low. The ineffectiveness of antibiotic therapy is thought to be related to nasopharyngeal bacterial biofilms. The prospect is to influence the bacteria stored in the biofilm, particularly using topical antibacterial agents.
Study Purpose: to determine the effect of topical antibacterial therapy with framycetin and add-on treatment on the progression of clinical symptoms in patients with exacerbation of CNP.
Methods: In a pragmatic, individually randomised, open-label, retrospective study, 143 patients receiving endonasal therapy with framycetin solution supplementary to conventional therapy with isotonic seawater solution or additional administration of antihistamines and antiviral agents were randomised. Assessment criteria: reduction in symptom severity: nasal discharge, postnasal drip, hyperaemia, mucosal oedema, assessed using 5-point MSS scale at each visit compared to visit 1, and the course of hyperthermia.
Results: Endonasal therapy with framycetin solution in exacerbation of CNP contributes to the reduction in the severity of the main clinical symptoms in patients of the treatment and control groups. Reduction in the severity of clinical symptoms correlates with a significant decrease in hyperthermia in patients of both groups. Additional administration of antiviral and antihistamine agents does not affect the course of clinical symptoms due to exacerbation of chronic nasopharyngitis.
Conclusion: Framycetin solution for endonasal therapy is a safe and effective agent for etiotropic treatment of exacerbation of chronic nasopharyngitis, and provides a significant therapeutic effect.
Keywords
nasopharyngitis, pharynx, respiratory infections, biofilms, treatment
nasopharyngitis articles, pharynx articles, respiratory infections articles, biofilms articles, treatment articles
Article Details
1. Introduction
Chronic inflammation of the nasopharyngeal tonsil (NT) is a common disease in the paediatric population, affecting the somatic and functional development of the child and quality of life. Until very recent times, most clinicians have used the term “chronic adenoiditis” [1, 2, 3]. However, such a term is empirical because current classifications do not use it. According to international classifications, the correct term is “chronic nasopharyngitis” (CNP). It is coded as J31.1 in ICD 10 and CA09.1 in ICD 11 [4, 5]. The NT surface has many folds or crypts with limited blood flow, and therefore microorganisms can easily integrate into them. Bacterial culture studies with 100 samples of removed adenoids found that only 7% of samples showed no bacterial growth [6, 7, 8]. Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and Moraxella catarrhalis were found to be the most common bacteria. Bacteria in adenoids usually include many other species and provide an indolent persistent inflammatory process with biofilm formation [9, 10]. In addition, biofilms can enhance antimicrobial resistance and bacterial anti-immune properties, which creates conditions for the progression of inflammation and frequent recurrence. Studies have found that impairment of the normal composition of the nasopharyngeal microbiome involving biofilm formation is associated with susceptibility to acute respiratory illnesses and exacerbation of chronic URT diseases, in particular nasopharyngitis [11, 12, 13].
To date, there is no consensus on the treatment of chronic nasopharyngitis, so these patients are often undergoing surgical intervention [14]. However, adenotomy frequently fails to resolve the problem, as symptoms are maintained in 19–26% of patients [15]. Therefore, the development of effective non-surgical treatments for this disease remains relevant. CNP is etiopathogenetically based on chronic inflammation, associated primarily with impaired nasopharyngeal microbiome. The above situation raises numerous questions on whether to choose systemic or local aetiotropic therapy. The treatment efficacy of CNP with systemic antibacterial agents is low. The ineffectiveness of antibiotic therapy in the paediatric population with chronic adenoiditis (nasopharyngitis) is thought to be related to nasopharyngeal bacterial biofilms [16, 17, 18]. Biofilm is formed of different microbial communities, and prevents the penetration of antimicrobial agents, so microorganisms in biofilms can be 500–1000 times more tolerant to systemic antibacterial agents than their planktonic equivalents [19]. In this regard, effective eradication of pathogenic agents using conventional antibacterial therapy is impossible. The prospect is to influence the bacteria stored in the biofilm, particularly using topical antibacterial agents. The results of randomised clinical trials show evidence-based therapeutic efficacy of topical antibacterial therapy associated with an effect on biofilms in patients with chronic rhinosinusitis [20]. However, no such studies have been conducted in patients with chronic nasopharyngitis, especially during exacerbations.
There is a problem with choosing a broad-spectrum agent that targets a wide range of bacteria types involved in biofilm formation. Efficiency and high safety are important requirements for such agents. Framycetin, an aminoglycoside antibiotic for topical use, has these properties. The concentration of framycetin, which is achieved during topical application, provides its bactericidal activity against a wide range of Gram-positive and Gram-negative microorganisms responsible for the development of infectious processes in the upper respiratory tract [21]. Add-on therapy for CNP is commonly used in these patients [22]. Nasal steroids, antileukotriene agents, and topical hyaluronic acid products have been suggested as add-on pharmacological treatment [23, 24, 25]. Overall, the evidence for the efficacy of these medications is equivocal.
Study purpose: to determine the effect of topical antibacterial therapy with framycetin and add-on treatment on the progression of clinical symptoms in patients with exacerbation of CNP.
2. Materials and methods
2.1 Trial design
A pragmatic, individually randomised, open-label, retrospective study was conducted in six outpatient facilities in Ukraine from September 2022 to April 2023. The study was conducted in accordance with GCP standards and the Declaration of Helsinki.
2.2 Participants
The study included 143 outpatients diagnosed with exacerbation of chronic nasopharyngitis. The criteria for diagnosis were:
- Objective clinical and endoscopic signs of the inflammatory process in the nasopharynx (hyperaemia, mucosal oedema, nasal discharge/postnasal drip);
- hyperthermia of the body;
- 3 or more disease episodes in the last 6 months;
Inclusion criteria:
- male and female subjects aged over 2 years who were on outpatient treatment and diagnosed with exacerbation of chronic nasopharyngitis:
- absence of diagnostic criteria for acute rhinosinusitis;
- severity of clinical symptoms not less than 2 points according to the 5-point MSS scale (Main Symptoms Severity Score, from 0— no symptoms to 5— severe symptoms);
- no exclusion criteria.
Exclusion criteria:
- use of study drug within 30 days prior to enrolment in the study;
- diagnosed with allergic rhinitis;
- known drug intolerance;
- indications for systemic antibacterial therapy;
- presence of immunodeficiency states, other chronic pathology and anatomical anomalies of ENT-organs, including grade 2–3 adenoid hypertrophy, which could affect the disease course.
The treatment group was randomised n – 58: patients who received endonasal therapy with framycetin solution (Isofra).
The control group was randomised n – 85: patients who received endonasal therapy with framycetin solution (Isofra) and additionally oral antihistamines (cetirizine, levocetirizine) and antivirals (inosine pranobex, enisamium iodide). The treatment group (n – 58) included 22 (37.9%) men and 36 (62.1%) women (average age 10.72 years). The control group (n – 85) included 43 (50.6%) men and 42 (49.4%) women (average age 18.21 years). Patients in both groups were comparable in sex and age (p<0.05).
2.3 Interventions
All patients were treated with: endonasal therapy with framycetin solution (Isofra) and isotonic seawater solution and, if indicated, the antipyretic paracetamol. Isofra is a nasal spray of 8000IU/ml framycetin solution in a 15mL spray bottle. Name of the marketing authorization holder: Laboratories Bouchara Recordati, France. The product is authorised in Ukraine and is available on prescription. The composition, manufacture, packaging and labelling of the product comply with the principles of good manufacturing practice and the current national requirements of Ukraine. A detailed description covering all quality and safety aspects of Isofra spray is part of the relevant product characteristics. Approved indications for use are treatment as a part of combined therapy for infectious-inflammatory diseases of the upper respiratory tract, including rhinitis, rhinopharyngitis, sinusitis. The control group patients were additionally treated with oral antihistamines (cetirizine, levocetirizine) and antivirals (inosine pranobex, enisamium iodide) in dosage according to the instructions for use. The patients included in the study were treated by specialists experienced for at least 5 years.
2.4 Outcome measures
All data were evaluated at the treatment initiation and over 10days (Table1).
Table 1: Visit schedule
|
V (visit) |
V1 |
V2 |
V3 |
|||||||
|
Day |
0 |
1 |
2 |
3 |
4 |
5 |
6–9 |
10 |
||
|
|
|
Treatment gr. |
||||||||
|
framycetin |
||||||||||
|
|
|
Control gr. |
||||||||
|
framycetin + add-on treatment |
||||||||||
V1 Day 0 Enrolment in the study, treatment assignment
V2 Day 5±1 Evaluation of condition, treatment efficiency
V3 Day 10±1 Evaluation of condition, treatment efficiency
Symptoms were assessed by the physicians. Four main symptoms were assessed at each visit: nasal discharge, postnasal drip, hyperaemia, mucosal oedema using 5-point MSS scale ((from 0— no symptoms to 5— severe symptoms) for each symptom). In addition, the physician assessed the presence of body hyperthermia. The main treatment efficacy endpoint was the reduction in the severity of the disease symptoms assessed using 5-point MSS scale at each visit compared to visit 1, and the course of hyperthermia.
2.5 Sample size
The clinical trial was designed to provide a reliable description of the in vivo efficacy of Isofra nasal spray monotherapy compared to the use of add-on treatment. Several exploratory descriptive and statistical evaluations were performed depending on the data so that a biometric assessment of sample size is not required. However, to ensure a sufficient sample size for analyses of the data obtained, a sample size of N = 140 was chosen.
2.6 Randomization
The clinical part of the pragmatic individually randomised retrospective study is open label. Subjects with symptomatic exacerbation of CRS were selected into one of two possible groups according to the study objectives. All patients meeting the inclusion criteria were randomised.
2.7 Statistical methods
In order to analyze homogeneity of groups, descriptive statistics methods were used for description of the baseline condition of the treatment and control group (for quantitative parameters— n, mean arithmetic, median, standard deviation, minimum and maximum va-lues; for qualitative parameters — incidence and share as %). Verification of normality of data distribution in groups was performed for quantitative parameters using Shapiro-Wilk test. If the data in groups showed normal distribution according to certain parameters, the groups were compared by these parameters via Student's test for in-dependent samples. Otherwise (if the data distribution was different from normal), comparison of groups was performed according to Mann-Whitney test. For categorical parameters, the groups were compared using Pearson's chi-squared test or Fisher's exact test. For analysis of efficacy, descriptive statistics parameters were calculated in each group (n, mean arithmetic, median, standard deviation, minimum and maximum values) for all visits in accordance with patients' examination scheme.
Analysis of dynamics of the said parameters in each group was performed via two-way analysis of variance (ANOVA) according to the following scheme: “Visit” factor is fixed (levels: visit 1… visit n); “Subjects” factor is random. Results of the subsequent visits were compared against the data of visit 1 via contrast analysis using simple contrasts. Comparison between groups in dynamics of tested parameters was performed by differences dTi = (TVisit n − TVisit 1) of assessed parameters using Mann-Whitney test. The level of confidence for Shapiro-Wilk test was accepted equal to 0.01, and for the rest of the criteria it was accepted equal to 0.05. The analysis was performed in software environment IBM SPSS 22.0
3. Results
3.1 Study Sample
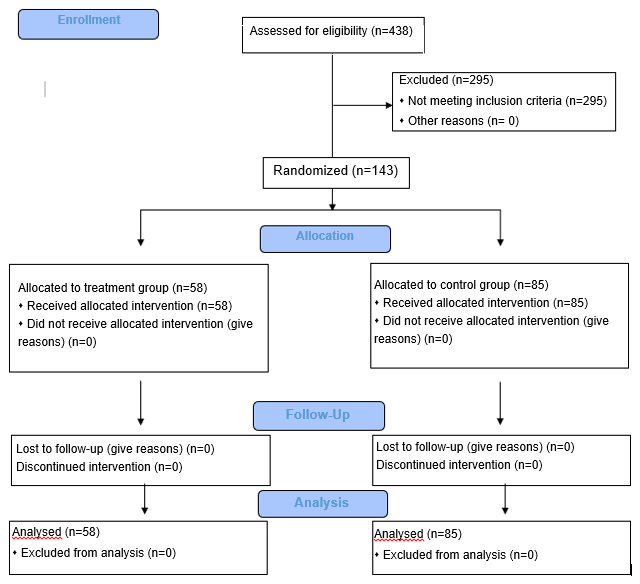
A total of 438 outpatients were screened, and 143 outpatients were randomised to participate in the study (Fig. 1).
Of the 438 selected patients diagnosed with exacerbation of chronic nasopharyngitis, 295 (67.4%) were not included in the study. The reason was meeting the inclusion criteria: allergy (n=124), administration of systemic antibacterial therapy (n=96), adenoid hypertrophy (n=75). Of the 143 patients meeting the inclusion criteria, n-58 were randomised to the treatment group and n-85 to the control group. All randomised patients were included in the outcome analysis. Table 2 shows patient distribution by gender. 22 (37.9%) males and 36 (62.1%) females were included in the treatment group and 43 (50.6%) males and 42 (49.4%) females were included in the control group.
Table 2: Comparison of groups by gender
|
Group |
Men |
Women |
Total |
|||
|
n |
% |
n |
% |
n |
% |
|
|
Treatment |
22 |
37.9 |
36 |
62.1 |
58 |
100 |
|
Control |
43 |
50.6 |
42 |
49.4 |
85 |
100 |
|
χ2=1.746 |
||||||
|
P=0.186 |
||||||
|
df=1 |
||||||
Comparison of groups by gender using the chi-square test at the significance level of 0.05 showed that the groups were formed statistically homogeneous by gender. The average age of the patients was 10.72 years in the treatment group and 18.21 years in the control group (Table 3).
Table 3: Analysis of groups by age by methods of descriptive statistics
|
Parameter |
Group |
n |
Average |
Median |
Standard deviation |
Min |
Max |
|
Age |
Treatment |
58 |
10.72 |
8 |
10.44 |
2 |
65 |
|
Control |
85 |
18.21 |
9 |
17.95 |
1 |
82 |
Since the data in the groups by “age” are not normally distributed and differ in the number of patients, comparison of groups by age was performed using the Mann-Whitney test adjusted for independent samples at a significance level of 0.05 (Table4).
Table 4: Comparison of groups by age using the Mann-Whitney test
|
Parameter |
Group |
U Mann-Whitney |
W Wilcoxon |
Z-statistics |
p -value |
|
Age |
Treatment |
2024 |
3735 |
-1.817 |
0.069 |
|
Control |
The analysis shows that the groups were formed statistically homogeneous by age. In general, there were no significant differences in demographic characteristics between the patients in the treatment group and the control group at baseline (V1).
3.2 Outcomes and estimation
Typical clinical symptoms of CNP are nasal discharge, postnasal drip, hyperaemia, mucosal oedema. Table5 shows the group comparison according to the main symptoms by the descriptive statistics at study baseline (V1).
Table 5: Group analyses by main symptoms at V1
|
Parameter |
Group |
n |
Average |
Median |
Standard deviation |
Min |
Max |
|
Nasal discharge |
Treatment |
58 |
3.33 |
4 |
1.3 |
0 |
5 |
|
Control |
85 |
4.13 |
5 |
1.23 |
0 |
5 |
|
|
Postnasal drip |
Treatment |
58 |
2.19 |
3 |
1.81 |
0 |
5 |
|
Control |
85 |
3.07 |
3 |
1.65 |
0 |
5 |
|
|
Mucosal hyperaemia |
Treatment |
58 |
3.31 |
4 |
1.25 |
0 |
5 |
|
Control |
85 |
4.35 |
4 |
0.8 |
0 |
5 |
|
|
Mucosal oedema |
Treatment |
58 |
1.9 |
2 |
1.83 |
0 |
5 |
|
Control |
85 |
3.76 |
4 |
1.32 |
0 |
5 |
As can be seen from the presented analysis, the average nasal discharge symptom score was 3.33 in the treatment and 4.23 in the control group. Postnasal drip was 2.19 and 3.07 scores, respectively. Mucosal hyperaemia was 3.31 in the treatment group and 4.35 in the control group. The severity of mucosal oedema was 1.90 in the treatment group and 3.76 in the control group. Since the groups differed in the number of patients and the scores were not normally distributed, group comparison at baseline for the main symptoms was performed using the Mann-Whitney test adjusted for independent samples at a significance level of 0.05 (Table6).
Table 6: Group comparison by main symptoms at V1 using the Mann-Whitney test
|
Parameter |
Group |
U Mann-Whitney |
W Wilcoxon |
Z-statistics |
p -value |
|
Nasal discharge |
Treatment |
1406 |
3117 |
-4.567 |
0 |
|
Control |
|||||
|
Postnasal drip |
Treatment |
1785.5 |
3496.5 |
-2.87 |
0.004 |
|
Control |
|||||
|
Mucosal hyperaemia |
Treatment |
1136 |
2847 |
-5.826 |
0 |
|
Control |
|||||
|
Mucosal oedema |
Treatment |
1062.5 |
2773.5 |
-5.922 |
0 |
|
Control |
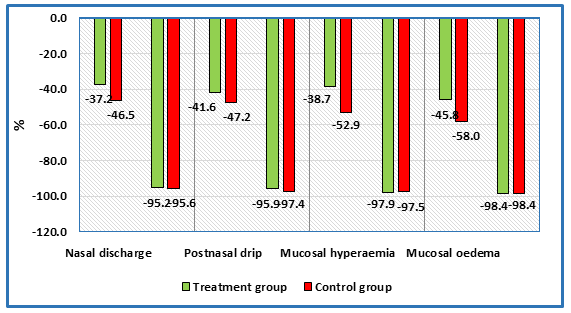
As can be seen from the analysis, at V1 the groups differed statistically significantly in the severity of the main symptoms. Figure 2 shows the relative course of regression of symptom severity assessed on a 5-point scale (from 0 to 5 points). The regression degree of symptom severity in % at V2 and V3 compared to the first visit (V1) was determined.
Regression of nasal discharge in patients of the treatment group by 37.2% versus 46.5% in the control group at V2 and by 95.2% versus 95.6% at V3 was observed. When evaluating the symptom of postnasal drip during treatment, regression of postnasal discharge by 41.6% at V2 and 95.9% at V3 was observed in patients in the treatment group. Comparable regression was observed in patients of the control group: by 47.2% at V2 and up to 97.4% at V3. Mucosal hyperaemia: regression of 38.7% at V2 in the treatment group and 52.9% in the control group was observed. At V3, regression was 97.9% in the treatment group and 97.5% in the control group. Mucosal oedema is the main symptom representing the degree of nasal breathing difficulty. On-therapy, regression of symptom severity was observed by 45.8% in the treatment group and 58.0% in the control group at V2 and 98.4% at V3 in the treatment and control groups, respectively. Since the groups were formed not statistically homogeneous by the above symptoms, to analyse the course of change in the severity of the main symptoms, individual differences dTi = Tvisit2 — Tvisit1, ...., Tvisit3 — Tvisit1 for each subject and symptom were calculated. Further comparison between groups on the course of severity of the main symptoms (differences dTi) was performed using the Mann-Whitney test. The set significance level was 0.05. Table7 shows the data of comparative analysis of the course of symptom characteristics between the groups.
Table 7: Comparison between groups in the course of the symptoms using the Mann-Whitney test
|
Parameter |
Visit |
U Mann-Whitney |
W Wilcoxon |
Z |
p-value* |
|
Nasal discharge |
V 2 |
2417 |
4128 |
-0.207 |
0.836 |
|
V 3 |
2402 |
4113 |
-0.414 |
0.679 |
|
|
Postnasal drip |
V 2 |
2114 |
3825 |
-1.487 |
0.137 |
|
V 3 |
2435.5 |
4146.5 |
-0.263 |
0.793 |
|
|
Mucosal hyperaemia |
V 2 |
2388.5 |
6043.5 |
-0.329 |
0.742 |
|
V 3 |
2401 |
4112 |
-0.548 |
0.584 |
|
|
Mucosal oedema |
V 2 |
1776.5 |
3487.5 |
-2.933 |
0.003* |
|
V 3 |
2405 |
4116 |
-0.66 |
0.509 |
The data show that the groups did not statistically differ in the course of severity of symptoms such as nasal discharge, postnasal drip, mucous membrane hyperaemia at V2 and V3 (p>0.05). Differences in the course of the symptom: mucosal oedema were significant between groups at visit 2 (p<0.05). According to the study design, thermometry was performed at all visits to determine body hyperthermia in patients (Table8). Comparison of groups on the course of body hyperthermia was performed using chi-square test in combination with Fisher t-test at a significance level of 0.05.
Table 8: Analysis of groups according to the course of body hyperthermia
|
Visit |
Treatment group |
Control group |
p-value |
||
|
Hyperthermia present |
No hyperthermia present |
Hyperthermia present |
No hyperthermia present |
||
|
V 1 |
24 (41.4%) |
34 (58.6%) |
39 (45.9%) |
46 (54.1%) |
0.718 |
|
V 2 |
4 (6.9%) |
54 (93.1%) |
14 (16.5%) |
71 (83.5%) |
0.124 |
|
V 3 |
0 (0%) |
58 (100%) |
0 (0%) |
85 (100%) |
1 |
The table data show that there were no statistically significant differences between the groups for the course of body hyperthermia at all patient visits. The analysis of the results of tolerability assessment showed that the treatment was well or very well tolerated in all cases. No side effects were reported in any patient during treatment.
3.3 Safety and tolerability
An analysis of the tolerability assessment findings showed that the treatment was well tolerated or very well tolerated in all cases. No on- treatment side effects were observed in any patient.
4. Discussion
The NT surface has been known to have many folds or crypts with limited blood flow and therefore microorganisms can easily integrate into them. Bacteria in adenoids usually include many species and provide an indolent persistent inflammatory process with biofilm formation [9,10]. Biofilms can create conditions for the progression of inflammation and frequent recurrence. Studies have found that impairment of the normal composition of the nasopharyngeal microbiome involving biofilm formation is associated with susceptibility to frequent exacerbation of chronic nasopharyngitis [11, 12, 13]. The inclusion criteria for patients were the presence of 3 or more episodes of disease within the last 6 months, which was clinical evidence of biofilms on the nasopharyngeal tonsil. The treatment efficacy with systemic antibacterial agents is low. The ineffectiveness of antibiotic therapy in the paediatric population with chronic nasopharyngitis is thought to be related to nasopharyngeal bacterial biofilms [16, 17, 18].
The prospect is to influence the bacteria stored in the biofilm, in particular their planktonic forms, using topical antibacterial agents. However, the efficacy of such treatment remains insufficiently studied. The study shows that the use of endonasal therapy with framycetin solution (Isofra) complementary to background therapy with isotonic seawater solution has an evidence-based clinical effect. Patients in the treatment and control groups demonstrated a clinically significant reduction in the severity of the main symptoms (nasal discharge, postnasal drip, mucosal hyperaemia, mucosal oedema) at V2 and V3. The obtained findings reflect the few data published in the literature showing evidence-based therapeutic efficacy of topical antibacterial therapy associated with an effect on biofilms in patients with chronic rhinosinusitis [20]. Add-on therapy for CNP is commonly used in these patients [22]. Nasal steroids, antileukotriene agents, and topical hyaluronic acid products have been suggested as add-on pharmacological treatment [23, 24, 25]. Overall, the evidence for the efficacy of this agent is equivocal, so the potential for additional treatment requires further investigation. According to the study design, the control group included patients who received complementary therapy with antihistamines and antivirals. Obviously, the reason for their administration was the greater severity of symptoms in the control group patients at V1, as the groups differed statistically significantly by this parameter. In our opinion, a higher score of symptom severity in the control group patients is rather related to the subjective assessment of the patient's condition by the clinician.
The patients did not show statistically significant differences between groups by the “body hyperthermia” parameter at V1, so the difference in the patient scores was not related to the disease severity. Add-on therapy did not improve the treatment outcomes of the control group patients. The data show that the groups did not statistically differ in the course of severity of symptoms such as nasal discharge, postnasal drip, mucous membrane hyperaemia at V2 and V3 (p>0.05). Differences in the course of the “mucous oedema” symptom were significant between the groups at visit 2 (p<0.05). This difference can be explained by a mild and not lasting antiedematous effect of antihistamines. The above agents can be used for self-treatment in the first day or two of the disease [20]. Add-on therapy showed no effect on body hyperthermia. There were no statistically significant differences between the groups for the course of the indicated symptom at the follow-up patient visits.
The results obtained confirm the data published in the literature reflecting the lack of therapeutic efficacy of add-on treatment in patients with chronic nasopharyngitis [23, 24, 25]. Thus, at the ten-day follow-up period in the treatment and control group patients against the background of endonasal therapy with framycetin solution, regression of symptoms of chronic nasopharyngitis exacerbation is observed, which allows us to evaluate the course of the disease as “significantly positive”. Reduction in the severity of symptoms correlates with the course of hyperthermia. The design was a comparative study, comparing additional administration of oral antihistamines and antivirals. The effect of this treatment did not lead to an improvement in the course of symptoms in the control group patients versus the treatment group. In this regard, the positive trends in treatment outcomes can be attributed to the clinical effects of framycetin.
5. Conclusions
Endonasal therapy with framycetin solution in exacerbation of CNP contributes to the reduction in the severity of the main clinical symptoms in patients of the treatment and control groups. Reduction in the severity of clinical symptoms correlates with a significant decrease in hyperthermia in patients of both groups. Additional administration of antiviral and antihistamine agents does not affect the course of clinical symptoms due to exacerbation of chronic nasopharyngitis.
Funding Statement
This research was supported in part by Laboratories Bouchara Recordati, Recordati Ireland Ltd. The costs of statistical processing of the data and translation of the article into English were covered.
Conflict of Interest Statement
There is no conflict of interest.
References
- Wang H. Chronic adenoiditis. J Int Med Res 48 (2020): 300060520971458.
- Purnell PR, Ramadan JH, Ramadan HH. Can Symptoms Differentiate Between Chronic Adenoiditis and Chronic Rhinosinusitis in Pediatric Patients. Ear Nose Throat J 98 (2019): 279-282.
- Smiianov YV, Smiianov VA, Sniehirova IA, Smiianova OI. Algorithm of adenoiditis treatment in adults, depending on the pharyngeal tonsil hypertrophy stage. Wiad Lek 71 (2018): 564-568.
- https://icd.who.int/browse10/2019/en#/J30-J39
- https://icd.who.int/browse11/l-m/en
- Rajeshwary A, Rai S, Somayaji G, Pai V. Bacteriology of symptomatic adenoids in children. N Am J Med Sci 5 (2013): 113-8.
- Badran H, Salah M, Fawzy M, Sayed A, Ghaith D. Detection of Bacterial Biofilms in Chronic Pharyngitis Resistant to Medical Treatment. Ann Otol Rhinol Laryngol 124 (2015): 567-71.
- Ren T, Glatt DU, Nguyen TN, Allen EK, Early SV, Sale M, et al. 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids. Environ Microbiol 15 (2013): 535-47.
- Van Hoecke H, De Paepe AS, Lambert E, Van Belleghem JD, Cools P, Van Simaey L, et al. Haemophilus influenzae biofilm formation in chronic otitis media with effusion. Eur Arch Otorhinolaryngol 273 (2016): 3553-3560.
- Kania R, Vironneau P, Dang H, Bercot B, Cambau E, Verillaud B, et al. Bacterial biofilm in adenoids of children with chronic otitis media. Part I: a case control study of prevalence of biofilms in adenoids, risk factors and middle ear biofilms. Acta Otolaryngol 139 (2019): 345-350.
- Dubourg G, Edouard S, Raoult D. Relationship between nasopharyngeal microbiota and patient's susceptibility to viral infection. Expert Rev Anti Infect Ther 17 (2019): 437-447.
- Tozzi AE, Del Chierico F, Pandolfi E, Reddel S, Gesualdo F, Gardini S. et al. Nasopharyngeal microbiota in hospitalized children with Bordetella pertussis and Rhinovirus infection. Sci Rep 11 (2021): 22858.
- Edouard S, Million M, Bachar D, Dubourg G, Michelle C, Ninove L, et al. The nasopharyngeal microbiota in patients with viral respiratory tract infections is enriched in bacterial pathogens. Eur J Clin Microbiol Infect Dis 37 (2018): 1725-1733.
- Miller BJ, Gupta G. Adenoidectomy. 2023 Feb 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023).
- Byars SG, Steams SC, Boomsma JJ. Association of Long-Term Risk of Respiratory, Allergic, and Infectious Diseases with Removal of Adenoids and Tonsils in Childhood JAMA Otolaryngol Head Neck Surg 144 (2018): 594-603.
- Chao Y, Bergenfelz C, Håkansson AP. In Vitro and In Vivo Biofilm Formation by Pathogenic Streptococci. Methods Mol Biol 1535 (2017): 285-299.
- Bair KL, Campagnari AA. Moraxella catarrhalis Promotes Stable Polymicrobial Biofilms With the Major Otopathogens. Front Microbiol 10 (2020): 3006.
- Luke NR, Jurcisek JA, Bakaletz LO, Campagnari AA. Contribution of Moraxella catarrhalis type IV pili to nasopharyngeal colonization and biofilm formation. Infect Immun 75 (2007): 5559-64.
- Abebe GM. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int J Microbiol 25 (2020): 1705814.
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020.- Rhinology 29 (2020): 1-464.
- Bowers I, Shermetaro C. Adenoiditis. [Updated 2022 Jan 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2022).
- Sakarya EU, Bayar Muluk N, Sakalar EG, Senturk M, Aricigil M, Bafaqeeh SA, et al. Use of intranasal corticosteroids in adenotonsillar hypertrophy. J Laryngol Otol 131 (2017): 384-390.
- Kar M, Altıntoprak N, Muluk NB, Ulusoy S, Bafaqeeh SA, Cingi C. Antileukotrienes in adenotonsillar hypertrophy: a review of the literature. Eur Arch Otorhinolaryngol 273 (2016): 4111-4117.
- Lou Z. "Commentary to: 'Endoscopic and clinical benefits of hyaluronic acid in children with chronic adenoiditis and middle ear disease'"? Eur Arch Otorhinolaryngol 275 (2018): 827-828.