A Personalized Real-time Model of Drug Resistance in Gynecological Cancers: Not to Leave CAF Unturned
Article Information
Pradip De1,2,3, Raed Sulaiman4, Kris Gaster5, Nandini Dey1,2*
1Translational Oncology Laboratory, Avera Cancer Institute, Sioux Falls, SD 57108, USA
2Department of Internal Medicine, University of South Dakota SSOM, Sioux Falls, SD 57108, USA
3Viecure, Greenwood Village, CO 80111
4Department of Pathology, Avera Research Institute, Sioux Falls, SD 57105, USA
5Assistant VP Outpatient Cancer Clinics, Avera Cancer Institute
*Corresponding Author: Nandini Dey, Senior Scientist, Director of Translational Oncology Laboratory, Avera Cancer Institute, 1000 E 23rd Street, Sioux Falls, South Dakota, USA.
Received: 24 August 2023; Accepted: 21 September 2023; Published: 03 October 2023
Citation: Pradip De, Raed Sulaiman, Kris Gaster, Nandini Dey. A Personalized Real-time Model of Drug Resistance in Gynecological Cancers: Not to Leave CAF Unturned. Journal of Cancer Science and Clinical Therapeutics 7 (2023): 164-168.
View / Download Pdf Share at FacebookAbstract
Evidence suggests the involvement of cancer-associated fibroblasts (CAFs) in developing treatment resistance in various solid tumors. These findings emphasize the importance of understanding the biology and function of CAFs in the context of therapy resistance to develop improved treatment strategies for these solid tumors. The function of CAFs in promoting therapy resistance is organ-type specific. In the published literature, we observed an organ-type-specific imbalance regarding CAF’s role in endometrial neoplasms. Here we present a commentary on the innovative and promising approach to studying endometrial and ovarian CAFs in therapy resistance. Our personalized real-time model allows a more comprehensive understanding of CAFs' involvement in developing resistance to specific drugs or combinations. By adopting a tumor-tumor microenvironment (TME) holistic approach, we recognized the importance of studying tumor cells and the surrounding TME to understand disease progression better. Our CAF-based Two-Cell Hybrid Co-Culture model established using CAFs from resected tumor tissue from patients with gynecological cancers provides a relevant and patient-specific context for testing drug resistance. This personalized real-time model has the potential to shed light on the mechanisms by which CAFs contribute to therapy resistance. By studying the interactions between tumor cells and CAFs in this model, one can gain valuable insights into the role of CAFs in driving resistance and identify potential therapeutic targets to overcome it.
Keywords
Cancer-associated Fibroblasts; Drug-Resistance; 2-Cell Model; Gynecological Cancers
Cancer-associated Fibroblasts articles; Drug-Resistance articles; 2-Cell Model articles; Gynecological Cancers articles
Cancer-associated Fibroblasts articles Cancer-associated Fibroblasts Research articles Cancer-associated Fibroblasts review articles Cancer-associated Fibroblasts PubMed articles Cancer-associated Fibroblasts PubMed Central articles Cancer-associated Fibroblasts 2023 articles Cancer-associated Fibroblasts 2024 articles Cancer-associated Fibroblasts Scopus articles Cancer-associated Fibroblasts impact factor journals Cancer-associated Fibroblasts Scopus journals Cancer-associated Fibroblasts PubMed journals Cancer-associated Fibroblasts medical journals Cancer-associated Fibroblasts free journals Cancer-associated Fibroblasts best journals Cancer-associated Fibroblasts top journals Cancer-associated Fibroblasts free medical journals Cancer-associated Fibroblasts famous journals Cancer-associated Fibroblasts Google Scholar indexed journals Drug-Resistance articles Drug-Resistance Research articles Drug-Resistance review articles Drug-Resistance PubMed articles Drug-Resistance PubMed Central articles Drug-Resistance 2023 articles Drug-Resistance 2024 articles Drug-Resistance Scopus articles Drug-Resistance impact factor journals Drug-Resistance Scopus journals Drug-Resistance PubMed journals Drug-Resistance medical journals Drug-Resistance free journals Drug-Resistance best journals Drug-Resistance top journals Drug-Resistance free medical journals Drug-Resistance famous journals Drug-Resistance Google Scholar indexed journals 2-Cell Model articles 2-Cell Model Research articles 2-Cell Model review articles 2-Cell Model PubMed articles 2-Cell Model PubMed Central articles 2-Cell Model 2023 articles 2-Cell Model 2024 articles 2-Cell Model Scopus articles 2-Cell Model impact factor journals 2-Cell Model Scopus journals 2-Cell Model PubMed journals 2-Cell Model medical journals 2-Cell Model free journals 2-Cell Model best journals 2-Cell Model top journals 2-Cell Model free medical journals 2-Cell Model famous journals 2-Cell Model Google Scholar indexed journals Gynecological Cancers articles Gynecological Cancers Research articles Gynecological Cancers review articles Gynecological Cancers PubMed articles Gynecological Cancers PubMed Central articles Gynecological Cancers 2023 articles Gynecological Cancers 2024 articles Gynecological Cancers Scopus articles Gynecological Cancers impact factor journals Gynecological Cancers Scopus journals Gynecological Cancers PubMed journals Gynecological Cancers medical journals Gynecological Cancers free journals Gynecological Cancers best journals Gynecological Cancers top journals Gynecological Cancers free medical journals Gynecological Cancers famous journals Gynecological Cancers Google Scholar indexed journals solid tumors articles solid tumors Research articles solid tumors review articles solid tumors PubMed articles solid tumors PubMed Central articles solid tumors 2023 articles solid tumors 2024 articles solid tumors Scopus articles solid tumors impact factor journals solid tumors Scopus journals solid tumors PubMed journals solid tumors medical journals solid tumors free journals solid tumors best journals solid tumors top journals solid tumors free medical journals solid tumors famous journals solid tumors Google Scholar indexed journals endometrial neoplasms articles endometrial neoplasms Research articles endometrial neoplasms review articles endometrial neoplasms PubMed articles endometrial neoplasms PubMed Central articles endometrial neoplasms 2023 articles endometrial neoplasms 2024 articles endometrial neoplasms Scopus articles endometrial neoplasms impact factor journals endometrial neoplasms Scopus journals endometrial neoplasms PubMed journals endometrial neoplasms medical journals endometrial neoplasms free journals endometrial neoplasms best journals endometrial neoplasms top journals endometrial neoplasms free medical journals endometrial neoplasms famous journals endometrial neoplasms Google Scholar indexed journals therapeutic targets articles therapeutic targets Research articles therapeutic targets review articles therapeutic targets PubMed articles therapeutic targets PubMed Central articles therapeutic targets 2023 articles therapeutic targets 2024 articles therapeutic targets Scopus articles therapeutic targets impact factor journals therapeutic targets Scopus journals therapeutic targets PubMed journals therapeutic targets medical journals therapeutic targets free journals therapeutic targets best journals therapeutic targets top journals therapeutic targets free medical journals therapeutic targets famous journals therapeutic targets Google Scholar indexed journals cytotoxic chemotherapy articles cytotoxic chemotherapy Research articles cytotoxic chemotherapy review articles cytotoxic chemotherapy PubMed articles cytotoxic chemotherapy PubMed Central articles cytotoxic chemotherapy 2023 articles cytotoxic chemotherapy 2024 articles cytotoxic chemotherapy Scopus articles cytotoxic chemotherapy impact factor journals cytotoxic chemotherapy Scopus journals cytotoxic chemotherapy PubMed journals cytotoxic chemotherapy medical journals cytotoxic chemotherapy free journals cytotoxic chemotherapy best journals cytotoxic chemotherapy top journals cytotoxic chemotherapy free medical journals cytotoxic chemotherapy famous journals cytotoxic chemotherapy Google Scholar indexed journals immunotherapy articles immunotherapy Research articles immunotherapy review articles immunotherapy PubMed articles immunotherapy PubMed Central articles immunotherapy 2023 articles immunotherapy 2024 articles immunotherapy Scopus articles immunotherapy impact factor journals immunotherapy Scopus journals immunotherapy PubMed journals immunotherapy medical journals immunotherapy free journals immunotherapy best journals immunotherapy top journals immunotherapy free medical journals immunotherapy famous journals immunotherapy Google Scholar indexed journals tumor microenvironment articles tumor microenvironment Research articles tumor microenvironment review articles tumor microenvironment PubMed articles tumor microenvironment PubMed Central articles tumor microenvironment 2023 articles tumor microenvironment 2024 articles tumor microenvironment Scopus articles tumor microenvironment impact factor journals tumor microenvironment Scopus journals tumor microenvironment PubMed journals tumor microenvironment medical journals tumor microenvironment free journals tumor microenvironment best journals tumor microenvironment top journals tumor microenvironment free medical journals tumor microenvironment famous journals tumor microenvironment Google Scholar indexed journals
Article Details
Cancer treatment has been one of the most challenging journeys in the history of medical sciences in the past century, bringing waves of excitement every decade and revolutionizing the very approach of clinical practice. In today's world of traditional patient care, the state-of-the-art method of disease management predominantly revolves around the direct killing or resection of tumor cells by surgeries, radiotherapy, cytotoxic chemotherapy, and selected categories of genomics/biomarker-driven systemic therapies, including combinations of targeted therapy, and immunotherapy. All these different approaches aim to eliminate already existing tumor cells to achieve PFS (progression-free survival) and, finally, OS (overall survival). Nevertheless, even under the best possible scenario of clinical management of the disease, a considerable percentage of patients across a wide range of solid tumors develop resistance to the treatment and remain nonresponsive to the available treatment options in the face of exhausted clinical options.
The collective knowledge revealing the hard fact that the "perfect treatment option(s) for a perfect genomics/biomarker-driven" disease is lacking drew our attention to the cells nearest to the tumor cells within the host's body, cells of the tumor microenvironment (TME), ignoring the vulnerability of CAF-mediating treatment resistance. In recent years, utilizing single-cell technology has revealed an extensively diverse cellular component and dissected cellular heterogeneity of almost all solid tumors, including the rare ones [1]. Pancreatic ductal adenocarcinoma (PDAC) has been at the forefront of the organ type for our "gain-of-knowledge" journey in this context. Multimodal mapping of PDAC using spatially resolved single-cell transcriptomics and imaging techniques by Schreyer et al. [2] has identified new potentially therapeutically actionable cellular targets and has provided new insights into PDAC tumor heterogeneity. Their study deconstructed PDAC at single-cell resolution to define actionable segments using scRNAseq, spatial transcriptomics, and state-of-the-art multiplexed imaging platforms. Their study, along with studies by Raghavan et al. [3] and Steele et al. [4], has thrown new insights about (1) neoplastic phenotypes, (2) the signaling dialogue between neoplastic and stromal cell populations, and (3) the evolution of resident tumor/residual tumor cells' cell populations during disease progression, metastasis and in response to therapy.
A recent scRNA-seq study profiled CAFs from lung, colorectal, breast, and ovarian cancers [5]. They profiled 233,591 single cells from patients with lung, colorectal, ovary, and breast cancers and constructed a pan-cancer blueprint of stromal cell heterogeneity using different single-cell RNA and protein-based technologies to identify 68 stromal cell populations, of which 46 are shared between cancer types, while 22 are unique. These technologies have enabled us to understand the TME, and it's by far the most abundant cellular component by volume, CAF [6, 7], based on parallel digital transcriptional profiling of single cells. Although as a component of TME, CAF's origin, markers subtypes, and functions have been studied in several solid tumors [8], the quest for knowledge of the functional meaningfulness of CAFs has just begun acknowledging the clinical and therapeutic relevance of CAF [9, 10]. Results of the studies demonstrating the prognostic value of CAF-related gene signatures in solid tumors like hepatocellular carcinoma [11] and CAF-mediated chemoresistance in bladder cancers [12] powerfully portray the role CAFs in the clinical context beyond initially identified organ-type cancers, including breast, pancreas, and lung. More than 13,446 articles related to CAF, its biology, a wide range of its functions, and clinical relevance have been published in PubMed (early August 2023).
Digging deep into the literature on the organ-type-specific origin, subtype(s), heterogeneity, activation, and functions of CAFs [13, 14], it appears that the involvement of CAF in the development of treatment resistance is undeniable in a wide range of solid tumors [9,15-17]. In recent years, CAF has been recognized as the non-redundant component of TME that individually interacts with tumor cells as well as the rest of TME, endothelial cells, and immune cells. Not difficult to comprehend that several studies establish the mechanistic events of CAF-mediated drug resistance in the context of tumor cells of different solid tumors [18, 19]). In our recent article, we reviewed the biology and function of CAF in the context of the development of therapy resistance in solid tumors, including radiotherapy, chemotherapy, targeted therapy, and immunotherapy [15]. In desmoplastic breast, lung, and pancreatic cancers, various signaling mediators that establish interaction between CAF, tumor cells, and the rest of the component cells of TME is multimodal and include (1) paracrine, (2) autocrine-paracrine loop, (3) exosomal cargo for miRNAs, (4) extracellular vesicles cargo, (5) a direct cell-to-cell contact, (6) extracellular matrix remodeling (7) neovascularization / vascular mimicry involving CAF secretome, (8) immune cell modulation, and (9) metabolic reprogramming. Resistance to radiotherapy has been reported in colorectal, nasopharyngeal, and esophageal cancers.
Resistance to chemotherapy has been reported in breast, gastric, head-neck, pancreatic, lung, ovarian, and bladder cancers. Resistance to radiotherapy has been reported in breast, lung, prostate, hepatocellular cancers, and melanoma. Finally, immunotherapy resistance was reported in pancreatic, breast, lung, hepatocellular, intrahepatic cholangiocarcinoma, urothelial, and esophageal carcinomas (See review [15]). Detailed interrogation of the function of CAFs in bringing out the therapy resistance in the above-mentioned organ-type cancers makes it evident that the signal transduction in CAFs is organ-type specific [10].
One of the reasons for the organ-type specificity of CAF signals is their tissue-specific activation, which in turn reflects molecular pathways in the activated CAF subtypes, leading to the presentation of different CAF subtypes in a particular stage of the disease progression.
As we appraise the participatory role of CAFs in mediating therapy resistance in solid tumors, it becomes apparent that there is an organ-type specific imbalance in the understanding of the role of CAF in therapy resistance. The uterine neoplasms are among the tumors with much lesser representation in CAF-mediated resistance. We observed that out of a total of 13,446 published articles related to CAF quarried from PubMed, articles related to the neoplasms of the breast, pancreas, lung, and colorectum are predominant, while organ-types uterine (endometrial) cancers are disproportionately limited. Table 1 presents conditional formatting of the number of published articles listed in PubMed on CAFs in common organ-type solid tumors.
Table 1: An organ-type specific imbalance in the published literature regarding CAF’s role in endometrial neoplasms: Conditional formatting of the number of published articles listed in PubMed on CAFs in common organ-type solid tumors*
*Organ-type cancers are presented alphabetically. 5-rating conditional formatting of data has been presented using a 3-color scale (green as the lowest, yellow as the medium, and red as the highest). The data was quarried on 7th August 2023.
There exists a substantial actionable gap between the role of CAF and the utilization of this knowledge in overcoming treatment resistance in clinical settings in endometrial neoplasms. The impact of the role of CAF on the development of resistance in response to therapy, chemotherapy, targeted therapy, and immune therapy needs to be systematically investigated in endometrial neoplasms on a patient-to-patient basis. A deeper understanding of this knowledge gap led us to pinpoint the fact that an absence of a patient-tailored experimental model limits our understanding of the mechanism underlying CAF-mediated drug resistance in real-time. As the pertinent question that remains unanswered is whether CAF-inclusive therapy can improve knowledge-guided platforms for better management/disease outcomes in patients with resistance to therapy in endometrial neoplasms, we are in demand for a patient-specific real-time model.
To address this issue, my colleagues and I have developed a personalized real-time model to test the role of CAF in developing resistance to a specific drug (combinations). In our research, we, in a more Tumor-TME holistic approach, embarked on a journey to understand the role of a specific cell type(s) from the supporting TME in progressive disease, cancer-associated fibroblast (CAF). We established a CAF-based Two-Cell Hybrid Co-Culture model for testing drug resistance using CAFs from resected tumor tissue from patients with endometrial cancers [20]. The overall theme of our model is presented in the diagram (Figure 1).
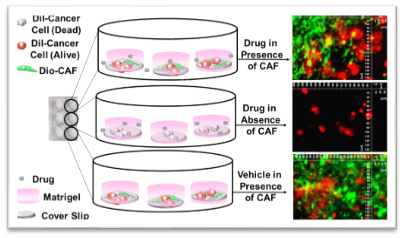
Figure 1: Model to test CAF functionality in drug resistance: The presence of CAFs resists the effect of anticancer drugs on tumor cells in 3D clonogenic culture format.
Primary CAFs cultured ex vivo from the resected tumor tissues from patients with endometrial cancer were characterized for CAF-specific markers and tested for their role in resisting the effect of anti-tumor drugs using a novel 2-cell-hybrid co-culture system. Primary CAFs were live stained with DiO-stain on coverslips. Tumor cells were live stained with DiI-stain and plated on the DiO-stained CAF monolayer or coverslips in the presence and absence of the drug. The photomicrograph shows the comparable growth of DiI-stained tumor cells in the presence of DiO-stained CAFs, irrespective of drug treatments (upper and lower panel), as compared to the drug-treated tumor cells plated in the absence of DiO-stained CAFs (middle panel).
Our patient-specific, laboratory-friendly, cost-effective, and time-sensitive novel tumor-TME two-cell ex vivo model tests endometrial CAF's role in resisting the anti-tumor drug paclitaxel. Endometrial CAFs, both NCAFs (tumor-adjacent normal-tissue-derived CAFs) and TCAFs (tumor-tissue-derived CAFs) were validated by CAF expression markers, including the positive markers like SMA, FAP, TE-7, and S100A4, which in varying degrees depending on the patients, while consistently lacking the negative markers, EpCAM and CK8, 9, 18. When tested using the model, CAFs resisted the growth inhibitory effect of paclitaxel on endometrial tumor cells in 2D and 3D formats as compared to the tumoricidal effect of paclitaxel in the absence of CAFs. The model helped us to test the role of CAFs in developing drug resistance and contribute to understanding tumor cell-CAF dialogue in endometrial cancers. We demonstrated that NCAF and TCAF from the same patient had the protective action to resist paclitaxel's tumoricidal effect on endometrial tumor cells. Endometrial CAFs expressed the immune marker PD-L1. We extended our study in laying out the tumor-TME landscape of PD-1/PD-L1 in endometrial cancers, demonstrating the mechanistic contribution of PD-L1/L2 and PD-1 signaling within the host's TME (CAF and immune cells) [21]. The results of our study will be utilized to understand the role of CAF in developing immune therapy resistance.
To understand the functional connection between the organ-type origin and activation of CAFs, we chose ovarian CAFs since the trans-differentiation of tumor endothelium is one of the major sources of CAFs. Our model can simultaneously evaluate the role of CAF from a particular patient on tumor cell growth as well as tumor angiogenesis [22]. We chose epithelial ovarian cancers, diagnosed at advanced/metastatic stages, commonly with a high angiogenesis index, presenting a rapid progression and poor outcome often due to the development of resistance to anti-angiogenic therapy. We hypothesized that ovarian CAF would play a critical role in resisting anti-angiogenic effects via direct crosstalk with endothelium and hence plays a direct role in the development of resistance to anti-angiogenic drugs. We employed our ex vivo Hybrid Co-Culture model to test co-culture comprising patient-derived primary CAFs from ovarian tumor samples and human umbilical vein endothelial cells [22]. Ovarian CAFs were characterized by the mRNA and protein expression of positive (SMA, S100A4, TE-7, FAP-A, CD90/THY1), negative (EpCAM, CK 8,18, CD31, CD44, CD45), functional (PDGFRA, TGFB1, TGFB2, TGFRA) and immunological markers (PD-L1, PD-L2, PD-1) associated with CAFs by qRT-PCR, flow cytometry, Western blot, and ICC. Our HyCC model proved that CAFs impart resistance to the anti-angiogenic drug lenvatinib, demonstrating for the first time the ability of patient-derived primary CAFs to resist lenvatinib's (an oral multi-targeted TKI of VEGFR1-3, FGFR, PDGFR, RET, and KIT) effect. Considering the various autocrine/paracrine modes of signaling between CAFs and the rest of the component cells of TME, our HyCC model also provided an opportunity to evaluate the manner of crosstalk between ovarian CAFs and endothelium during the course of developing resistance to anti-angiogenic drugs. CAF-derived ECM was found to be sufficient to initiate cord formation in HUVEC independent of matrigel ("on-plate"). As we demonstrated that CAF-derived ECM is enough to initiate cord formation at 18 hours of co-culture in HUVEC, and the signaling occurs via paracrine, most likely secretomic manner. We also demonstrated that the cord formation is protected from the anti-angiogenic effect of lenvatinib. As expected, we recorded no cord formation for HUVEC cells "on-plate" without CAF.
We finally tested the utility of the model by evaluating its clinical significance [23]. CAFs partner with tumor cells and all components of TME in a solid tumor, working in favor of the progression despite therapy. We hypothesize that CAF-rich endometrial tumors have clinical relevance from the viewpoint of post-surgery events (PSE). We tested our hypothesis by generating patient-derived endometrial CAFs from resected tumor tissues (TCAF) and tumor-adjacent normal tissues (NCAF) and stratified them into aggressive CAFs (based on the time of establishment and a higher passage number in the primary culture). Designating the aggressiveness of the patient-specific CAFs, we demonstrated that the PSE in patients with a high grade and stage of the disease is directly correlated to the aggressive nature of the CAFs in endometrial cancers. In identifying the mechanistic role of CAF, we demonstrated that aggressive CAFs from patients with PSE resisted the effects of paclitaxel and lenvatinib in a hybrid-co-culture (HyCC), indicating an effect mediated via secretomic paracrine signals as compared to direct contact. Our model was validated by our first report of a correlation between the PSE and the aggressive nature of CAFs and provided an undeniable reason to study the in-depth mechanism of CAF function towards the development of treatment resistance in endometrial cancers [23].
The purpose of the development of the model of resistance was to determine if it is logical to combine conventional tumor-cell-targeted therapy with TME-cell-targeted therapy, more specifically, CAF-targeted complementary therapy. The analytical and experimental tools that constitute our ex vivo research platform open an option to tailor future combating of drug resistance at the TME/CAF level.
Although our model primarily utilized endometrial and ovarian CAFs, the model can be extended even beyond gynecological cancers to combat drug resistance, employing complementary therapy targeted to CAFs. By selecting optimal combinations of drugs targeting tumor cells and cells of TME/CAF to overcome treatment resistance, one may envision to approach true precision therapy: disease by disease, stage by stage, and patient by patient. We are not there yet [24], but the model will test whether it is worth investing ourselves in that direction in a patient-tailored manner. There is more to TME than CAF to model, but this is the start.
Funding
The study was funded by Avera Cancer Institute.
Institutional Review Board Statement
Anonymized tissue samples were collected at surgery from patients with endometrial cancers following their Informed (IRB approved: Protocol Number Study: 2017.053?100399_ExVivo001) consent.
Informed Consent Statement
Informed (IRB approved: Protocol Number Study: 2017.053?100399_ExVivo001) consents to receiving resected tissue from enrolled patients with endometrial and ovarian cancers.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge Avera Cancer Institute for funding the entire study. We acknowledge every patient and their family for their participation in the ex vivo study at the Avera Cancer Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ren, X.; Kang, B.; Zhang, Z. Understanding tumor ecosystems by single-cell sequencing: promises and limitations. Genome Biol 19 (2018): 211.
- Schreyer D, Neoptolemos JP, Barry ST, et al. Deconstructing Pancreatic Cancer Using Next Generation-Omic Technologies-From Discovery to Knowledge-Guided Platforms for Better Patient Management. Front Cell Dev Biol 9 (2021): 795735.
- Raghavan S, Winter PS, Navia AW, et al. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell 184 (2021): 6119-6137.
- Steele NG, Carpenter ES, Kemp SB, et al. Multimodal Mapping of the Tumor and Peripheral Blood Immune Landscape in Human Pancreatic Cancer. Nat Cancer 1 (2020): 1097-1112.
- Qian J, Olbrecht S, Boeckx B, et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res 30 (2020): 745-762.
- Zheng GX, Terry JM, Belgrader P, et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun 8 (2017): 14049.
- Lavie D, Ben-Shmuel A, Erez N, et al. Cancer-associated fibroblasts in the single-cell era. Nat Cancer 3 (2022): 793-807.
- Nurmik M, Ullmann P, Rodriguez F, et al. In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer 146 (2020): 895-905.
- Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol 18 (2021): 792-804.
- Galbo PM, Zang X, Zheng D. Molecular Features of Cancer-associated Fibroblast Subtypes and their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin Cancer Res 27 (2021): 2636-2647.
- Dong W, Xie Y, Huang H. Prognostic Value of Cancer-Associated Fibroblast-Related Gene Signatures in Hepatocellular Carcinoma. Front Endocrinol (Lausanne) 13 (2022): 884777.
- Zhuang J, Shen L, Li M, et al. Cancer-Associated Fibroblast-Derived miR-146a-5p Generates a Niche That Promotes Bladder Cancer Stemness and Chemoresistance. Cancer Res 83 (2023): 1611-1627.
- Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 16 (2016): 582-598.
- Pereira BA, Vennin C, Papanicolaou M, et al. CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends Cancer 5 (2019): 724-741.
- De P, Aske J, Dey N. Cancer-Associated Fibroblast Functions as a Road-Block in Cancer Therapy. Cancers (Basel) 13 (2021).
- De P, Aske J, Sulaiman R, et al. Bete Noire of Chemotherapy and Targeted Therapy: CAF-Mediated Resistance. Cancers (Basel) 14 (2022).
- Saw PE, Chen J, Song E. Targeting CAFs to overcome anticancer therapeutic resistance. Trends Cancer 8 (2022): 527-555.
- Pradip D, Jennifer A, Nandini D. Cancer-Associated Fibroblasts in Conversation with Tumor Cells in Endometrial Cancers: A Partner in Crime. Int J Mol Sci 22 (2021).
- Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol 17 (2020): 487-505.
- Sulaiman R, De P, Aske JC, et al. A CAF-Based Two-Cell Hybrid Co-Culture Model to Test Drug Resistance in Endometrial Cancers. Biomedicines 11 (2023).
- Sulaiman R, De P, Aske JC, et al. Tumor-TME Bipartite Landscape of PD-1/PD-L1 in Endometrial Cancers. Int J Mol Sci 24 (2023).
- Sulaiman R, De P, Aske JC, et al. Patient-Derived Primary Cancer-Associated Fibroblasts Mediate Resistance to Anti-Angiogenic Drug in Ovarian Cancers. Biomedicines 11 (2023).
- Sulaiman R, De P, Aske JC, et al. Characterization and Clinical Relevance of Endometrial CAFs: Correlation between Post-Surgery Event and Resistance to Drugs. Int J Mol Sci 24 (2023).
- Papait A, Romoli J, Stefani FR, et al. Fight the Cancer, Hit the CAF! Cancers (Basel) 14 (2022).
