A Novel Mutation (c.122T>A) of PEX11B Gene in a Female Adolescent with Congenital Cataract and Clubfoot
Article Information
Vasiliki Rengina Tsinopoulou1*, Liana Fidani2, Styliani Giza1, Eleni I Sakellari1, Angeliki Beslika1, Stergianna Ntouma1, Spyridon Gerou3, Assimina Galli-Tsinopoulou1
1Unit of Paediatric Endocrinology and Metabolism, 2nd Department of Paediatrics, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, AHEPA University Hospital, Thessaloniki, Greece
2Laboratory of Genetics, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Greece
3Analysis Iatriki S.A., Biopathological Diagnostic Research Laboratories, Thessaloniki, Greece
*Corresponding Author: Vasiliki Rengina Tsinopoulou, Academic Research Fellow in Pediatric Endocrinology and Metabolism, Unit of Paediatric Endocrinology and Metabolism, 2nd Department of Paediatrics, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, AHEPA University Hospital, Stilponos Kyriakidi 1, 54636, Thessaloniki, Greece.
Received: 21 May 2023; Accepted: 29 May 2023; Published: 05 June 2023
Citation:
Vasiliki Rengina Tsinopoulou, Liana Fidani, Styliani Giza, Eleni I Sakellari, Angeliki Beslika, Stergianna Ntouma, Spyridon Gerou, Assimina Galli-Tsinopoulou. A Novel Mutation (c.122T>A) of PEX11B Gene in a Female Adolescent with Congenital Cataract and Clubfoot. Journal of Pediatrics, Perinatology and Child Health. 7 (2023): 107-110.
View / Download Pdf Share at FacebookAbstract
Objective: Peroxisome biogenesis disorder type 14B (PEX14B, OMIM #614920) is an autosomal recessive disorder characterized clinically by mild mental retardation, congenital cataract, progressive hearing loss, and polyneuropathy. We aimed to report a new mutation in a female adolescent of Greek origin with prominent clinical signs of congenital cataract and clubfoot.
Material: A 134/12-year-old girl having overweight, mild psychomotor retardation, congenital cataract, clubfoot from infancy progressively worsening with accompanying unsteadiness of gait, episodes of faecal incontinence in the dolichosigmoid setting and non-autoimmune hypothyroidism was presented. On clinical examination, furrows, lowered eyelid fissures and corners of the mouth, a deep philtrum and micrognathia were noted and neurologically found reduced muscle strength in the peripheral parts of the upper and lower limbs with absence of tendon reflexes and atrophy of the thenar, peroneal and tibial muscles both, findings indicative of peripheral polyneuropathy, which was confirmed in the neurophysiological examination. Neuroimaging, metabolic control and karyotype reveled normal findings. Whole Exome Sequencing (WES) was performed.
Results: The unreported nucleotide substitution c122T>A in the PEX11B gene was found in the homozygous state, causing premature termination of protein synthesis and impaired biogenesis of type 14B peroxisomes. Heterozygosity for the same mutation was found in parents.
Conclusions: DNA sequencing of all genes is an excellent tool for the diagnosis of phenotypically and genetically heterogeneous conditions such as as disorders of hyperhyxosome biogenesis. The detection of new mutations helps to complete the clinical and genetic spectrum of rare diseases and constitutes a valuable learning resource for future research.
Keywords
PEX11β; Peroxisome biogenesis disorder; Congenital cataract; Clubfoot
Article Details
1. Objective
Peroxisomes are cellular organelles in eukaryotic human cells that are mainly involved in catabolic and anabolic metabolic pathways, such as fatty acid alpha- and beta-oxidation, glyoxylate detoxification, biosynthesis of ether phospholipids; docosahexaenoic acid (DHA); bile acids and cellular redox metabolism [1].
Consequently, two main categories of peroxisomal disorders can be distinguished; the isolated peroxisomal enzyme deficiencies (PEDs) and the peroxisome biogenesis disorders (PBDs). Peroxisome biogenesis disorders are defects in different processes of peroxisome biogenesis affecting the peroxins and caused by biallelic mutations in one of the 14 known different PEX genes [2,3].
The phenotypes of patients with a PBD range from a severe and lethal multisystem disorder to milder diseases with slow progression or isolated visual and/or hearing problems [1]. Of these, the best known are the Zellweger spectrum disorders, which include three distinct branches: Zellweger syndrome, infantile Refsum disease, and neonatal adrenoleukodystrophy. Zellweger syndrome has the most severe manifestation, while in all forms of the spectrum functional peroxisomes are absent in the nervous system, suggesting severe central nervous system (CNS) involvement in the Zellweger spectrum phenotype [2].
Consistent with CNS involvement in Zellweger spectrum disorders, gastrointestinal tract involvement and renal disturbances accompany the entity.
In recent decades, some studies have reported new homozygous mutations in PEX11B causing Zellweger spectrum disorders such as a clinical phenotype with bilateral congenital cataract, polyneuropathy, intellectual disability, progressive deafness and gastrointestinal problems [4-6].
Here we report a novel mutation in PEX11B gene in an adolescent girl with PBD-like features.
2. Case presentation
The patient was born by vaginal delivery in 40 weeks of gestation with an Apgar score of 9 at 1st minute and 10 at 5th minute; at birth her weight was 3900 gr (75th -90th percentile), length 52 cm (75th percentile) and head circumference 35,5 cm (75th-90th percentile). She is the second child of two phenotypically healthy parents, while the father recently was diagnosed with sarcoidosis; it is noteworthy a consanguinity in the family since the parents are relatives. Her past medical history, until her referral to the Unit of Pediatric Endocrinology, included a surgery for congenital bilateral cataract at the age of 40 days, primary nocturnal enuresis and mild intellectual disability. Since then, she has been under the supervision of a pediatric neurologist. At the age of 66/12 years, she first was hospitalized because of a severe headache accompanied by vomiting and dysarthria. Thereafter, the headaches continued to be present and a diagnosis of migraine was established based on the known familiar history of migraine.
Additionally, she presented with clubfoot since toddler age with a progressive unsteadiness in walking. She had episodes of encopresis, which were investigated by a pediatric gastroenterologist and her rectosigmoidoscopic examination attributed them to an elongation of the colon with flexures, loops and kinks in the lumen of the gut compatible with dolichocolon. At the age of 89/12 years, she was diagnosed with mild non-autoimmune hypothyroidism and treated with levothyroxine since then. An early menarche at the age of 10 years was noted.
At the age of 134/12 years, she was admitted on the Unit of Pediatric Endocrinology for further investigation for obesity (BMI 23.9 kg/m2), hirsutism, downward lid margins and lip angles, deep philtrum, a dimpled chin, micrognathia, generalized hirsutism and stretch marks of the abdominal wall. Her neurological examination revealed decreased peripheral muscle strength of the upper and lower extremities with absence of tendon reflexes and bilateral atrophy of thenar, fibular and tibial muscles, findings suggestive of peripheral polyneuropathy. A detailed neurological investigation was performed including nerve conduction velocity (NCV) and panel for inborn metabolic diseases. The NCV test confirmed sensorimotor polyneuropathy, while the investigation for metabolic diseases was negative. Magnetic resonance imaging (MRI) of the Brain and cerebellum revealed normal findings.
A complete endocrinological investigation conducted, which revealed a normal metabolic profile, without insulin-resistance (HOMA-IR: 2.62) or hyperinsulinemia, normal level of glycosylated hemoglobin (5.3%), normal adrenal function with maintenance of circadian rhythm of cortisol levels (morning cortisol 8.21 μg/dl and evening cortisol 1.42 μg/dl) and non-autoimmune hypothyroidism.
The karyotype analysis revealed a normal finding 46,XX. Biochemical, hematological and immunological analyses were normal.
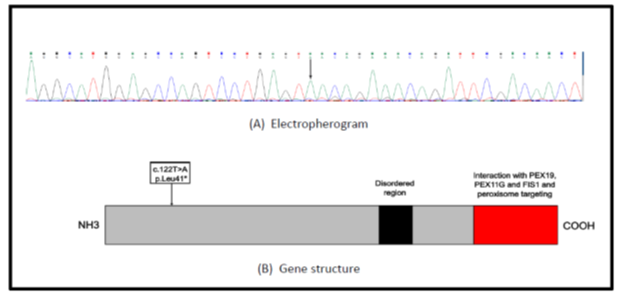
The notable facial characteristics in combination with the interesting medical history raised the suspicion for a possible genetic disease, so a genetic evaluation with Whole Exome Sequencing (WES) analysis by Next Generation Sequencing ThermoFisher S5/S5x1 platform was performed. Data analysis was conducted with Ion Reporter, with comparison to referral genome hg19. Genetic analysis discovered a homozygous c.122T>A nucleotide replacement in the PEX11B gene, responsible for the formation of peroxismal membrane protein 11B (Figure 1).
The protein is involved in regulation of peroxisome abundance and overexpression of PEX11B gene can induce peroxisome proliferation, in absence of external stimuli [7].
3. Discussion
In this case report we describe a female adolescent with a novel mutation (c.122T>A) of the PEX11B gene. This is the first time to our knowledge, the mutation is reported and mutates the corresponding codon to a stop codon, resulting in a change in protein function and the premature termination of protein synthesis [8].
Pathogenic homozygous mutations in this gene have been associated with peroxisome biogenesis disorders (PBD) type 14B (Peroxisome biogenesis disorder 14B, PEX14B, OMIM#614920). Peroxisome biogenesis disorder (PBD) type 14B is inherited in an autosomal recessive manner and characterized by polyneuropathy, congenital cataract, intellectual disability and progressive hearing loss [6].
Indeed, our patient had most of the specific clinical characteristics, with early diagnosed congenital cataract and mild intellectual disability. It is of great importance to mention that early diagnosis of congenital cataract could raise the suspicion of a possible underlying genetic disorder and a genome analysis could promote targeted counselling and putative treatment and in such patients [9]. Indeed, most of the patients described in the literature had an early sign of congenital cataract without any specific characteristic in early life. Thus, the single feature of congenital cataract could be indicative of PEX11B gene mutation. Furthermore, the patient developed progressively signs of polyneuropathy and gastrointestinal disorders with encopresis, both features of the disease spectrum; However, no signs of progressive hearing loss were mentioned.
Interestingly, our patient had migraine episodes since the early years of life. This finding coincides with a previously reported patient by Ebberink et al. [6], who had severe migraine attacks and could be a possible additional sign to the phenotypic features of PBDs.
To our knowledge, only few studies have linked peroxisome biosynthesis disorders to PEX11B mutations. In the first published study the authors found a homozygous variant in the PEX11B gene c.46C>T., in a 26-year-old male patient with mild intellectual disability, history of bilateral congenital cataract, bilateral perceptive hear loss and neurological involvement (nystagmus, diminished muscular strength-sensory abnormalities and areflexia in lower extremities) [6]. Accordingly, another study identified three diverse mutations in the PEX11B gene in three patients from different families. All of them suffered from congenital bilateral cataract, mild intellectual disability and neurological symptomatology, dysmorphic characteristics, while only one of them suffer from gastrointestinal symptoms. In terms of endocrinology all three patients appeared with height below the normal range and one patient had central obesity. The mutations in the PEX11B gene identified in the above-mentioned patients where a homozygous c.277C>G in patient 1, a homozygous c.136C>T in patient 2, and a heterozygous c.136C>T in patient 3 [5].
In another study the authors described a previously reported homozygous mutation of the PEX11B gene c.277C>T [4]. In this case the patient appeared with bilateral congenital cataract, nystagmus and strabismus, increased muscle tone and intellectual disability. No mentions regarding phenotypic dysmorphic characteristics or endocrinological involvement has been reported.
In all previously cited studies, the subjects involved appeared with clinical features similar to PBDs -like phenotypic characteristics. However, recently a study by Malekzadeh et al. [10] a novel mutation in PEX11β Gene has been reported a patient with episodic migraine-like attacks, delirium, mood and behavior change, polyneuropathy, and history of congenital cataract; in contrast to previously reported patients, this case presented with milder features and extended the spectrum of the clinical phenotype of this mutation [10].
Nevertheless, so far there are few reports on endocrinological involvement in patients with PBDs. Interestingly, in this study the patient presented with non-autoimmune hypothyroidism, hirsutism, obesity and early menarche. Possible mechanisms underlying those endocrinological manifestations can be linked to the novel homozygous mutation, and expand the clinical phenotype, providing evidence for future studies.
It is crucial, thought, to highlight the fact that patient’s parents are relatives. Despite the fact of consanguinity, it’s interesting that both other siblings of the patient have no signs and physical characteristics of possible underlying PBDs. Nonetheless, none of the other sibling has undergone targeted analysis of the finding, which is a limitation to our study.
As in this case, many diseases can be diagnosed based on thorough clinical examination and complete medical history, which can raise clinical suspicion. Our aim was to highlight the importance of increased clinical suspicion for unusual clinical findings, laboratory and imaging findings. Since PBDs appear most often with signs such as bilateral congenital cataract, especially in the early years of life, initial recognition and correlation with a possible underlying pathological condition is crucial. Ιt is of great importance to refer to clinical genetics and subsequent genetic analysis in order to diagnose rare genetic disorders.
4. Conclusion
This is the first case reported of a mutation c.122T>A in PEX11B gene, linked to a phenotype of peroxisome biogenesis disorder 14B (Peroxisome biogenesis disorders 14B, PEX14B, OMIM#614920). Althought, up to date the available data concerning the therapeutic approach are scarce, early diagnosis could have benefited the patient with the highest contribution of multiple medical specialties, in order to avoid further deterioration and to improve the quality of life.
Acknowledgments:
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest:
None declared.
References
- Waterham HR, Ferdinandusse S, Wanders RJA. Human disorders of peroxisome metabolism and biogenesis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1863 (2016): 922-933.
- Steinberg SJ, Dodt G, Raymond G V, et al. Peroxisome biogenesis disorders. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1763 (2006): 1733-1748.
- Honsho M, Okumoto K, Tamura S, et al. Peroxisome Biogenesis Disorders (2020): 45-54.
- Tian Y, Zhang L, Li Y, et al. Variant analysis of PEX11B gene from a family with peroxisome biogenesis disorder 14B by whole exome sequencing. Mol Genet Genomic Med 8 (2020): mgg3.1042.
- Taylor RL, Handley MT, Waller S, et al. Novel PEX11B Mutations Extend the Peroxisome Biogenesis Disorder 14B Phenotypic Spectrum and Underscore Congenital Cataract as an Early Feature. Investigative Opthalmology and Visual Science 58 (2017): 594.
- Ebberink MS, Koster J, Visser G, et al. A novel defect of peroxisome division due to a homozygous non-sense mutation in the PEX11β gene. J Med Genet 49 (2012): 307-313.
- Schrader M, Reuber BE, Morrell JC, et al. Expression of PEX11β Mediates Peroxisome Proliferation in the Absence of Extracellular Stimuli. Journal of Biological Chemistry 273 (1998): 29607-29614.
- Theodoulou FL, Bernhardt K, Linka N, et al. Peroxisome membrane proteins: multiple trafficking routes and multiple functions? Biochemical Journal 451 (2013): 345-352.
- Gillespie RL, Urquhart J, Anderson B, et al. Next-generation Sequencing in the Diagnosis of Metabolic Disease Marked by Pediatric Cataract. Ophthalmology 123 (2016): 217-220.
- Malekzadeh H, Shakiba M, Yasaei M. A novel mutation in PEX11β gene. Iran J Child Neurol 15 (2021): 93-100.