A Novel Late Effect of Chemotherapy: Fibro-Sclerotic Panniculopathy
Article Information
Carlesimo M MD1, Framarino Dei Malatesta M MD2, Fortuna MC MD1, Caro G MD1*, Pigliacelli F MD1, D’Arino A MD1, Rossi A Prof.1
1Department of Internal Medicine and Medical Specialties, “Sapienza” University of Rome, Italy
2Department of Gynecologic Obstetrics and Urologic Sciences, “Sapienza” University of Rome, Italy
*Corresponding Author: Dr. Gemma Caro, Section of Dermatology, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome, Viale del Policlinico, Rome, Italy
Received: 29 May 2019; Accepted: 06 June 2019; Published: 12 August 2019
Citation: Carlesimo M, Framarino Dei Malatesta M, Fortuna MC, Caro G, Pigliacelli F, D’Arino A, Rossi A. A Novel Late Effect of Chemotherapy: Fibro-Sclerotic Panniculopathy. Archives of Clinical and Medical Case Reports 3 (2019): 158-161.
View / Download Pdf Share at FacebookAbstract
Recently, increasing attention has been paid to late effects of chemotherapy. Even if cardiovascular and nervous toxicity is more often described [1-2], also skin and soft tissues are involved. Herein we reported 2 cases of chemotherapy-induced fibro-sclerotic panniculopathy.
Keywords
Panniculopathy, Chemotherapy, Vascular, Lymphatic
Panniculopathy articles Panniculopathy Research articles Panniculopathy review articles Panniculopathy PubMed articles Panniculopathy PubMed Central articles Panniculopathy 2023 articles Panniculopathy 2024 articles Panniculopathy Scopus articles Panniculopathy impact factor journals Panniculopathy Scopus journals Panniculopathy PubMed journals Panniculopathy medical journals Panniculopathy free journals Panniculopathy best journals Panniculopathy top journals Panniculopathy free medical journals Panniculopathy famous journals Panniculopathy Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals Vascular articles Vascular Research articles Vascular review articles Vascular PubMed articles Vascular PubMed Central articles Vascular 2023 articles Vascular 2024 articles Vascular Scopus articles Vascular impact factor journals Vascular Scopus journals Vascular PubMed journals Vascular medical journals Vascular free journals Vascular best journals Vascular top journals Vascular free medical journals Vascular famous journals Vascular Google Scholar indexed journals health articles health Research articles health review articles health PubMed articles health PubMed Central articles health 2023 articles health 2024 articles health Scopus articles health impact factor journals health Scopus journals health PubMed journals health medical journals health free journals health best journals health top journals health free medical journals health famous journals health Google Scholar indexed journals Lymphatic articles Lymphatic Research articles Lymphatic review articles Lymphatic PubMed articles Lymphatic PubMed Central articles Lymphatic 2023 articles Lymphatic 2024 articles Lymphatic Scopus articles Lymphatic impact factor journals Lymphatic Scopus journals Lymphatic PubMed journals Lymphatic medical journals Lymphatic free journals Lymphatic best journals Lymphatic top journals Lymphatic free medical journals Lymphatic famous journals Lymphatic Google Scholar indexed journals chemotherapy articles chemotherapy Research articles chemotherapy review articles chemotherapy PubMed articles chemotherapy PubMed Central articles chemotherapy 2023 articles chemotherapy 2024 articles chemotherapy Scopus articles chemotherapy impact factor journals chemotherapy Scopus journals chemotherapy PubMed journals chemotherapy medical journals chemotherapy free journals chemotherapy best journals chemotherapy top journals chemotherapy free medical journals chemotherapy famous journals chemotherapy Google Scholar indexed journals cardiovascular articles cardiovascular Research articles cardiovascular review articles cardiovascular PubMed articles cardiovascular PubMed Central articles cardiovascular 2023 articles cardiovascular 2024 articles cardiovascular Scopus articles cardiovascular impact factor journals cardiovascular Scopus journals cardiovascular PubMed journals cardiovascular medical journals cardiovascular free journals cardiovascular best journals cardiovascular top journals cardiovascular free medical journals cardiovascular famous journals cardiovascular Google Scholar indexed journals patient articles patient Research articles patient review articles patient PubMed articles patient PubMed Central articles patient 2023 articles patient 2024 articles patient Scopus articles patient impact factor journals patient Scopus journals patient PubMed journals patient medical journals patient free journals patient best journals patient top journals patient free medical journals patient famous journals patient Google Scholar indexed journals fibrotic tissue articles fibrotic tissue Research articles fibrotic tissue review articles fibrotic tissue PubMed articles fibrotic tissue PubMed Central articles fibrotic tissue 2023 articles fibrotic tissue 2024 articles fibrotic tissue Scopus articles fibrotic tissue impact factor journals fibrotic tissue Scopus journals fibrotic tissue PubMed journals fibrotic tissue medical journals fibrotic tissue free journals fibrotic tissue best journals fibrotic tissue top journals fibrotic tissue free medical journals fibrotic tissue famous journals fibrotic tissue Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals
Article Details
1. Case 1
Case one is a 68 year old woman affected by breast cancer (left breast), treated surgically. Sentinel lymph node was negative. She underwent 4 cycles of epirubicine and cyclophosfamide (January-February 2017), followed by 12 cycles of paclitaxel (March-June 2017). In anamnesis she referred Raynaud phenomenon. The patient presented on April 2017 with asymptomatic non-inflammatory nodules intermixed with raised and depressed areas of her right forearm (Figure 1). The diagnosis of a chemotherapy-induced fibro-sclerotic panniculopathy, grade III according to Nürnberger and Müller classification [3], was made.
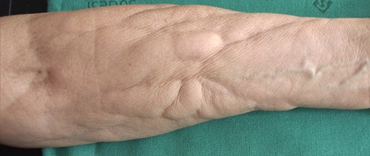
Figure 1: Non-inflammatory nodules intermixed with raised and depressed areas of the patient’s right forearm.
2. Case 2
Case two is a 43 year old woman, affected by breast cancer (left breast) surgically treated in February 2018, followed by 4 cycles of fluoruracile, cyclophosfamide and epirubicine (May-June 2018) and subsequently 12 cycles of paclitaxel (July-November 2018). Anamnesis was negative, except for a Hashimoto’s thyroiditis and a thrombotic phlebitis of her right arm after the first chemotherapy infusion. The patient presented on July 2018 with asymptomatic non-inflammatory nodules intermixed with raised and depressed areas of her right forearm. The diagnosis of a chemotherapy-induced fibro-sclerotic panniculopathy, grade III [3], was made (Figure 2).
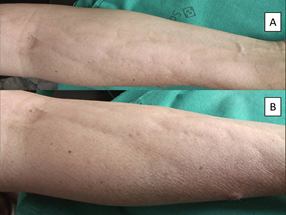
Figure 2: Non-inflammatory nodules intermixed with raised and depressed areas of the patient’s right forearm. A: minor magnification. B: greater magnification.
To our knowledge these are the first cases of fibro-sclerotic panniculopathy in patients receiving chemotherapy. The cases reported above are not characterized by inflammation, and no inflammatory signs, such as erythema or warmth, were present, having a clinical aspect similar to lipodystrophy [3-5]. Even though drug-induced panniculitis have been reported in association to chemotherapy [6-7], our cases do not enter in differential diagnosis with panniculitis, because of the lack of an inflammatory component, and because clinical aspect is completely different.
Curri et al. [5] described the pathogenesis of lipodystrophy asserting that alterations in venous and lymphatic micro-circle leads to a tissutal hypo-oxygenation, and glycosaminoglycan (GAG) deposition in capillary walls with a subsequently stasis of circulation and interstitial accumulation of fluid and catabolites [4-5]. These alterations drive to a cell suffering, with more and more accumulation of toxic molecules, interfering with cell oxygenation and nutrition. As a response, it is possible that, in predisposed patients, pro-fibrotic cytokines are released, eliciting neovascularization and thickening and sclerosis of fibrous septae with the production of collagen fiber, which arrange adipocytes, producing clusters of adipocytes pads [4-5]. Moreover, some gene polymorphisms have been associated with lipodystrophy, validating the role of a subjective predisposition [8].
Standing to this clinical similitude, we hypothesize that also the pathogenesis of our cases could full fit with the one descripted by Curri, with the crucial difference in the causative agent, that in our cases is chemotherapy. It is well known that chemotherapeutic agents are able to induce an endothelium damage with different mechanisms: inducing apoptosis of endothelium cells (cyclophosphamide and 5-FU); through a direct cytotoxic effect (5-FU); producing reactive oxygen species (5-FU and doxorubicin); influencing angiogenesis (taxanes) [9].
It is important to underline that panniculopathy in our patients is contralateral respect to the cancer site, but ipsilateral to the peripherally inserted central catheter (PICC). For this reason, we suggest that its pathogenesis is due to drug accumulation, that could be worsened by inflammatory events such as thrombotic phlebitis in the second case. Even if it is reasonable that the presence of autoimmune traits such as Reynaud’s phenomenon and Hashimoto Thyroiditis may have contributed to cause the fibro-sclerotic panniculopathy after chemotherapy, we do not consider it a determinant factor. Firstly, because these autoimmune conditions are very frequent in women, conversely not so many fibro-sclerotic panniculopathy have been described, and anyway up to date no data is available. Surely a skin biopsy should have been the correct procedure to be carried out, in order to highlight the cellular infiltration or the fibrotic tissue, but both our patients refused it.
3. Conclusion
The one we describe should be considered a late effect of chemotherapy, and a higher attention must be paid, because they are less alarming than acute ones, but they could have severe sequel even so. Moreover, cutaneous involvement could be a sentinel of damage to other organs, which are not so evident but have to be investigated.
4. Declaration of Interest
All the authors disclose any financial, consulting, and personal relationships with other people or organizations that could influence (bias) the author’s work. We also disclose any scientific writing assistance, any grant support and numbers (including NIH/Wellcome-funded papers), and any statements of employment.
References
- Meinardi MT, Gietema JA, van Veldhuisen DJ, et al. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat Rev 26 (2000): 429-447.
- Duffner PK. The long term effects of chemotherapy on the central nervous system. J Biol 5 (2006): 21.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol 4 (1978): 221-229.
- Curri Cellulite and fatty tissue microcirculation. Cosmet. Toilet. 108 (1993): 51-58.
- Curri SB, Bombardelli Local lipodystrophy and districtual micro-circulation. Cosmet. Toilet 109 (1994): 51-65.
- Kismet E, Demirkaya E, Koseoglu V, et al. Panniculitis induced by a chemotherapy regimen consisting of topotecan and cyclophosphamide. Pediatr Blood Cancer 44 (2005): 98-99.
- Maubec E, Oberlin O, Belhadj K, et al. Subcutaneous inflammatory edema induced by MINE chemotherapy. Ann Dermatol Venereol 128 (2001): 534-537.
- Emanuele E, Bertona M, Geroldi D. A multilocus candidate approach identifies ACE and HIF1A as susceptibility genes for cellulite. J Eur Acad Dermatol Venereol 24 (2010): 930-935.
- Svilaas T, Lefrandt JD, Gietema JA, et al. Long-term arterial complications of chemotherapy in patients with cancer. Thromb Res 140 (2016): S109-S118.
