A New Anthropometric Model for Body Composition Estimation in the Assessment of Metabolic Risk Factors of Obese Women
Article Information
Nicolaus Dahlmann1, Dietrich Klingmüller²
1Institut für Klinische Chemie, Universitätsklinikum Schleswig-Holstein, Germany
2Endokrinologie, Diabetologie und Stoffwechselmedizin, Universitätsklinik Bonn, Germany
*Corresponding Author: Dr. N. Dahlmann, Institut für Klinische Chemie, Arnold-Heller-Straße 3, Haus U30, D-24105 Kiel, Germany.
Received: 20 May 2023; Accepted: 29 May 2023; Published: 06 June 2023
Citation: Nicolaus Dahlmann, Dietrich Klingmüller. A new anthropometric model for body composition estimation in the assessment of metabolic risk factors of obese women. Archives of Internal Medicine Research. 6 (2023): 44-54.
View / Download Pdf Share at FacebookAbstract
Background:
Excessive body fat is associated with disorders defined as Metabolic Syndrome (MetS). The study sets out to uncover the underlying mechanisms of MetS pathogenesis comparing metabolic and inflammatory variables with increasing amounts of fat mass. Aiming to solve this issue, the study refers to an anthropometric model named Dahlmann-Body- Analysis (DBA) to define the percentage of fat mass (%FM).
Methods:
A data set of 61 severely obese women were analysed. All subjects had a BMI ≥29.7 kg/m². Body weight (W), body height (Ht), hand circumference (HdC) and the circumference of the abdomen (AC) were measured and processed by the DBA model. The result is the percentage of fat mass (%FM), which is compared to data, produced by a bioelectrical impedance analysis (BIA) device. Anthropometric data are statistically compared with systolic blood pressure (SBP) and the MetS risk factors triglyceride (TG), HDL cholesterol (HDL-C), fasting plasma glucose (FPG) and the parameters C-reactive protein (CRP) and low-density lipoprotein (LDL-C) using receiver operating curves (ROC) based on sensitivity and specificity, area under curve (AUC), correlation coefficients and regression analysis.
Results:
The average %FM was about 50%, meaning that 44% of subjects suffered from MetS. The overall pattern of correlation coefficients revealed that none of the adiposity indices like BMI, AC, AC/Ht and %FM (BIA or DBA) is of crucial advantage to detect metabolic risk factors. AUC values of the different obesity indices detecting MetS reached values between 0.63 and 0.75 representing a low discrimination power in the diagnose of MetS. Associations between body fat mass measured by the DBA system (%FMDBA) and the systolic blood pressure and seven metabolic risk factors showed a significantly rising linear relationship for the parameters Insulin, HOMA-IR, HDL-C and CRP. The corresponding correlation coefficients are r > 0.30. The parameters Glucose, TG, LDL-C and SBP had correlation coefficients r < 0.12.
Conclusions:
To our knowledge, it is the first time that biochemical parameters and blood pressure are associated with increasing amounts of fat mass in human adults. The waist circumference (WC) as part of the MetS definition should be replaced by direct or indirect measurements of body fat estimation to give reliable information on individuals.
Keywords
Body composition analysis; Dahlmann-Body-Analysis (DBA); Body fat mass; Metabolic syndrome; ROC curve analysis
Body composition analysis articles; Dahlmann-Body-Analysis (DBA) articles; Body fat mass articles; Metabolic syndrome articles; ROC curve analysis articles
Article Details
Abbreviations
ABSI: A new Body Shape Index; AC: Abdomen Circumference; ADP: Air-displacement Plethysmography; BIA: Bioelectrical Impedance Analysis; BMI: Body Mass Index; BRI: Body Roundness Index; CRP: C-reactive Protein; CVD: Cardiovascular Disease; CUN-BAE: Clinica Universidad de Navarra-body Adiposity Estimator; DBA: Dahlmann-Body-Analysis; %FM: Percentage Fat Mass; HDL: High-density Lipoprotein; Ht: height; Hd: hand; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; LDL: Low-density Lipoprotein; MetS: Metabolic Syndrome; MHO: Metabolic Health Obesity; Ref-W: Reference Weight; SBP: Systolic Blood Pressure; TG: Triglycerides; W: weight; WC: Waist Circumference
Introduction
The prevalence of obesity has increased globally during the past few decades as an established risk factor for cardiovascular disease[1] and is associated with significantly higher all-cause mortality[2]. Notably, excessive body fat is associated with the occurrence of clinical complications that compromise the quality of life and survival of individuals, based on metabolic disorders[3]. The complex of these metabolic abnormalities is defined as Metabolic Syndrome (MetS), which is a complex disorder defined by a cluster of interconnected factors that increase the risk of hyperglycemia, dyslipidemia and elevated blood pressure, indicating a high-risk condition for type 2 diabetes and cardiovascular disease. The diagnostic criteria have been modified several times. The most widely used definitions are critically discussed with particular focus on waist circumference as a proxy for abdominal obesity[4]. Beside waist circumference, different obesity indices are used to define MetS and it is still an ongoing debate, which anthropometric indices best reflect the risk potential[5-8].
Although research has been carried out in recent decades on MetS, the exact underlying etiology is still not completely understood. Many contributing factors and mechanisms have been proposed, including insulin resistance, adipose tissue dysfunction, chronic inflammation, etc.[9]. For that purpose, the study sets out to uncover the underlying mechanisms of MetS pathogenesis comparing metabolic and inflammatory variables with increasing amounts of fat mass (kg), which is given as percentage of body weight.
Aiming to solve this issue, the study refers to an anthropometric model named Dahlmann-Body-Analysis (DBA) involving simple anthropometric parameters to define a reference weight (Ref-W). It is based on the hand circumference as a proxy for skeleton frame[10,11] and the circumference of the abdomen as a proxy for central obesity[12]. The processed data of the DBA model represent the percentage of fat mass (%FM). The association of biochemical traits with individual fat mass offers the opportunity to identify risk factors rather than to focus on the diagnosis of MetS.
Materials and Methods
Subjects
For this cross-sectional study subjects were recruited in the obesity consultation-hour of the endocrinological department in the University Hospital Bonn, Germany from January 2019 to May 2021. All patients were candidates for bariatric surgery and each of whom had a BMI ≥30 kg/m2 with one exception (29.7 kg/m²). All participants were females of European descendent ranging in age from 18-65 years. Exclusion criteria were pregnancy, oedema, skeletal malformation, and acute diseases (i.e. overt organ failure) and patients with known essential hypertension, diabetes Type I or genetic verified dyslipidemia. All study procedures were performed according to the ethical standards of the World Medical Association’s Declaration of Helsinki, approved by the institutional ethics review board. Written informed consent was obtained from each patient prior to trial participation.
Measurements
Body composition was assessed by single-frequency BIA device (Omron BF511, Kyoto, Japan). Body weight was measured to the nearest ±0,1 kg using the body weight scale of the BIA device with the patient standing in the centre of the scale platform, bare foot, wearing underwear. Body height (Ht) was obtained with a stadiometer (seca, Hamburg, Germany) with the patient standing, barefoot with the heels together, back upright, and arms stretched next to the body. Hand circumference (HdC) was measured by positioning a non-stretchable measuring tape in the horizontal plane over the base joints of the 2nd to 5th finger. The hand should be strained and the thumb splayed. The left hand is chosen for right-handed people, the right hand for left-handed people. The circumference of the abdomen (AC) was measured at the level of the iliac crest passing it along the umbilical level with the patients lying supine. Measurements were taken by fitting the tape snugly without compressing the underlying soft tissue. Readings of all measurements were taken to the nearest mm (±0.1 cm). BMI was calculated as weight divided by height in square meters (kg/m²). AC/Ht is calculated as the abdomen circumference divided by the height (cm/cm). The data of weight, height, HdC and AC are processed by the DBA model, based on a couple of algorithms, which were described in part previously [11,12]. One result is the percentage of fat mass (%FM).
The study was performed in a double-blind form. Measurements were taken in Bonn, send to the first author, processed by the Dahlmann-Body-Analysis (DBA) system and send back.
Metabolic variables
Blood pressure was measured in seated position with a standard manual sphygmomanometer. Blood samples were obtained after overnight-fasting and analysed by standard procedures: C-reactive protein (CRP) was measured by a particle-enhanced immunoturbidimetric assay with a limit of quantitation = 0,6 mg/l (CRP4, cobas c702, Roche Diagnostics). Elevated levels were defined as ≥3,0 mg/l [13,14]. Fasting plasma glucose (FPG) was assayed by hexokinase enzymatic method (GLUC3, cobas c702, Roche Diagnostics) and plasma insulin by electrochemiluminescence immunoassay (ECLIA) (Elecsys Insulin, cobas e801, Roche Diagnostics). After complete hydrolysis, triglycerides (TG) were measured by an enzymatic colorimetric assay (TRIGL, cobas c702). High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured on the basis of a cholesterol enzymatic method (LDL-CC3, HDLC4, cobas c702, Roche Diagnostics), setting the limit for LDL-C to a borderline high level in terms of risk for coronary heart disease as ≥130 mg/dl.
The homeostasis model assessment (HOMA-IR) was used to calculate insulin resistance (IR) according to the equation: HOMA-IR = fasting plasma glucose (mg/dl) x fasting insulin (mU/l)/405. Subjects were classified as having IR if the calculated value was ≥2.61[15].
MetS was defined according to the Joint Scientific Statement with waist circumference as a measure for central obesity being an obligatory component [9,16]. Persons are qualified for metabolic syndrome, if further two abnormal findings out of four are present: (1) high TG concentration, ≥150 mg/dl (≥1.69 mmol/l), (2) low HDL cholesterol: <50 mg/dl (<1.29 mmol/l) in women, (3) high fasting plasma glucose: ≥100 mg/dl (≥5.5 mmol/l) and (4) elevated systolic blood pressure ≥130 mmHg and/or ≥85 mmHg diastolic blood pressure, according to the National Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP-ATP-III) update from 2005[17].
Statistical analyses
The characteristics of the study population were presented as means ± standard deviations (SD) for continuous variables or percentages for categorical variables. Outliers are identified by calculating the trimmed mean on the 5% level and then were checked to be significant on the 5% level by t-test according to the formula (xi-µ)/σ, where µ and σ are replaced by the mean and the standard deviation, respectively. If significant they were taken out. The remaining observations were controlled to be normal distributed by the Jarque-Bera test based on skewness and kurtosis[18]. Non-normalised parameters were logarithmic transformed (CRP, Insulin, HOMA-IR, TG and LDL-C).
Differences between parameters of body composition assessed by different methods were tested by paired samples t-test. Association between the metabolic variables and %FM-DBA were analysed by linear regression analysis. The slope of the regression line was tested to be significant by t-test. Relationships between variables were examined using Pearson`s product-moment correlation coefficients (r). Means of correlation coefficients of each anthropometric index were compared using the Friedman test[19]. The Friedman test is a non-parametric test to repeated measures. Here, it is used to determine whether or not there is a statistical difference between the means of all groups of obesity indices in which the same subjects show up in each group. The Q statistic of the Friedman test gives an answer, whether paired samples (here correlation coefficients) come from the same population. The p-value associated with a given Q-value is approximated by a Chi² distribution.
Observed correlation coefficients were analysed to be different to zero using Fisher t-distribution. Prevalence was calculated according to the border values described above. To examine diagnostic ability of obesity indices for assessment of metabolic risk, we calculated sensitivity and specificity of obesity indices for creation of receiver operating characteristic (ROC) curves. Sensitivity was defined as the percentage of true positives meeting the proportion of individuals, fulfilling the criteria of MetS definition. Specificity is calculated as the number of true-negative test results divided by the number of persons without MetS. It is to emphasize that both parameters depend on the selected cut-off values of the metabolic risk factors, as pointed out in “Metabolic variables”. The corresponding Youden index is calculated as the sum of sensitivity and specificity in percentage points minus 100. Here, the index is weighting false-positive and false-negative test results of blood pressure and metabolic risk factors on the basis of the described cut-off thresholds. The diagnostic odds ratio (DOR) is the ratio of odds of a positive test result in an individual with MetS to the odds of a positive test result in an individual without MetS. A DOR of 1 is uninformative[20]. Values are calculated according to the formula: DOR = (Se/(100-Se))/((100-Sp)/Sp). Based on the same assumption of thresholds, the false-positive test (FP) represents the false-positive test result in a target population defined as MetS-negative.
The area under the ROC curve (AUC) is computed as a single measure of overall accuracy that is not dependent upon a particular threshold. It indicates the probability that a person with MetS has a higher test value than a person without MetS, providing the means to compare the discriminative power of each anthropometric index. AUC values are usually used as criteria to compare overall performance of different screening tests. To compare the overall performance of AUC values we paraphrased the rules of Hosmer and Lemeshow[21], indicating that values between 0.8-0.7 are considered acceptable, values between 0.7-0.6 are considered poor and a value of 0.5 has no discriminative power at all.
Cut-offs of obesity indices to calculate ROC curves were chosen as follows: for BMI in steps of 2 kg/m² in the range of 30-50 kg/m², resulting in 10 steps, for AC in steps of 5 cm in the range of 94 to 147 cm, resulting in 10 steps, for AC/Ht in steps of 0,05 cm/cm in the range of 0,5-0,9 cm/cm, resulting in 7 steps, for %FM-DBA and %FM-BIA in steps of 3% in the range of 35-60 %, resulting in 7 steps each.
All statistics were performed in Excel (Office 2019, Microsoft Corporation, USA). Tests not available in Excel were calculated by hand. A p-value < 0.05 was considered statistically significant.
Results
A data set of 63 severely obese Europid women were analysed and a group of 17 were identified as outliers by trimmed means on the 5% level. Of these, 10 proved to be significant. A set of two subjects with FPG values >230 mg/dl were completely taken out (n=61). Two had CRP values >24 mg/dl and were excluded, three data of CRP were missing (n=56). TG, LDL and HDL each had two values >310 mg/dl, >240 mg/dl, >100 mg/dl, respectively and were excluded (n=59). The remaining cases are summarized in Table 1.
|
n |
Mean |
±SD |
Median |
Min |
Max |
|
|
Age (years) |
61 |
41.6 |
12.1 |
42 |
18 |
65 |
|
Height (cm) |
61 |
166.5 |
6.3 |
166.1 |
153.7 |
179.2 |
|
Weight (kg) |
61 |
108.1 |
16.5 |
105.4 |
80.6 |
147 |
|
HdC (cm) |
61 |
19.4 |
1 |
19.5 |
16.5 |
21.4 |
|
AC (cm) |
61 |
118.3 |
12.2 |
118.7 |
94 |
147 |
|
AC/Ht (cm/cm) |
61 |
0.71 |
0.07 |
0.71 |
0.55 |
0.89 |
|
BMI (kg/m²) |
61 |
38.9 |
4.9 |
38.2 |
29.7 |
51.7 |
|
%FM-BIA (%) |
61 |
49.8 |
4.5 |
49.6 |
36.9 |
59.2 |
|
%FM-DBA (%) |
61 |
49.1 |
4.8 |
49.4 |
35.9 |
57.9 |
|
Glucose (mg/dl) |
61 |
96.4 |
14.5 |
93 |
76 |
149 |
|
Insulin (mU/l) |
61 |
19.1 |
9.8 |
18.2 |
3.8 |
56.9 |
|
HOMA-IR (mU/l, mg/dl) |
61 |
4.6 |
2.8 |
3.9 |
0.7 |
18.6 |
|
TG (mg/dl) |
59 |
141.7 |
55.9 |
134 |
43 |
286 |
|
HDL-C (mg/dl) |
59 |
52.6 |
13.1 |
51 |
32 |
85 |
|
LDL-C (mg/dl) |
59 |
126.7 |
25.9 |
127 |
76 |
190 |
|
CRP (mg/l) |
56 |
5.9 |
4.1 |
4.6 |
0.9 |
19 |
|
SBP (mmHg) |
61 |
131.4 |
17.2 |
130 |
100 |
179 |
AC: Abdomen circumference; BIA: Bioimpedance analysis; BMI: Body mass index; CRP: C-reactive protein; DBA: Dahlmann-Body-Analysis; HdC: Hand circumference; Ht: Height; %FM: Percentage fat mass; HDL: High-density lipoproteins; HOMA-IR: Homeostasis model assessment of insulin resistance; LDL: Low-density lipoproteins; TG: Triglycerides; SBP: Systolic blood pressure
Table 1: Anthropometric and metabolic risk factors of obese German women
Age ranged between 18 and 65 years. All subjects had a BMI ≥29.7 kg/m², an abdomen circumference ≥ 94 cm and a relationship AC/Ht ≥0.55 cm/cm. The mean of %FM was about 50%, measured by the two methods BIA and DBA, respectively. With the exception of Insulin, HOMA-IR, CRP and SBP the mean values of all the other metabolic risk factors are lying within the recommended normal range. The HOMA-IR value 4.6 is in the same order of magnitude (4.7) compared to a Brazilian study of obese women (BMI = 40.5 kg/m²) [22].
We furthermore performed correlation analyses to elucidate associations between obesity indices and age. The results are presented in Table 2.
|
Age |
Height |
Weight |
HdC |
AC |
AC/Ht |
BMI |
%FM-BIA |
%FM-DBA |
|
|
Age |
1 |
||||||||
|
Height |
-0.17 |
1 |
|||||||
|
Weight |
-0.13 |
0.56 |
1 |
||||||
|
HdC |
0.12 |
0.23 |
0.36 |
1 |
|||||
|
AC |
-0.05 |
0.15 |
0.7 |
0.39 |
1 |
||||
|
AC/Ht |
0.02 |
-0.21 |
0.49 |
0.3 |
0.93 |
1 |
|||
|
BMI |
-0.06 |
0.07 |
0.86 |
0.28 |
0.75 |
0.71 |
1 |
||
|
%FM-BIA |
-0.28 |
0.05 |
0.72 |
0.14 |
0.7 |
0.67 |
0.83 |
1 |
|
|
%FM-DBA |
-0.13 |
0.11 |
0.82 |
0.12 |
0.74 |
0.69 |
0.91 |
0.85 |
1 |
AC: Abdomen circumference; BMI: Body mass index; BIA: Bioimpedance analysis; DBA: Dahlmann-Body-Analysis; HdC: Hand circumference; Ht: Height; %FM: Percentage fat mass
Table 2: Results of the correlation analyses between obesity indices and age
The correlation coefficients between age and %FM are negative for the BIA as well as the DBA measurement, indicating that younger women are more obese than older ones. All obesity indices were highly correlated with each other. The correlations between BMI and the indices of central adiposity AC (r=0.75) and AC/Ht (r=0.71) were lower than the one between BMI and the general adiposity marker (%FM). The highest correlation coefficient was found between BMI and %FM-DBA (r=0.91). Fat mass measurement, either performed by a BIA device or the DBA model, showed an excellent agreement expressed by a correlation coefficient of 0.85 as a sign that both methods are equal to calculate the %FM.
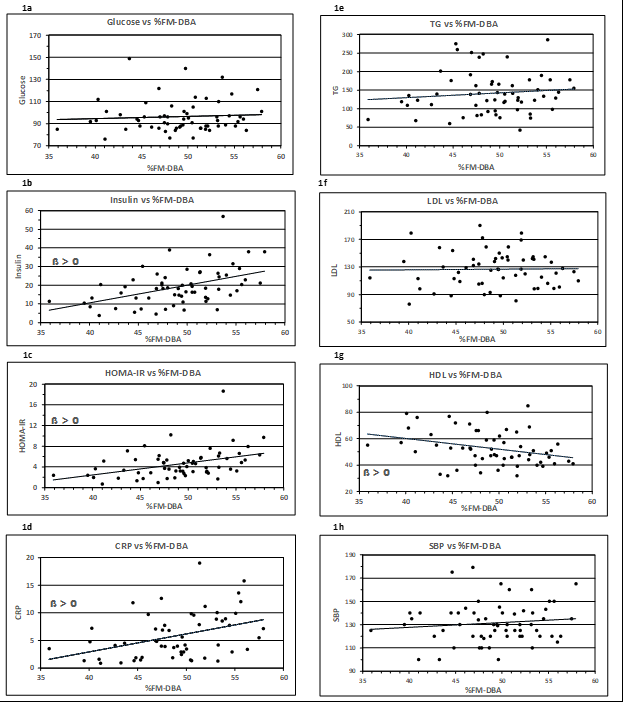
Associations between body fat mass measured by the DBA system (%FM-DBA) and the systolic blood pressure and seven metabolic risk factors are plotted in Figs. 1a – 1h.
Figure 1: Association of metabolic risk factors with %FM detected by the DBA method. Insulin, HOMA-IR, HDL-C and CRP correlated positively with %FM-DBA (1b-d, 1g). In contrast, Glucose, TG, LDL and SBP showed no significant correlation (1a, 1e-f, 1h). The corresponding regression equations, R², r and p values are displayed in Table 3. Trend lines were calculated as linear regressions. ß > 0, slope is significant to zero
The graphical representation shows a linear relationship. The equations of the corresponding regression lines are given in Table 3. The indicated regression coefficient represents the slope ß, which is a measure of the contribution of body fat volume toward the depending metabolic variables. A positive relationship is represented by a rising (ß > 0) or falling (ß < 0) regression line. The t-test (null hypothesis ß = 0) revealed significant rising slopes for the parameters Insulin, HOMA-IR, and CRP and HDL-C showing an inverse relation. The corresponding correlation coefficients are all I > 0,30 I. The traits Glucose, TG, LDL-C and SBP have correlation coefficients r < 0.12.
|
Metabolic Parameters |
Linear Regr. Equation |
R² |
r |
Slope |
p-value |
|
Glucose (mg/dl) |
y = 0,20x + 86,4 |
0,005 |
0,07 |
ß |
0,604 |
|
Insulin (mU/l) |
y = 0,95x - 27,5 |
0,217 |
0,47 |
ß* |
0,001 |
|
HOMA-IR (mU/l, mg/dl) |
y = 0,24x - 6,9 |
0,162 |
0,40 |
ß* |
0,001 |
|
TG (mg/dl) |
y = 1,20x + 79,6 |
0,012 |
0,11 |
ß |
0,411 |
|
HDL-C (mg/dl) |
y = -0,82x + 92,8 |
0,094 |
-0,31 |
ß* |
0,018 |
|
LDL (mg/dl) |
y = 0,08x + 122,7 |
0,000 |
0,02 |
ß |
0,909 |
|
CRP (mg/l) |
y = 0,33x - 10,1 |
0,153 |
0,39 |
ß* |
0,003 |
|
SBP (mmHg) |
y = 0,42x + 111,1 |
0,013 |
0,12 |
ß |
0,376 |
* a p-value < 0,05 was considered significant
Table 3: Regression analysis of metabolic parameters vs. %FM-DBA
All of the study participants were obese, independent of the obesity index measured (Table 4). Consequently, there was an alarming high prevalence of morbidity given that more than 80% of subjects were insulin resistant, about 40% had dyslipidemia, three quarters showed signs of inflammation and nearly half of them (44.3%) suffered from MetS (Table 4). Comparing subjects with MetS to subjects without MetS, there were no significant differences in age and obesity indices except AC and AC/Ht, who were different on a weak significant level. Mean values of FPG, TG, HDL-C and SBP were significantly impaired compared to subjects without MetS.
|
All |
Prevalence |
MetS |
Without-MetS |
|||||||
|
n |
n |
% |
n |
mean |
±SD |
n |
mean |
±SD |
p |
|
|
Age, years |
27 |
42.2 |
11.6 |
34 |
41.1 |
12.6 |
||||
|
AC, >88 cm |
61 |
61 |
100 |
27 |
122.4 |
13.1 |
34 |
115 |
10.7 |
* |
|
AC/Ht, ≥0,5 cm/cm |
61 |
61 |
100 |
27 |
0.74 |
0.08 |
34 |
0.69 |
0.06 |
* |
|
BMI, ≥30 kg/m² |
61 |
61 |
100 |
27 |
40.1 |
5.8 |
34 |
38 |
4.1 |
|
|
%FM-BIA, ≥30% |
61 |
61 |
100 |
27 |
50.7 |
4.5 |
34 |
48.9 |
4.4 |
|
|
%FM-DBA, ≥30% |
61 |
61 |
100 |
27 |
49.8 |
4.7 |
34 |
48.5 |
4.9 |
|
|
Glucose ≥100 mg/dl |
61 |
16 |
26.2 |
27 |
104.6 |
15.6 |
34 |
89.9 |
9.5 |
* |
|
Insulin ≥25 (mU/l) |
61 |
14 |
23 |
27 |
21.6 |
8 |
34 |
17.2 |
10.8 |
|
|
HOMA-IR ≥2,61 |
61 |
49 |
80.3 |
27 |
5.5 |
2.1 |
34 |
3.9 |
3.1 |
|
|
TG, ≥150 (mg/dl) |
59 |
21 |
35.6 |
26 |
177.5 |
51.3 |
33 |
113.5 |
38.6 |
* |
|
HDL-C < 50 (mg/dl) |
59 |
28 |
47.5 |
27 |
47.1 |
11.4 |
33 |
57.3 |
12.7 |
* |
|
LDL-C ≥130 mg/dl |
59 |
26 |
44.1 |
25 |
126.7 |
25.9 |
34 |
126.7 |
26.2 |
|
|
CRP ≥3,0 (mg/l) |
56 |
39 |
69.6 |
27 |
6.4 |
3.6 |
29 |
5.3 |
4.5 |
|
|
SBP ≥130 (mmHg) |
61 |
33 |
54.1 |
27 |
140.7 |
14.9 |
34 |
124.1 |
15.2 |
* |
|
MetS |
61 |
27 |
44.3 |
|||||||
AC: Abdomen circumference; BIA; Bioimpedance analysis; BMI: Body mass index DBA: Dahlmann-Body-Analysis; CRP, C-reactive protein; HC, Hand circumference; Ht, Height; HOMA-IR. Homeostasis model assessment of insulin resistance; HDL, High-density lipoproteins; LDL: Low-density lipoproteins; %FM: Percentage fat mass; TG: Triglycerides; SBP: Systolic blood pressure. * p > 0.05
Table 4: Prevalence and descriptive characteristics of metabolic risk factors in the study
population with and without MetS
The associations between different obesity indices and the logarithmically transformed metabolic risk factors are shown in Table 5 as a matrix of absolute values of correlation coefficients. The HOMA variable is excluded as a non-independent variable calculated out of Glucose and Insulin. The correlation coefficients were tested to be different to zero as a proof of a significant relationship. This holds true for all obesity indices and the parameters Insulin, HDL-C and CRP. There are two exceptions, namely the relationship TG vs. AC/Ht and SBP vs. AC. The overall pattern of correlation coefficients reveals that none of the adiposity indices is of crucial advantage to detect metabolic risk factors. Notably, BMI and the %FM measurements (BIA and DBA) spread out a homogeneous picture. This impression is confirmed by the Friedman test. The test statistic is Q = 1.60 and the corresponding p-value is p = 0.81 and with that exceeds the critical level of 0.05 by far. The result gives sufficient evidence to conclude that there is no significant difference between the means of all obesity indices calculated out of all metabolic parameters.
|
BMI |
AC |
AC/Ht |
%FM-BIA |
%FM-DBA |
|
|
CRP, log |
*0.41 |
*0.45 |
*0.41 |
*0.51 |
*0.41 |
|
Glucose |
0.1 |
0.18 |
0.26 |
0.07 |
0.07 |
|
Insulin, log |
*0.44 |
*0.35 |
*0.33 |
*0.48 |
*0.48 |
|
TG, log |
0.13 |
0.24 |
*0.34 |
0.19 |
0.14 |
|
HDL |
*-0.43 |
*-0.37 |
*-0.44 |
*-0.36 |
*-0.31 |
|
LDL, log |
0 |
-0.04 |
0.01 |
-0.07 |
0.04 |
|
SBP |
0.17 |
*0.27 |
0.25 |
0.18 |
0.12 |
|
Mean |
0.12 |
0.15 |
0.17 |
0.14 |
0.14 |
AC: Abdomen circumference; BMI: Body mass index; BIA, Bioimpedance analysis; DBA, Dahlmann-Body-Analysis; Ht, Height; %FM: Percentage fat mass; log: Values are logarithmic transformed; * p < 0.05
Table 5: Pearson correlation coefficients between anthropometric and metabolic risk factors, tested different to zero
The sensitivity, specificity, Youden index and the diagnostic odds ratio (DOR) of the systolic blood pressure and the metabolic risk factors to identify those classified as MetS positive are presented in Table 6.
|
Classsification based on: |
Sensitivity (%) |
Specificity (%) |
Youden-Index (%) |
DOR |
|
Glucose ≥100 (mg/dl) |
51.9 |
94.1 |
46 |
17.2 |
|
Insulin ≥25 (mU/l) |
33.3 |
85.3 |
18.6 |
2.9 |
|
HOMA-IR ≥2,61 |
92.6 |
29.4 |
22 |
5.2 |
|
TG ≥150 (mg/dl) |
69.2 |
90.9 |
60.1 |
22.5 |
|
HDL-C < 50 (mg/dl) |
77.8 |
78.1 |
55.9 |
12.5 |
|
LDL-C ≥130 mg/dl |
48 |
58.8 |
6.8 |
1.3 |
|
CRP ≥3,0 (mg/l) |
77.8 |
37.9 |
15.7 |
2.1 |
|
SBP ≥130 (mmHg) |
81.5 |
67.6 |
49.1 |
9.2 |
DOR: Diagnostic odds ratio
Table 6: The sensitivity, specificity, Youden-Index and the diagnostic odds ratio of metabolic risk factors classifying individuals as MetS positive
HOMA-IR showed good sensitivity but poor specificity resulting in a Youden Index of 22.0%. The Youden Index of Glucose, TG, HDL and SBP were all above 45% (printed in bold). This corresponds to DOR values about 9 and greater, meaning that the chance of a positive result in individuals with MetS is 10 times greater than in individuals without MetS. The values of all other risk factors like Insulin, LDL-C and CRP were below 22% corresponding to DOR values <5.2.
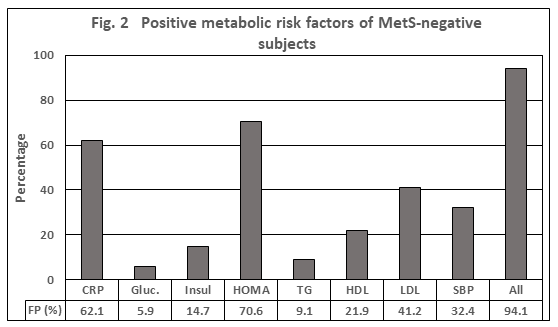
Based on the same assumption of thresholds, the false-positive tests (FP) represent the false-positive test results in a cohort of individuals defined as MetS-negative. As depicted in Figure 2, almost 95% of MetS-negative subjects had a positive test result of at least one parameter, in the average 1.9. The candidates with the highest values >40% were CRP, HOMA and LDL-C, which are the parameters not being involved in the definition of MetS. Compared to results based on the MetS definition that is, based on the traits Glucose, TG, HDL, and SBP, the FP-rate was still 67.7% with an average of 1.0 parameter.
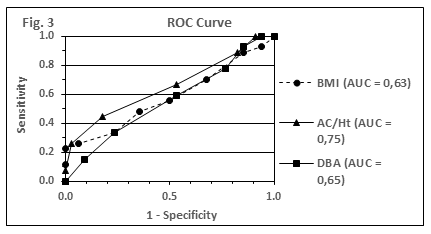
In Figure 3, the accuracy of adiposity indices with respect to the prediction of ≥2 component traits of MetS (elevated blood pressure, TG or glucose level) is compared by using plots of receiver operating curves (ROC). AUC values for the obesity indices BMI, AC/Ht and %FM-DBA are shown (0.63; 0.75; 0.65). The AUC values for AC and %FM-BIA were calculated as 0.70 for both parameters each.
AUC values of the different obesity indices were all in a similar range and reached values between 0.63 and 0.75. Furthermore, with the exception of AC/Ht (AUC = 0.75), all given AUC values fall below the discrimination level of 0.7
Discussion
The present study reports on the relationship of increasing amounts of overweight, estimated by different anthropometric indices in a cohort of hundred percent obese individuals and is focused on the investigative work into the proximal origins of the MetS. One main finding of our study is that %FM, either measured by the DBA system or a BIA device, had no advantage over indirect indices of obesity (BMI, AC, AC/Ht) in the assessment of obesity-related metabolic risk, at least at the population level and with that the study comes to the same result as shown before[25]. The significant difference of AC and AC/Ht between MetS positive and negative populations is not a contradiction as they are different by definition. This observation is in line with previous studies [23,24]. This holds true, independent of the inaccuracies of impedance measurements, since similar results were produced by the use of densitometry (ADP) for body composition analysis[25] or by underwater-weighing to assess fat mass, adding little additional information to BMI with respect to cardiovascular disease risk factors in females[26].
Comparing the accuracy of MetS prediction by %FM (DBA or BIA), BMI, AC, or AC/Ht, the analysis of ROC curves revealed similar AUC values for different obesity indices lying in a range between 0.63 and 0.75, suggesting an equivalent value of methods. Measurements taken from Han Chinese confirm these findings. The pairwise comparison indicated that the differences between the AUCs for waist circumference, waist-to-height ratio, and BMI - all lying below a value of 0.75 - were not statistically different for a comparable group of women [27,28]. Furthermore, the values are so low that they are considered to deliver no sustainable information. This is the second result, which agrees with the data of Bosy-Westphal [25] and two other studies, including the indices ABSI, BRI and CUN-BAE [7, 29]. Of all indices, AC/Ht was the main predictor of metabolic risk, a result which is conform to our study. However, at the end, they were all equivalent in their non-ability to predict MetS and no AUC analysis exceeded a value ≥0.75 in women, which is at the low end of acceptable discrimination[21]. These data, derived from Europid descendants, match with female Malay, Chinese and Indian results not exceeding AUC values ≥ 0.7 for the anthropometric indices BMI, waist circumference and waist-to-hip ratio, respectively[28]. In addition, the results are consistent with findings that waist indices do not perform better than BMI in the prediction of hypertension with AUCs lying in a range of 0.64 – 0.72[30]. The interpretation of AUC values according to the Hosmer-Lemeshow classification found entrance in clinical studies like the classification of breast lesions as benign versus malignant[31] and the association of adiposity with the risk of death[32].
Taken together, these results suggest that, at least at the basis of mass statistic, none of the discussed anthropometric indices was of superior diagnostic power compared with each other and was able to contribute significant information to the diagnosis of MetS. Furthermore, the data show that MetS is a composite of individual component traits, where each trait is differently associated with obesity indices. However, this result does not challenge the issue that the knowledge of body fat at the level of individuals is of minor interest.
To give an answer to this question, we compared the metabolic risk factors SBP, Glucose, TG and HDL-C and, in addition, the biochemical parameters Insulin, LDL-C and CRP, respectively, with increasing amounts of fat mass, expressed in percent. Insulin was measured as part of the HOMA index. CRP was included to the investigation as recent studies have shown that CRP is elevated in subjects with MetS and predicts the development of MetS[33]. LDL-C is part of the study as the primary driver of atherogenesis and the endpoint of MetS development leading to a transformation of macrophages to an inflammatory phenotype[34].
To our knowledge, it is the first time that biochemical parameters and blood pressure are associated with increasing amounts of fat mass in human adults. The third result is that a significant relationship could be proven for Insulin, HOMA-IR, HDL-C and CRP. These results are in accordance with data showing HOMA-IR and CRP increasing monotonously against the number of MetS components and HDL-C as a variable that contributes most to the level of CRP [33]. Elevated CRP levels reflect the macrophage activity, either in adipose tissue[35] or in the intima of arterial vessels [36] as an expression of an obesity induced low-grade inflammatory status.
In contrast to these results, the variables Glucose, TG, LDL-C and SBP had no significant association to the increasing amount of body fat, indicating that the atherosclerotic process is not yet as severe to result in an elevated blood pressure. Obviously, they don’t represent the patho-biochemical process, at least at this point of time axis as the metabolic health is a dynamic and continuous process with a metabolic deterioration over time[37]. Taken together, the data enrol the picture of a group that is, despite the seriousness of obesity, still in the state of a prediabetes. This interpretation is in conflict with the results of Table 6. The highest diagnostic values for MetS detection, calculated as Youden Index or DOR, are found for the traits Glucose, TG, HDL-C and SBP. But one has to keep in mind that these are exactly the parameters reflecting the MetS definition.
Usually, the false-positive rate is of minor interest. Here, it may contribute to a long-lasting, still open debate based on observations that a proportion of individuals with obesity has a significantly lower risk for cardiometabolic abnormalities. This led to the concept of metabolically healthy obesity (MHO). The actual harmonized definition of MHO is based on obesity (BMI ≥30 kg/m²), Glucose, TG, HDL-C and SBP with thresholds to be identical with MetS definition (for review see [38]). For the present cohort of obese MetS-negative individuals our data reveal a FP-rate of about 68% which ramps up to about 95% if the parameters Insulin, CRP and LDL are included into consideration. This leads to the conclusion that the concept of MHO is not supported by our data and adds to the evidence base that the majority of obese are metabolically not healthy.
As the whole patho-biochemical process takes decades from a prediabetic status to T2DM, atherosclerosis and cardiovascular disease, the MetS definition should be thought over with regard to a damage score including the variables Insulin, LDL-C and CRP to identify patients at risk. In any case, the field methods for body composition analysis, notably BMI[39] and AC, are of low advantage as an index of obesity related health risk. As a surrogate for abdominal obesity they lag behind direct assessment of adiposity and should be replaced by direct or indirect measurements of body fat estimation like DXA, ADP, BIA devices or the DBA model to give reliable information on individuals.
Study weakness includes possible limited generalization of the results to populations other than those included in this study. Future research, notably in men, should involve individuals with a wider weight range and other ethnicities.
Conclusion
To our knowledge, it is the first time that a linear relationship between biochemical parameters and blood pressure is associated with increasing amounts of fat mass in human adults. Significant relationship could be proven for Insulin, HOMA-IR, HDL-C and CRP. The MetS definition should be thought over with regard to a damage score including the variables Insulin, LDL-C and CRP. In any case, the waist circumference as part of the MetS definition is a surrogate for abdominal obesity and should be replaced by direct or indirect measurements of body fat estimation like DXA, BIA devices, or the DBA model to give reliable information on individuals.
Declarations
Acknowledgements
The authors are grateful to Dr. Vera Demond for assistance in anthropometric measurements and bioelectrical impedance analysis and Dr. A. Mayr, University Bonn, for discussion and support concerning statistical questions. Unconditional participation of patients and nurses is very much appreciated.
Authors ’contributions
ND is responsible for the conception and design of the study, drafted the manuscript and performed statistical analyses, data analysis and interpretation. DK is responsible for the conception and design of the study and the measurements on patients. All authors were engaged in interpretation and discussion of data and read and approved the final manuscript.
Funding
This research received no external funding.
Availability of data and materials
The data sets used during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All study procedures were performed according to the ethical standards of the World Medical Association’s Declaration of Helsinki and were approved by the medical ethics committee of the Rheinische-Friedrich-Wilhelm’s-Universität, Bonn, Germany (197/19). Written informed consent was obtained from each patient prior to trial participation.
Consent for publication
Not applicable.
Competing interests
ND is the owner of the website www.dahlmann-body-analysis.de
For scientific work, the system is free of charge.
References
- Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 9 (2009): 88.
- Flegal KM, Kit BK, Orpana H, et al. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories. JAMA 309 (2013): 71-82.
- Bastien M, Poirier P, Lemieux I, et al. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 56 (2004): 369-381.
- Kassi E, Pervanidou P, Kaltsas G, et al. Metabolic syndrome: definitions and controversies. BMC Med. 9 (2011): 48.
- Tseng CH. Waist-to-height Ratio and Coronary Artery Disease in Taiwanese Type 2 Diabetic Patients. Obesity 16 (2008): 2754-2759.
- He W, Li Q, Yang M, et al. Lower BMI cutoffs to define overweight and obesity in China. Obes Silver Spring Md 23 (2015): 684-691.
- Suliga E, Ciesla E, Gluszek-Osuch M, et al. The Usefulness of Anthropometric Indices to Identify the Risk of Metabolic Syndrome. Nutrients 11 (2019): 2598.
- Kim KY, Moon HR, Yun JM. Neck Circumference as a Predictor of Metabolic Syndrome in Koreans: A Cross-Sectional Study. Nutrients 13 (2021): 3029.
- Xu H, Li X, Adams H, et al. Etiology of Metabolic Syndrome and Dietary Intervention. Int J Mol Sci 20 (2019): 128.
- Dahlmann N, Schlegel W, Hölzer KH, et al. A simple method of determining the ideal weight 68 (1977): 265-74.
- Dahlmann N, Klingmüller D. The skeleton frame as a crucial part of body weight: Comparison to Metropolitan Life Insurance data. Intern J Sci Res 12 (2023): 369-376.
- Dahlmann N, Demond V. A new anthropometric model for body composition estimation: Comparison with a bioelectrical impedance consumer device. PLOS ONE 17 (2022): e0271880.
- Ajani UA, Ford ES, Mokdad AH. Prevalence of high C-reactive protein in persons with serum lipid concentrations within recommended values. Clin Chem 50 (2004): 1618-1622.
- Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem 43 (1997): 52-58.
- Matthews DR, Hosker JR, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and ß-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28 (1985): 412-419.
- Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome - a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23 (2006): 469-480.
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 112 (2005): 2735-2752.
- Jarque CM, Bera AK. Efficient tests for normality, homoscedasticity and serial independence of regression residuals. Econ Lett 6 (1980): 255-259.
- Friedman M. The Use of Ranks to Avoid the Assumption of Normality Implicit in the Analysis of Variance. J Am Stat Assoc 32 (1937): 675-701.
- Hoyer A, Zapf A. Studies for the Evaluation of Diagnostic Tests–Part 28 of a Series on Evaluation of Scientific Publications. Dtsch Arzteblatt Int 118 (2021): 555-560.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. Bd. 2nd ed. John Wiley & Sons; (2000): p156-164.
- Jamar G, Almeida FR de, Gagliardi A, et al. Evaluation of waist-to-height ratio as a predictor of insulin resistance in non-diabetic obese individuals. A cross-sectional study. Sao Paulo Med J Rev 135 (2017): 462-468.
- Tian S, Zhang X, Xu Y, et al. Feasibility of body roundness index for identifying a clustering of cardiometabolic abnormalities compared to BMI, waist circumference and other anthropometric indices. The China Health and Nutrition Survey, 2008 to 2009. Medicine (Baltimore) 95 (2016): e4642.
- Tulloch-Reid MK, Williams DE, Looker HC, Hanson RL, Knowler WC. Do measures of body fat distribution provide information on the risk of type 2 diabetes in addition to measures of general obesity? Comparison of anthropometric predictors of type 2 diabetes in Pima Indians. Diabetes Care 26 (2003): 2556–2561.
- Bosy-Westphal A, Geisler C, Onur S, et al. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes 30 (2006): 475-483.
- Tanaka S, Togashi K, Rankinen T, et al. Is adiposity at normal body weight relevant for cardiovascular disease risk? Int J Obes Relat Metab Disord 26 (2002): 176-183.
- Wang Jin-wen, Hu Da-yi, Sun Yi-hong, Wang Jia-hong, Wang G-lian, Xie J, et al. Obesity criteria for identifying metabolic risks. Asia Pac J Clin Nutr 18 (2009): 105-113.
- Liu Y, Tong G, Tong W, et al. Can body mass index, waist circumference, waist-hip ratio and waist-height ratio predict the presence of multiple metabolic risk factors in Chinese subjects? BMC Public Health 11 (2011): 35.
- Gluszek S, Ciesla E, Gluszek-Osuch M, et al. Anthropometric indices and cut-off points in the diagnosis of metabolic disorders 15 (2020): e0235121.
- Tuan NT, Adair LS, Stevens J, et al. Prediction of hypertension by different anthropometric indices in adults: the change in estimate approach. Public Health Nutr 13 (2010): 639–646.
- El Khouli RH, Macura KJ, Barker PB, et al. The Relationship of Temporal Resolution to Diagnostic Performance for Dynamic Contrast Enhanced (DCE) MRI of the Breast. J Magn Reson Imaging 30 (2009): 999-1004.
- Pischon T, Boeing H, Hoffmann K, et al. General and Abdominal Adiposity and Risk of Death in Europe. N Engl J Med 359 (2008): 2105-2120.
- Nakamura H, Ito H, Egami Y, et al. Waist circumference is the main determinant of elevated C-reactive protein in metabolic syndrome. Diabetes Res Clin Pract 79 (2008): 330-336.
- Borén J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 41 (2020): 2313-2330.
- Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112 (2003): 1796-1808.
- Libby P. Inflammation in atherosclerosis. Nature 420 (2002): 868-874.
- Elías-López D, Vargas-Vázquez A, Mehta R, et al. Natural course of metabolically healthy phenotype and risk of developing Cardiometabolic diseases: a three years follow-up study. BMC Endocr Disord 21 (2021): 85.
- Blüher M. Metabolically Healthy Obesity. Endocr Rev 41 (2020): bnaa004.
- Piers LS, Rowley KG, Soares MJ, et al. Relation of adiposity and body fat distribution to body mass index in Australians of Aboriginal and European ancestry. Eur J Clin Nutr 57 (2003): 956-963.