A Mini Review on Different Methods of Functional-MRI Data Analysis
Article Information
Karunanithi Rajamanickam*
Department of Biotechnology, Faculty of Allied Health Sciences, Chettinad Academy of Research and Education, Tamil Nadu, India
*Corresponding author: Karunanithi Rajamanickam, Department of Biotechnology, Faculty of Allied Health Sciences, Chettinad Academy of Research and Education, Kelambakkam, Chennai- 603103, Tamil Nadu, India
Received: 24 December 2019; Accepted: 09 January 2020; Published: 15 January 2020
Citation:
Karunanithi Rajamanickam. A Mini Review on Different Methods of Functional-MRI Data Analysis. Archives of Internal Medicine Research 3 (2020): 044-060.
View / Download Pdf Share at FacebookAbstract
Physiological changes due to blood oxygen level dependent (BOLD) signals from the brain can be probed by functional MRI (fMRI). Especially, several resting state fMRI (rs-fMRI) studies have evidenced the alterations in the default mode network (DMN), which is a fundamental network among the resting state networks (RSNs) with respect to progressing diminished brain function due to various disease conditions such as Alzheimer’s disease (AD). Recently, there are several techniques developed to analyze the rs-fMRI data such as voxel based morphometry (VBM), i.e., seed based analysis, independent component analysis (ICA), clustering algorithm, graph method, neural networks, pattern classification method and statistical parametric mapping. Though these techniques are promising, its application on routine clinical practice is not yet developed. However, it may play a vital role in future for diagnostic and prognosticating various dementia conditions. In this review, fundamentals of rs-fMRI, different data analysis techniques such as seed-based, independent component analysis and graph theory analysis, regional homogeneity analysis and amplitudes of low-frequency fluctuations of the rs-fMRI BOLD signal are discussed.
Keywords
Resting State fMRI, Seed-Based Analysis, Independent Component Analysis, Graph-Theory; Regional Homogeneity (ReHo), Amplitude Of Low-Frequency Fluctuation
Resting State fMRI articles Resting State fMRI Research articles Resting State fMRI review articles Resting State fMRI PubMed articles Resting State fMRI PubMed Central articles Resting State fMRI 2023 articles Resting State fMRI 2024 articles Resting State fMRI Scopus articles Resting State fMRI impact factor journals Resting State fMRI Scopus journals Resting State fMRI PubMed journals Resting State fMRI medical journals Resting State fMRI free journals Resting State fMRI best journals Resting State fMRI top journals Resting State fMRI free medical journals Resting State fMRI famous journals Resting State fMRI Google Scholar indexed journals Seed-Based Analysis articles Seed-Based Analysis Research articles Seed-Based Analysis review articles Seed-Based Analysis PubMed articles Seed-Based Analysis PubMed Central articles Seed-Based Analysis 2023 articles Seed-Based Analysis 2024 articles Seed-Based Analysis Scopus articles Seed-Based Analysis impact factor journals Seed-Based Analysis Scopus journals Seed-Based Analysis PubMed journals Seed-Based Analysis medical journals Seed-Based Analysis free journals Seed-Based Analysis best journals Seed-Based Analysis top journals Seed-Based Analysis free medical journals Seed-Based Analysis famous journals Seed-Based Analysis Google Scholar indexed journals Independent Component Analysis articles Independent Component Analysis Research articles Independent Component Analysis review articles Independent Component Analysis PubMed articles Independent Component Analysis PubMed Central articles Independent Component Analysis 2023 articles Independent Component Analysis 2024 articles Independent Component Analysis Scopus articles Independent Component Analysis impact factor journals Independent Component Analysis Scopus journals Independent Component Analysis PubMed journals Independent Component Analysis medical journals Independent Component Analysis free journals Independent Component Analysis best journals Independent Component Analysis top journals Independent Component Analysis free medical journals Independent Component Analysis famous journals Independent Component Analysis Google Scholar indexed journals Graph-Theory articles Graph-Theory Research articles Graph-Theory review articles Graph-Theory PubMed articles Graph-Theory PubMed Central articles Graph-Theory 2023 articles Graph-Theory 2024 articles Graph-Theory Scopus articles Graph-Theory impact factor journals Graph-Theory Scopus journals Graph-Theory PubMed journals Graph-Theory medical journals Graph-Theory free journals Graph-Theory best journals Graph-Theory top journals Graph-Theory free medical journals Graph-Theory famous journals Graph-Theory Google Scholar indexed journals Regional Homogeneity (ReHo) articles Regional Homogeneity (ReHo) Research articles Regional Homogeneity (ReHo) review articles Regional Homogeneity (ReHo) PubMed articles Regional Homogeneity (ReHo) PubMed Central articles Regional Homogeneity (ReHo) 2023 articles Regional Homogeneity (ReHo) 2024 articles Regional Homogeneity (ReHo) Scopus articles Regional Homogeneity (ReHo) impact factor journals Regional Homogeneity (ReHo) Scopus journals Regional Homogeneity (ReHo) PubMed journals Regional Homogeneity (ReHo) medical journals Regional Homogeneity (ReHo) free journals Regional Homogeneity (ReHo) best journals Regional Homogeneity (ReHo) top journals Regional Homogeneity (ReHo) free medical journals Regional Homogeneity (ReHo) famous journals Regional Homogeneity (ReHo) Google Scholar indexed journals Amplitude Of Low-Frequency Fluctuation articles Amplitude Of Low-Frequency Fluctuation Research articles Amplitude Of Low-Frequency Fluctuation review articles Amplitude Of Low-Frequency Fluctuation PubMed articles Amplitude Of Low-Frequency Fluctuation PubMed Central articles Amplitude Of Low-Frequency Fluctuation 2023 articles Amplitude Of Low-Frequency Fluctuation 2024 articles Amplitude Of Low-Frequency Fluctuation Scopus articles Amplitude Of Low-Frequency Fluctuation impact factor journals Amplitude Of Low-Frequency Fluctuation Scopus journals Amplitude Of Low-Frequency Fluctuation PubMed journals Amplitude Of Low-Frequency Fluctuation medical journals Amplitude Of Low-Frequency Fluctuation free journals Amplitude Of Low-Frequency Fluctuation best journals Amplitude Of Low-Frequency Fluctuation top journals Amplitude Of Low-Frequency Fluctuation free medical journals Amplitude Of Low-Frequency Fluctuation famous journals Amplitude Of Low-Frequency Fluctuation Google Scholar indexed journals brain imaging articles brain imaging Research articles brain imaging review articles brain imaging PubMed articles brain imaging PubMed Central articles brain imaging 2023 articles brain imaging 2024 articles brain imaging Scopus articles brain imaging impact factor journals brain imaging Scopus journals brain imaging PubMed journals brain imaging medical journals brain imaging free journals brain imaging best journals brain imaging top journals brain imaging free medical journals brain imaging famous journals brain imaging Google Scholar indexed journals pathophysiological changes articles pathophysiological changes Research articles pathophysiological changes review articles pathophysiological changes PubMed articles pathophysiological changes PubMed Central articles pathophysiological changes 2023 articles pathophysiological changes 2024 articles pathophysiological changes Scopus articles pathophysiological changes impact factor journals pathophysiological changes Scopus journals pathophysiological changes PubMed journals pathophysiological changes medical journals pathophysiological changes free journals pathophysiological changes best journals pathophysiological changes top journals pathophysiological changes free medical journals pathophysiological changes famous journals pathophysiological changes Google Scholar indexed journals ultrasonography articles ultrasonography Research articles ultrasonography review articles ultrasonography PubMed articles ultrasonography PubMed Central articles ultrasonography 2023 articles ultrasonography 2024 articles ultrasonography Scopus articles ultrasonography impact factor journals ultrasonography Scopus journals ultrasonography PubMed journals ultrasonography medical journals ultrasonography free journals ultrasonography best journals ultrasonography top journals ultrasonography free medical journals ultrasonography famous journals ultrasonography Google Scholar indexed journals magnetic resonance imaging articles magnetic resonance imaging Research articles magnetic resonance imaging review articles magnetic resonance imaging PubMed articles magnetic resonance imaging PubMed Central articles magnetic resonance imaging 2023 articles magnetic resonance imaging 2024 articles magnetic resonance imaging Scopus articles magnetic resonance imaging impact factor journals magnetic resonance imaging Scopus journals magnetic resonance imaging PubMed journals magnetic resonance imaging medical journals magnetic resonance imaging free journals magnetic resonance imaging best journals magnetic resonance imaging top journals magnetic resonance imaging free medical journals magnetic resonance imaging famous journals magnetic resonance imaging Google Scholar indexed journals
Article Details
1. Introduction
Recent developments in brain imaging have significantly enhanced our ability to detect pathophysiological changes in the brain network [1]. For understanding the structural and functional characteristics of the brain, different imaging techniques are used in clinical settings such as skull radiography, midline ultrasonography, isotope scan such as positron emission tomography (PET), angiography, computed tomography, structural and functional magnetic resonance imaging etc. [1, 2]. Although no one imaging modality serves all the purpose, as each has unique advantages and limitations. However, among these modalities, resting state fMRI (rs-fMRI) has been widely studied and reported to be a promising tool for assessing brain functional connectivity and its neurodegenerative conditions [3-15]. Several automated statistical approaches have been adopted to classify subjects based on these rs-fMRI signals [16, 17]. Rs-fMRI exploits the spontaneous low frequency fluctuations, which are generally lesser than 0.1 Hertz in the BOLD signals. There are two large contrasting systems in the brain; one is the default mode network (DMN) - a set of brain regions showing increased activity at rest, which is the most fundamental resting state network [18]. These DMN regions are consistently active when the participants at rest (the healthy volunteers are asked to quietly lie down in the MRI in supine position with their eyes being closed and are in awaken condition) [19]. The other is the task based network such as visual, auditory, somatosensory or attention, language networks, attention modulation and cognitive control networks, dorsal and ventral attention networks [20-23]. Dementia is a brain disease that causes diminish in ability to think and remember which gradually affect an individual’s daily functioning. There are many different causes leading to dementia [24, 25]; among them, the major contributing disorder is Alzheimer’s disease (AD) [26]. It is the most common form, leading to memory loss at an early stage of the disease and then developing into common decline in numerous cognitive spheres. It is a progressive neurodegenerative disorder and is found to be around 60% to 70% of cases worldwide [27, 28]. In AD, pathological changes neuro-degeneration, neuronal cell loss, and formation of neurofibrillary tangles, senile plaques in the brain are evidenced [29]. In the context of morphological changes, the AD brain on magnetic resonance imaging (MRI) shows atrophy changes mainly in the medial temporal and parietal cortices and hippocampus [30]. Other notable MRI morphological changes such as cortical thinning, enlargement of sulci are however not specific to AD [31]. With respect to the functional outcome, several studies have reported diminished function of DMNs in AD cohort [32-35]. In the recent decade, neuroimaging in AD have been utilized as a well-established tool by clinicians and scientists and it plays a central role in diagnostic and therapeutic interventions and serves as an outcome measure. The new modalities with ultrahigh speed and novelty in image acquisition and post-processing methods were the cause for this expansion of brain imaging. In this review, the basic principles of rs-fMRI, most common data processing techniques viz., seed based functional connectivity; regional homogeneity (ReHo); amplitude of low frequency fluctuation (ALFF); principal component analysis (PCA); independent component analysis (ICA) and graph theory functional connectivity measures, the strengths and limitations of these techniques are discussed, furthermore, the utility of rs-fMRI for probing AD is also deliberated.
2. Basics of Resting State-fMRI Technique
The low frequency fluctuations (<0.1 Hz) in the rs-fMRI are initially thought to be noise [36] was later recognized as signals that are originating as a result of neuronal activity [37]. Followed by this, several studies investigated and validated the space-time associations as a measure of functional connectivity [38, 39]. The rs-fMRI is a potential tool for analysing macro scale connectomics to characterize the intrinsic brain function, however, the main limitations in the data pre-processing are whole brain correlation and head motion correction. The whole brain correlation produces negative correlations, which are non-significant to physiological signals [40]. Inadequate head motion correction can produce false positive results in data processing [41, 42]. Followed by these preprocessing steps, various methods can be used to analyze the data, each with its own inherent advantages and disadvantages. In this section, a brief overview of some of the statistical and mathematic approaches previously applied to rs-fMRI data is discussed. There are several programs available for the processing of resting state fMRI data. Some of the widely used programs include Statistical Parametric Mapping (SPM), Analysis of Functional Neuro Images (AFNI), and Functional MRI of the Brain Software Library (FSL-specifically) for Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC), Functional Connectivity Toolbox (CONN) and Configurable Pipeline for the Analysis of Connectomes (CPAC). Though the above mentioned techniques are available to post-process rs-fMRI data, among which three most popular and widely used method are seed based/ region of interest (ROI) [43], Independent Component Analysis (ICA) method [44] and graph analysis method [45]. The seed based/ ROI method uses the signal from the cluster of voxels (seed or ROI) to calculate the correlation coefficient with other voxels of the brain. This approach provides detailed connectivity information in the brain areas of interest. Whereas the ICA technique involves statistical approach that maximize the statistical independence among its components in the process of detecting the resting state networks (RSNs). It has the capabilities to extract DMNs and other RSNs with high consistency. In graph theory, the RSNs are treated as a collection of nodes connected by edges. Other methods of analysis include clustering method (group voxels that are alike based on a set of related features of importance, dynamic time warping distance (measuring the similarity between the two temporal sequences, which vary in velocity) [46, 47] which are less popular.
Since the first report about rs-fMRI, in which the left and right hemispheric regions of the primary motor network showed a high correlation with the BOLD signal [37], it has attracted several researchers to evaluate the spontaneous activity of the brain using this technique [48]. Rs-fMRI has been potentially applied in studying the alterations in RSNs in both healthy volunteers and in patients with a number of clinical conditions [49, 50]. However, the application of rs-fMRI in the routine clinical setting needs the capabilities to interpret the results and to arrive at conclusions for utilizing this potential tool to its optimum level. Here, in this review, the basic methodologies, utility of the three widely used technical approaches (namely seed based, independent component and graph theory analysis) for assessing rs-fMRI, particularly applied in the evaluation of Alzheimer’s disease (AD) are discussed.
3. Data Processing Techniques in rs-fMRI
Varieties of methods have been used to assess the alterations in the rs-fMRI BOLD signals in the human brain. These computational methods are classified into two broad-spectrum: model-driven (seed-based) method and data-driven method (1. Independent Component Analysis (ICA), which comes under the data decomposition method and 2. Clustering algorithm which comes under data clustering) [51]. The main intent of these techniques is to assess the functional network of the brain into qualitatively and quantitatively or both, the strength and orientation of the information flow within the brain functional connectivity network. Impulsive variations of the BOLD signal from a seed region/ROI are correlated with BOLD signal fluctuations from all other voxels in the task-free brain to create a resting state connectivity map. This seed-based method is a suitable method for testing a priori hypotheses. Another method known as hierarchical clustering has been used to enhance seed-based analyses method by means of a correlation matrix (CM) built from the multiple seeds to resolve which regions are most closely connected [52]. One more method to seed-based analyses is the use of data-driven techniques in which, the simultaneous activation across all voxels in the brain is considered for analysis. The mathematical model with nodes (ROIs) and edges of network connectivity is called the graph theory, which measures both global (whole-brain region) and local (within the region) connectivity between brain regions (Figure 1) [53]. Alzheimer’s disease is the main cause of dementia, leading to memory loss in the earlier stage of the disease followed by an extensive decline in cognitive functions. Pathologically, AD progression results in neuronal cell loss and formation of neurofibrillary tangles and senile plaques [54]. Since AD is a major contributing factor for dementia, diagnosing this disease at an early stage is prerequisite. Rs-fMRI is a flexible method for clinicians to find out the neuropathology of patients suffering from various cognitive disorders. In the near future, rs-fMRI can be a method of choice to offer useful diagnostic information for the early stages of this disorder. However, this technique requires highly skilled personnel for MR image acquisition, image pre and post-processing methods and also require a unique method of image analysis to get optimal results.
|
Methods |
Purposes |
Strengths |
Limitations |
|
Seed-based FC analysis |
Estimating correlations between the predefined voxel and the rest of the brain voxels |
(i) Easy to understand and compute |
(i) Requires a priori selection of ROI, which may require skill and lead to potential biases |
|
Regional homogeneity |
Using Kendall's coefficient concordance to measure the homogeneity of a given voxel with its nearest neighbors based on the BOLD time-series |
(i) Easy to understand and compute |
(i) Potential biases in prior seed selection |
|
Amplitude of low-frequency fluctuations |
Estimating the intensity of spontaneous brain activity in specific regions by calculating the voxel-wise magnitude within a defined low-frequency range |
(i) Can serve as a potential variable while investigating functional connectivity and network |
(i) fractional ALFF (fALFF) approach, a better choice as it is sensitive to physiological noise |
|
Principal component analysis |
Finding spatial and temporal components that capture as much of the variability of the data based on decorrelation as possible |
(i) Can verify the quality of difference in the activations between conditions without specifying any prior knowledge of BOLD response |
(i) Based on strong assumptions like linearity, orthogonal principal components, and high signal noise ratio (SNR) |
|
Independent component analysis |
Identifying noise within the BOLD signal by separating distinct resting-state networks that are spatially or temporally independent of each other |
(i) Can generate spatially or temporally distributed default mode functional connectivity patterns with some priori assumptions |
(i) Less sensitive to inter-individual variation in the composition of such networks. Changes of errors at the group level if a network is presented across multiple components |
|
Graph theory |
Explaining the functional brain networks topology by calcu-lating connectional characteristics of the graph comprised of nodes (voxels) and edges (connections between voxels) |
(i) Directly describes and compares different brain networks using topological parameters |
(i) Difficult to interpret |
* table reused under Creative Commons Attribution License from [55]
Table 1: Comparison among resting-state fMRI analysis methods*.
4. Seed Based ROI Data Processing
Seed based analysis is a traditional and most popular method as it is straight forward and provides broad results. The seed based ROI analysis method involves selecting the ROI and correlating within the ROI and spatio-temporal BOLD signals from the rest of the brain. This method requires a prior knowledge about the selection of ROI. The advantage of seed-based method for analyzing functional connectivity of the rs-fMRI BOLD signal is that the results are focused on particular ROIs and hence it could be easier to understand in relation to neuropathology. The following text in this review discusses some of the studies that used this technique to evaluate the resting state networks in the brain. Functional connectivity between the hippocampus and other brain regions in healthy age matched controls and AD patients were assessed by Wang et al., [56]. In which, they used a seed-based ROI selections on the hippocampus region and evaluated the connectivity to other brain regions. They found a decreased activity of DMNs in AD and disrupted hippocampus connectivity to some set of regions indicating reduced integrity of hippocampus cortical networks in AD. An assessment of functional connectivity with three distinct functional segments of the hippocampus (hippocampal head, body and tail) to the prefrontal cortex (PFC), posterior cingulate cortex (PCC) and thalamus showed stronger connectivity with PFC and weaker functional connectivity with PCC in the case of AD cohorts and correlated well with Mini Mental State Examination (MMSE) Score [57]. PCC is at risk to get segregated from the rest of the brain in AD [58]. Zhang et al tested the PCC connectivity with the rest of the brain using RS fMRI and found disrupted and compensational functional connectivity [59]. Dillen et al documented the difference in the functional connectivity of the PCC to the rest of the brain which is distinct from the functional connectivity of the retrosplenial cortex (RSC) to the rest of the brain [60]. Another study by Zhang et al 2010 documented disruption of connectivity has increased with the progression of AD and demonstrated alterations in the DMN [61]. The connectivity disruption is demonstrated in amygdala structure in the early stage of AD and the results correlated with MMSE score [62]. In contradiction to the above findings, greater connectivity in the hippocampus was noted by Kenny et al in AD cohort (n=16) [63]. In another study Galvin et al showed unique pattern of connectivity in dementia with lewy bodies when compared to AD that has different pattern of functional connectivity [64]. This increased connectivity is due to compensational functional connectivity and due to salience networks [65, 66]. Chen et al showed that RSN properties in subjects with amnestic MCI (aMCI) and AD could classify them from CN. In this study, for classifying the groups, they calculated Pearson product moment correlation coefficient of pairwise 116 ROIs [67].
Figure 1: Functional connectivity determined by seed-based correlation analysis centering on ROIs in an 8-mm diameter circle (reproduced under the terms of the Creative Commons Attribution License [68]).
The advantage of seed-based correlation analysis (SCA) is that it provides the network of regions most strongly functionally connected with the region of interest. Interpretation by this SCA is a straight forward and attracted many researchers. However, the noise due to the influence of other structural spatial resting networks caused by head motion or scanner induced artefacts is the major limitation of this technique. These artefacts can be removed by a pre-processing technique such as temporal filtering. The final result depends on the choice of the seed size and location spatial normalization and functional localization. Hence as caution, a careful selection of seed regions and identification of specific RSNs might play a major role in managing and understanding these effects.
5. Regional Homogeneity (ReHo) Data Processing
This method evaluates the brain activity by synchronization between the time series of the cluster of voxels of interest and its nearest neighbours [69]. No priori ROI selection is required for assessing the regional activity of the brain. ReHo method is based on Kendall’s coefficient of concordance technique [69]. Under specific condition the regions of the functional brain were homogenous at the given moment. Considering other model-driven methods, ReHo analysis seems to less sensitive hemodynamic response under active conditions. However, this method is capable of detecting unexpected hemodynamic response [69].
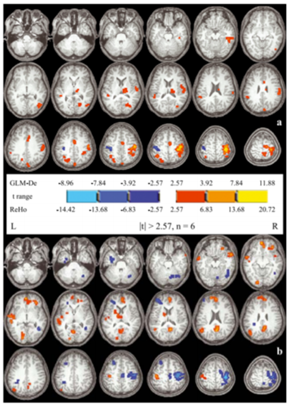
Figure 2: (a): Z maps (Paired t test between left and right hand movement conditions); (b): Redness in left> right side; Blue on right > left [69].
6. Independent Component Analysis (ICA) Data Processing
ICA method is another widely used approach which is a mathematic technique that gets the most out of statistical choice among its components [70]. ICA can be used to spatially classify distinctive RSNs from rs-fMRI data. ICA has the advantage of a priori assumptions but it requires manual selection of important components and discriminate signals that originate from low frequency fluctuation from the physiological origin. Functional connectivity measures by ICA technique decomposes a 2D data (voxel and time viz., spatio-temporal changes) into a set of time-courses and gives related spatial maps of the hidden signals [71]. Compared to seed based method, ICA has some advantages like automated components that identify RSNs, however both the techniques yielded similar results [72] when conducted in a group of healthy subjects. In a follow-up study Binnewijzend et al reported a decreased functional connectivity within default mode network in AD [73]. Another two-state study (resting and task based) by Schwindt et al showed that disrupted connectivity in the DMNs in AD is a non-enduring phenomena [74] and reported the degree of apparent change in the FC in DMN could distinctively predict cognitive state. Wu et al reported connecting directions were in opposite side in AD compared to CN using Bayesian Network [75] adding with that, they showed an increased pathological and functional connectivity burden on the right side of the hippocampus for AD cohort. Another study revealed increased local (within hippocampus) and decreased global connectivity (within DMN) in the hippocampus in AD [76]. Default mode networks showed increased functional connectivity in AD [77]. Functional connectivity in the DMNs was disrupted more in the early onset of AD compared to late onset patients [3]. Another study has confirmed the abnormal reduction of functional connectivity in PCC and right hemisphere in DMNs of the AD cohorts compared to CN [32]. Lowther et al documented a difference in resting state functional connectivity between AD and dementia with Lewy bodies (DLB) subjects [78]. Koch et al [12] investigated differences in the DMN as a marker for prognosticating Alzheimer disease. In another study, parameters from structural MR imaging and resting-state functional MRI were used to discriminate patients with AD from CN [79]. Another interesting study by Zhou et al used ICA for assessing the intrinsic functional connectivity in salience network and DMN [80]. In which, they documented decreased connectivity in salience network and increased connectivity in DMN in patients with frontotemporal dementia, whereas; in the case of AD, increased connectivity in salience network and decreased connectivity in DMN was noted.
7. Graph Theory Data Processing
Graph method uses the ball and stick model to connect the RSNs as group of ‘nodes’ (ROIs) with ‘edges’ (correlation between ROIs). This method is unique and can be an alternative method of seed-based analysis and ICA [81-83]. In this method, the region of interest corresponds to nodes and the correlation between the ROIs corresponds to the connectivity of the edges. Quantification of these connectivity parameters can be done using this graph theory [82]. Few of the parameters include the average path length, global efficiency (the average length of the shortest connection between all pairs of nodes), local efficiency (the average global efficiency of subgraphs for each node containing the neighbours of that node) clustering coefficient (connectedness of neighbouring nodes). The following study highlights the results achieved by utilizing graph theory technique. Alterations in global distant functional connectivity in AD were addressed by Sanz-Arigita et al using graph theory [45]. Quantified functional connectivity derived by graph theory in four DMN subsystem revealed a decreased antero-posterior and increased (high local clustering) frontal and parietal subsystem [84]. Another graph theory study by Kim et al, documented the alterations in the functional network that was quite dynamic with the progression of AD [85]. AD related degeneration of brain network hubs was well discussed in the study conducted by Dai et al. Rs-fMRI has been widely used as a potential tool in identifying patients with Alzheimer’s disease. Clustering coefficients at hippocampus were significantly decreased in patients compared to controls in a study conducted by Supekar et al., [11].
8. Discussions
This review provides an overview of three widely used ways of rs-fMRI analysing techniques applied particularly to assess Alzheimer’s disease. Other significant rs-fMRI methods applied to AD/non AD cohorts includes hierarchical clustering, regional homogeneity (ReHo) [69], the amplitude of low frequency fluctuation (ALFF) [86] Granger Causality Analysis (GCA) [87]. Rs-fMRI has a number of advantages when compared to other functional imaging methods. Mainly it does not demand the study participants to undergo any activity, thus it helps in imaging uncooperative or inattentive patients demanding only their immobility while acquiring images. There may be circumstances to perform rs-fMRI by sedating the participants to achieve an adequate level of immobility [88-91]. Since no task is demanded, rs-fMRI is free from differences in the level
AD patients progressed drastically in the recent time since its first study by Supekar et al [11]. With numerous hypotheses, results were achieved by researchers using this rs-fMRI BOLD signal analysis technique. Some of the key findings reported in the literature are discussed here, deterioration of the small-world networks (– quantified by high clustering coefficient, low characteristic path length [92]), status of the hippocampal connectivity to the rest of the brain, posterior cingulated cortex (PCC – connectivity), retrosplenial cortex, longitudinal studies to document AD progression, decreased functional connectivity of amygdala, loss of intra and inter network in AD progression, decreased functional connectivity in cortical regions, decreased connectivity between precuneus and other RSNs, lower functional connectivity within DMN in precuneus and PCC, alterations in DMN connectivity, alterations in hippocampus connectivity - local (within hippocampus) and global (within DMN), compensatory connectivity mechanisms, functional connectivity in early and late onset of AD, reduced PCC activity in AD compared to dementia with lewy bodies (DLB), anterior-posterior disconnection, functional differences between occipital, parietal and frontal lobes in AD, psychiatry assessments – uncontrolled flow of information through the entire network, network hub connectivity etc., These findings show clinical relevance in AD patients at various stages of the disease. However, the results derived from these studies have to be understood with a warning since they have arrived with reasonably small sample sizes, diverse characteristics of patients, and variance in the analysis procedure. The majority of the studies considered in this review have relatively small sample size. However, in theory, larger samples should provide more consistent results. Large data base studies can avoid yielding substantially false negative results [21, 93-95]. Next challenge in the rs-fMRI analysis is the motion artefacts and physiological artefacts resulting from respiration and cardiac activity. Recent advances in MRI acquisition and pre-processing techniques have facilitated the successful elimination of movement artifacts [96, 97] . However, the low frequency noise from these involuntary movements never the less affects the results of rs-fMRI. ICA based noise removing methods for physiological artefacts removal has been reported [98]. Inspite of these limitations, improvement of acquisition protocols and analysis methods is currently under intensive investigation. Apart from rs-fMRI, combining methods like EEG and other neurophysiological measurements to examine the resting state of the brain would become a more potent tool to search for a biomarker of the neurological and psychological disorder [99-101]
9. Summary
The existing studies in the literature have used various methods for assessing resting state functional connectivity using rs-fMRI BOLD signal. The reported studies collectively propose functional alterations in various regions of the resting brain in those with AD. This functional alteration extends beyond DMN to entire RSNs. Each approach has their own advantages and limitations, however; all the techniques discussed in this review are extensively used because of their ability to provide an insight on the functional networks of healthy controls and patients with early/late mild cognitive impairment and AD. Different RSNs have been identified such as DMN, sensorimotor, attentional, visual, auditory networks. However, clinical applications of rs-fMRI are limited to-date especially in circumstances like presurgical planning for patients with brain pathophysiological diseases such as tumour or epilepsy. Understanding the BOLD contrast mechanisms; implementing increased acquisition protocols can increase sensitivity; the use of new optimized preprocessing and analytic techniques; extensive data and more powerful computational methods; design of studies with large population is the potential ways to analyse functional neuroimaging.
10. Conclusion
Rs-fMRI technique is non-invasive and needs only resting state to assess the patients, its application in future will be certain to identify patients with earlier onset of Alzheimer disease and other neurological or psychiatric disorders. Thus, rs-fMRI is a promising tool that can identify functional damage at an early stage of AD and could bridge the gap between molecular level pathological changes (amyloid plaques, neurofibrillary tangles), physiological functional loss (functional connectivity) and macro level tissue loss and neurodegeneration (atrophy).
Acknowledgment
The author would like to thank Director – Research, Principal – Faculty of Allied Health Sciences, Chettinad Academy of Research and Education for their valuable support and guidance.
References
- Johnson K A, Fox N C, Sperling R A, et al. Brain Imaging in Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine 2 (2012): a006213.
- Oldendorf W H. The quest for an image of brain: a brief historical and technical review of brain imaging techniques. Neurology 28 (1978): 517-533.
- Adriaanse S M, Binnewijzend M A, Ossenkoppele R, et al. Widespread disruption of functional brain organization in early-onset Alzheimer's disease. PloS one 9 (2014): e102995.
- Xiao H, Yang Y, Xi J-h, et al. Structural and functional connectivity in traumatic brain injury. Neural Regeneration Research 10 (2015): 2062-2071.
- Sbardella E, Petsas N, Tona F, et al. Resting-State fMRI in MS: General Concepts and Brief Overview of Its Application. BioMed Research International (2015): pg8.
- Barkhof F, Haller S, Rombouts S A. Resting-state functional MR imaging: a new window to the brain. Radiology 272 (2014): 29-49.
- Craddock R C, Holtzheimer P E 3rd, Hu X P, et al. Disease state prediction from resting state functional connectivity. Magnetic resonance in medicine 62 (2009): 1619-1628.
- Lee M H, Smyser C D, Shimony J S. Resting-state fMRI: a review of methods and clinical applications. AJNR. American journal of neuroradiology 34 (2013): 1866-1872.
- van den Heuvel M P, Hulshoff Pol H E. Exploring the brain network: a review on resting-state fMRI functional connectivity. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 20 (2010): 519-534.
- Zhang D, Johnston J M, Fox M D, et al. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: initial experience. Neurosurgery 65 (2009): 226-236.
- Supekar K, Menon V, Rubin D, et al. Network Analysis of Intrinsic Functional Brain Connectivity in Alzheimer's Disease. PLoS Computational Biology 4 (2008): e1000100.
- Koch W, Teipel S, Mueller S, et al. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer's disease. Neurobiology of aging 33 (2012): 466-478.
- Wang K, Liang M, Wang L, et al. Altered functional connectivity in early Alzheimer's disease: A resting-state fMRI study. Human brain mapping 28 2007): 967-978.
- Rombouts S A, Barkhof F, Goekoop R, et al. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Human brain mapping 26 (2005): 231-239.
- Sheline Y I, Raichle M E. Resting State Functional Connectivity in Preclinical Alzheimer’s Disease: A Review. Biological psychiatry 74 (2013): 340-347.
- Challis E, Hurley P, Serra L, et al. Gaussian process classification of Alzheimer's disease and mild cognitive impairment from resting-state fMRI. NeuroImage 112 (2015): 232-243.
- Cole D, Smith S, Beckmann C. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in Systems Neuroscience (2010): 4.
- Raichle M E, MacLeod A M, Snyder A Z, et al. A default mode of brain function. Proceedings of the National Academy of Sciences 98 (2001): 676-682.
- Greicius M D, Krasnow B, Reiss A L, et al. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America 100 (2003): 253-258.
- Power J D, Cohen A L, Nelson S M, et al. Functional network organization of the human brain. Neuron 72 (2011): 665-678.
- Smith S M, Fox P T, Miller K L, et al. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America 106 (2009): 13040-13045.
- Yeo B T, Krienen F M, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology 106 (2011): 1125-1165.
- Tomasi D, Volkow N D. Resting functional connectivity of language networks: characterization and reproducibility. Molecular psychiatry 17 (2012): 841-854.
- Schneider J A, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69 (2007): 2197-2204.
- Burns A, Iliffe S Dementia. BMJ (Clinical research ed.) 338 (2009): b75.
- McKhann G M, Knopman D S, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 7 (2019): 263-269.
- Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues in Clinical Neuroscience 11 (2009): 111-128.
- Sachdev P S, Lo J W, Crawford J D, et al. STROKOG (stroke and cognition consortium): An international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring 7 (2017): 11-23.
- Selkoe D J. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer's disease. Annual review of cell biology 10 (1994): 373-403.
- Sluimer J D, van der Flier W M, Karas G B, et al. Accelerating regional atrophy rates in the progression from normal aging to Alzheimer's disease. European radiology 19 (2009): 2826-2833.
- Dubois B, Hampel H, Feldman H H, et al. Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 12 (2016): 292-323.
- Franciotti R, Falasca N W, Bonanni L, et al. Default network is not hypoactive in dementia with fluctuating cognition: an Alzheimer disease/dementia with Lewy bodies comparison. Neurobiology of aging 34 (2013): 1148-1158.
- Katell Mevel, Gaël Chételat, Francis Eustache, et al. The Default Mode Network in Healthy Aging and Alzheimer's Disease. International Journal of Alzheimer's Disease (2011).
- James G A, Tripathi S P, Ojemann J G, et al. Diminished default mode network recruitment of the hippocampus and parahippocampus in temporal lobe epilepsy. Journal of neurosurgery 119 (2013): 288-300.
- Weiler M, Teixeira C V, Nogueira M H, et al. Differences and the relationship in default mode network intrinsic activity and functional connectivity in mild Alzheimer's disease and amnestic mild cognitive impairment. Brain connectivity 4 (2014): 567-574.
- Purdon P L, Weisskoff R M. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel-level false-positive rates in fMRI. Human brain mapping 6 (1998): 239-249.
- Biswal B, Yetkin F Z, Haughton V M, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine 34 (1995): 537-541.
- Biswal B, Hudetz A G, Yetkin F Z, et al. Hypercapnia reversibly suppresses low-frequency fluctuations in the human motor cortex during rest using echo-planar MRI. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 17 (1997): 301-308.
- Biswal B B, Van Kylen J, Hyde J S. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in biomedicine 10 (1997): 165-170.
- Anderson J S, Druzgal T J, Lopez-Larson M, et al. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Human brain mapping 32 (2011): 919-934.
- Power J D, Barnes K A, Snyder A Z, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59 (2012): 2142-2154.
- Van Dijk K R, Sabuncu M R, Buckner R L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59 (2012): 431-438.
- Margulies D S, Kelly A M, Uddin L Q, et al. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage 37 (2007): 579-588.
- Beckmann C F, DeLuca M, Devlin J T, et al. Investigations into resting-state connectivity using independent component analysis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 360 (2005): 1001-1013.
- Sanz-Arigita E J, Schoonheim M M, Damoiseaux J S, et al. Loss of 'small-world' networks in Alzheimer's disease: graph analysis of FMRI resting-state functional connectivity. PloS one 5 (2010): e13788.
- Chang C, Glover G H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage 50 (2010): 81-98.
- Faria A V, Joel S E, Zhang Y, et al. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies. NeuroImage 61 (2012): 613-621.
- Shimony J S, Zhang D, Johnston J M, et al. Resting State Spontaneous Fluctuations in Brain Activity: A New Paradigm for Presurgical Planning using fMRI. Academic radiology 16 (2009): 578.
- Zhang D, Raichle M E. Disease and the brain's dark energy. Nature reviews. Neurology 6 (2010): 15-28.
- Takamura T, Hanakawa T. Clinical utility of resting-state functional connectivity magnetic resonance imaging for mood and cognitive disorders. Journal of neural transmission (Vienna, Austria: 1996) (2017).
- Li K, Guo L, Nie J, et al. Review of methods for functional brain connectivity detection using fMRI. Computerized medical imaging and graphics: the official journal of the Computerized Medical Imaging Society 33 (2009): 131-139.
- Cordes D, Haughton V, Carew J D, et al. Hierarchical clustering to measure connectivity in fMRI resting-state data. Magnetic resonance imaging 20 (2002): 305-317.
- Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Frontiers in systems neuroscience 4 (2010): 16.
- Serrano-Pozo A, Frosch M P, Masliah E, et al. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine 1 (2011): a006189.
- Zhan X, Yu R. A Window into the Brain: Advances in Psychiatric fMRI. Biomed Res Int (2015): 542467-542467.
- Wang L, Zang Y, He Y, et al. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. NeuroImage 31 (2006): 496-504.
- Zarei M, Beckmann C F, Binnewijzend M A, et al. Functional segmentation of the hippocampus in the healthy human brain and in Alzheimer's disease. NeuroImage 66 (2013): 28-35.
- Leech R, Sharp D J. The role of the posterior cingulate cortex in cognition and disease. Brain 137 (2014): 12-32.
- Zhang H Y, Wang S J, Xing J, et al. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behavioural brain research 197 (2009): 103-108.
- Dillen K N, Jacobs H I, Kukolja J, et al. Aberrant functional connectivity differentiates retrosplenial cortex from posterior cingulate cortex in prodromal Alzheimer's disease. Neurobiology of aging 44 (2016): 114-126.
- Zhang H Y, Wang S J, Liu B, et al. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology 256 (2010): 598-606.
- Yao H, Liu Y, Zhou B, et al. Decreased functional connectivity of the amygdala in Alzheimer's disease revealed by resting-state fMRI. European journal of radiology 82 (2013): 1531-1538.
- Kenny E R, Blamire A M, Firbank M J, et al. Functional connectivity in cortical regions in dementia with Lewy bodies and Alzheimer's disease. Brain 135 (2012): 569-581.
- Galvin J E, Price J L, Yan Z, et al. Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology 76 (2011): 1797-1803.
- Balthazar M L, Pereira F R, Lopes T M, et al. Neuropsychiatric symptoms in Alzheimer's disease are related to functional connectivity alterations in the salience network. Human brain mapping 35 (2014): 1237-1246.
- Zhou J, Greicius M D, Gennatas E D, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133 (2010): 1352-1367.
- Chen G, Ward B D, Xie, et al. Classification of Alzheimer disease, mild cognitive impairment, and normal cognitive status with large-scale network analysis based on resting-state functional MR imaging. Radiology 259 (2011): 213-221.
- Martins D L N, Valiatti T D d S, D'Ávila J, et al.. Extrinsic functional connectivity of the default mode network in crack-cocaine users. Radiol Bras 51 (2018): 1-7.
- Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. NeuroImage 22 (2004): 394-400.
- Esposito F, Aragri A, Pesaresi I, et al. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magnetic resonance imaging 26 (2008): 905-913.
- McKeown M J, Makeig S, Brown G G, et al. Analysis of fMRI data by blind separation into independent spatial components. Human brain mapping 6 (1998): 160-188.
- Rosazza C, Minati L, Ghielmetti F, et al. Functional connectivity during resting-state functional MR imaging: study of the correspondence between independent component analysis and region-of-interest-based methods. AJNR. American journal of neuroradiology 33 (2012): 180-187.
- Binnewijzend M A, Schoonheim M M, Sanz-Arigita E, et al. Resting-state fMRI changes in Alzheimer's disease and mild cognitive impairment. Neurobiology of aging 33 (2012): 2018-2028.
- Schwindt G C, Chaudhary S, Crane D, et al. Modulation of the default-mode network between rest and task in Alzheimer's Disease. Cerebral cortex (New York, N.Y. : 1991) 23 (2013): 1685-1694.
- Wu X, Li R, Fleisher A S, et al. Altered default mode network connectivity in Alzheimer's disease--a resting functional MRI and Bayesian network study. Human brain mapping 32 (2011): 1868-1881.
- Pasquini L, Scherr M, Tahmasian M, et al. Link between hippocampus' raised local and eased global intrinsic connectivity in AD. Alzheimer's & dementia : the journal of the Alzheimer's Association 11 (2015): 475-484.
- Castellazzi G, Palesi F, Casali S, et al. A comprehensive assessment of resting state networks: bidirectional modification of functional integrity in cerebro-cerebellar networks in dementia. Frontiers in neuroscience 8 (2014): 223.
- Lowther E R, O'Brien J T, Firbank M J, et al. Lewy body compared with Alzheimer dementia is associated with decreased functional connectivity in resting state networks. Psychiatry research 223 (2014): 192-201.
- Dai Z, Yan C, Li K, et al. Identifying and Mapping Connectivity Patterns of Brain Network Hubs in Alzheimer's Disease. Cerebral cortex (New York, N.Y. : 1991) 25 (2015): 3723-3742.
- Zhou J, Greicius M D, Gennatas E D, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain : a journal of neurology 133 (2010): 1352-1367.
- Salvador R, Suckling J, Coleman M R, et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cerebral cortex (New York, N.Y. : 1991) 15 (2005): 1332-1342.
- van den Heuvel M P, Stam C J, Boersma M, et al. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. NeuroImage 43 (2008): 528-539.
- Power J D, Cohen A L, Nelson S M, et al. Functional network organization of the human brain. Neuron 72 (2011): 665-678.
- Toussaint P J, Maiz S, Coynel D, et al. Characteristics of the default mode functional connectivity in normal ageing and Alzheimer's disease using resting state fMRI with a combined approach of entropy-based and graph theoretical measurements. NeuroImage 101 (2014): 778-786.
- Kim H, Yoo K, Na D L, et al. Non-monotonic reorganization of brain networks with Alzheimer's disease progression. Frontiers in aging neuroscience 7 (2015): 111.
- Zang Y F, He Y, Zhu C Z, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & development 29 (2007): 83-91.
- Delaveau P, Salgado-Pineda P, Fossati P, et al. Dopaminergic modulation of the default mode network in Parkinson's disease. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 20 (2010): 784-792.
- Greicius M D, Kiviniemi V, Tervonen O, et al. Persistent default-mode network connectivity during light sedation. Human brain mapping 29 (2008): 839-847.
- Kiviniemi V, Kantola J H, Jauhiainen J, et al. Independent component analysis of nondeterministic fMRI signal sources. NeuroImage 19 (2003): 253-260.
- Wei Z, Alcauter S, Jin K, et al. Graph Theoretical Analysis of Sedation's Effect on Whole Brain Functional System in School-Aged Children. Brain connectivity 3 (2013): 177-189.
- Liang P, Zhang H, Xu Y, et al. Disruption of cortical integration during midazolam?induced light sedation. Human brain mapping 36 (2015): 4247-4261.
- Watts D J, Strogatz S H. Collective dynamics of /`small-world/' networks. Nature 393 (1998): 440-442.
- Laird A R, Fox P M, Eickhoff S B, et al. Behavioral interpretations of intrinsic connectivity networks. Journal of cognitive neuroscience 23 (2011): 4022-4037.
- Biswal B B, Mennes M, Zuo X N, et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America 107 (2010): 4734-4739.
- Laird A R, Eickhoff S B, Rottschy C, et al. Networks of task co-activations. NeuroImage 80 (2013): 505-514.
- Satterthwaite T D, Elliott M A, Gerraty R T, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage 64 (2013): 240-256.
- Feis R A, Smith S M, Filippini N, et al. ICA-based artifact removal diminishes scan site differences in multi-center resting-state fMRI. Frontiers in neuroscience 9 (2015): 395.
- Griffanti L, Dipasquale O, Laganà M M, et al. Effective artifact removal in resting state fMRI data improves detection of DMN functional connectivity alteration in Alzheimer's disease. Frontiers in Human Neuroscience 9 (2015): 449.
- Omata K, Hanakawa T, Morimoto M, et al. Spontaneous Slow Fluctuation of EEG Alpha Rhythm Reflects Activity in Deep-Brain Structures: A Simultaneous EEG-fMRI Study. PloS one 8 (2013): e66869.
- Brueggen K, Fiala C, Babiloni C, et al. SIMULTANEOUS EEG-FMRI IN PATIENTS WITH ALZHEIMER’S DISEASE: ARE BOLD SIGNAL FLUCTUATIONS IN THE DEFAULT MODE NETWORK CORRELATED WITH ALPHA BAND POWER? Alzheimer's & Dementia: The Journal of the Alzheimer's Association 12 (2019): P544.
- Mulert C. Simultaneous EEG and fMRI: towards the characterization of structure and dynamics of brain networks. Dialogues in Clinical Neuroscience 15 (2013): 381-386.
