A Coronary Artery Perforation: Successful Complication Management after Intravascular Ultrasound Imaging Guided Covered Stent Implantation
Article Information
Aiste Zebrauskaite1, 3*, Greta Ziubryte1, 2, Petras Medzevicius1, Kristina Morkunaite1, Ramunas Unikas1, 3, Gediminas Jarusevicius1, 2, 3
1Department of Cardiology, Hospital of Lithuanian University of Health Sciences Kaunas Clinics, Kaunas, Lithuania
2Institute of Cardiology, Lithuanian University of Health Sciences, Kaunas, Lithuania
3Academy of Medicine, Lithuanian University of Health Sciences, Kaunas, Lithuania
*Corresponding Author: Aiste Zebrauskaite, Department of Cardiology, Hospital of Lithuanian University of Health Sciences Kaunas Clinics; Eiveniai street 2, LT-50161, Kaunas, Lithuania
Received: 17 January 2022; Accepted: 27 January 2022; Published: 28 February 2022
Citation: Aiste Zebrauskaite, Greta Ziubryte, Petras Medzevicius, Kristina Morkunaite, Ramunas Unikas, Gediminas Jarusevicius. A Coronary Artery Perforation: Successful Complication Management after Intravascular Ultrasound Imaging Guided Covered Stent Implantation. Archives of Clinical and Medical Case Reports 6 (2022): 131-136.
View / Download Pdf Share at FacebookAbstract
A coronary artery perforation is a rare, but dangerous periprocedural complication. In this case the perforation stented with two covered stents, balloon inflations applied. Despite the treatment extravasation persisted. Intravascular ultrasound (IVUS) imaging identified the reasons of persisting extravasation. IVUS guided third covered stent implantation leaded to the successful treatment.
Keywords
Coronary Artery Disease; Intravascular Ultrasound
Coronary Artery Disease articles; Intravascular Ultrasound articles
Coronary Artery Disease articles Coronary Artery Disease Research articles Coronary Artery Disease review articles Coronary Artery Disease PubMed articles Coronary Artery Disease PubMed Central articles Coronary Artery Disease 2023 articles Coronary Artery Disease 2024 articles Coronary Artery Disease Scopus articles Coronary Artery Disease impact factor journals Coronary Artery Disease Scopus journals Coronary Artery Disease PubMed journals Coronary Artery Disease medical journals Coronary Artery Disease free journals Coronary Artery Disease best journals Coronary Artery Disease top journals Coronary Artery Disease free medical journals Coronary Artery Disease famous journals Coronary Artery Disease Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals cancer articles cancer Research articles cancer review articles cancer PubMed articles cancer PubMed Central articles cancer 2023 articles cancer 2024 articles cancer Scopus articles cancer impact factor journals cancer Scopus journals cancer PubMed journals cancer medical journals cancer free journals cancer best journals cancer top journals cancer free medical journals cancer famous journals cancer Google Scholar indexed journals Disease articles Disease Research articles Disease review articles Disease PubMed articles Disease PubMed Central articles Disease 2023 articles Disease 2024 articles Disease Scopus articles Disease impact factor journals Disease Scopus journals Disease PubMed journals Disease medical journals Disease free journals Disease best journals Disease top journals Disease free medical journals Disease famous journals Disease Google Scholar indexed journals Ultra Sound articles Ultra Sound Research articles Ultra Sound review articles Ultra Sound PubMed articles Ultra Sound PubMed Central articles Ultra Sound 2023 articles Ultra Sound 2024 articles Ultra Sound Scopus articles Ultra Sound impact factor journals Ultra Sound Scopus journals Ultra Sound PubMed journals Ultra Sound medical journals Ultra Sound free journals Ultra Sound best journals Ultra Sound top journals Ultra Sound free medical journals Ultra Sound famous journals Ultra Sound Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Radiology articles Radiology Research articles Radiology review articles Radiology PubMed articles Radiology PubMed Central articles Radiology 2023 articles Radiology 2024 articles Radiology Scopus articles Radiology impact factor journals Radiology Scopus journals Radiology PubMed journals Radiology medical journals Radiology free journals Radiology best journals Radiology top journals Radiology free medical journals Radiology famous journals Radiology Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Angiography articles Angiography Research articles Angiography review articles Angiography PubMed articles Angiography PubMed Central articles Angiography 2023 articles Angiography 2024 articles Angiography Scopus articles Angiography impact factor journals Angiography Scopus journals Angiography PubMed journals Angiography medical journals Angiography free journals Angiography best journals Angiography top journals Angiography free medical journals Angiography famous journals Angiography Google Scholar indexed journals
Article Details
1. History of Presentation
A 82 year-old patient admitted with dyspnea on mild exertion progressing for the last 12 months. On physical examination vital signs were within normal limits and a grade III/VI systolic ejection murmur was auscultated at the right sternal border. ECG revealed sinus rhythm, left ventricle (LV) hypertrophy signs and ST depression in V5-V6. High sensitivity Troponin T was negative.
2. Past Medical History
A history of stabile coronary artery disease, stent implant-tation to a right coronary artery (RCA) in 2013. A perma-nent pacemaker implanted in 2016 for sick sinus syndrome.
3. Differential Diagnosis
Differential diagnoses included coronary artery disease, valvular heart disease, and chronic heart failure.
4. Investigations
A transthoracic echocardiogram (TTE) revealed severe aortic stenosis with an aortic valve (AV) mean gradient of 52 mm Hg, peak velocity of 5.0 m/s, and AV area of 1.0 cm2 - 0.52cm2/m2, preserved LV function. Diagnostic ang-iogram demonstrated severe in-stent restenosis of proximal-mid RCA, moderate left anterior descending and proximal circumflex artery lesions, and severe obtuse marginal (OM) 2st lesion. After discussion in a multidisciplinary team meet-ing, percutaneous coronary intervention (PCI) to RCA and OM2 followed by transcatheter aortic valve replacement (TAVR) was recommended.
5. Management
The patient referred for PCI. OM2 lesion appeared to be ca-
lcified (Figure 1A, Video1). OM2 lesion predilated with a semi-compliant 2.0mm balloon 10atm. Resolute Onyx 2.25 × 12mm DES implanted, at the nominal 12atm pressure the stent appeared under-expanded at the mid portion, pressure increased up to 16atm, which was followed by the stent balloon rupture. Immediate angiogram revealed a type III perforation (Classification of Ellis et al [1]) of OM2 with blood extravasation into a pericardium cavity below the distal stent edge and no blood flow to the distal segment (Figure 1B, Video2). The patient remained hemodynamic-cally stabile. A 2.0mm balloon was inflated immediately at the site of perforation for stopping the extravasation. A BeGraft covered stent 2.5x8mm was deployed at nominal pressure (11atm), overlapping it with the distal third of DES. After covered stent implantation the extravasation persisted (Figure 1C, Video3), prolonged inflations with 2.5 non-compliant (NC) balloon was performed. Despite this, extravasation still persisted, an additional BeGraft covered stent 2.5x18mm was deployed at nominal pressure (11atm), covering the angiographically visible perforation site. However, the extravasation jet was smaller, but still significant (Figure 1D, Video4). Repeated 2.5mm NC balloon inflations were ineffective. TTE showed pericardial effusion up to 8mm without signs of tamponade. Due to the stable patient’s condition, it was decided to perform an intravascular ultrasound (IVUS) to identify reasons of persisting blood leakage. IVUS identified the exact size of the vessel lumen - 3.0mm, and the site of perforation, which described as a break of the external elastic (EEL) membrane with communication between a vessel lumen and a perivas-cular-extravascular space, a tear of the covered stent was visible at the site of the perforation (Figure 2, Video5). A BeGraft covered stent 2.5x16mm (15atm 2.73mm diameter) was implanted covering the area of perforation. A minimal extravasation was still present (Figure 1E, Video6). A balloon inflation was adjusted per IVUS measurements, using a 3.0mm NC balloon was applied. Angiography confirmed a good result, with the perforation site successs-fully sealed, no extravasation was visible. The TIMI3 blood flow to the distal vessel was restored (Figure 1F, Video7). In stable condition the patient was transferred to an intensive coronary care unit (ICU).
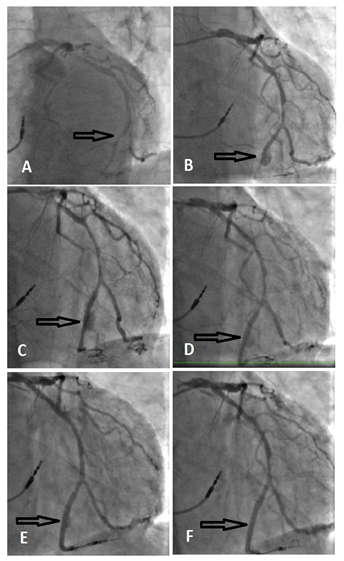
Figure 1: Angiogram pictures. A: a severe lesion in OM2 (black arrow); B: CAP after DES implantation (black arrow); C: persistent extravasations after 1st covered stent implantation (black arrow); D: persistent extravasations after 2st covered stent implantation (black arrow); E: Decreased size extravasation after IVUS guided 3rd covered stent implantation (black arrow); F: final result, perforation sealed after dilation with 3.0mm NC balloon (black arrow).
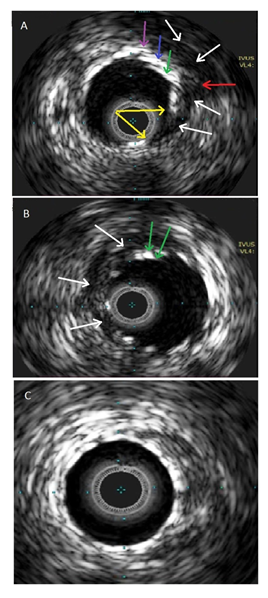
Figure 2: IVUS imaging. A: CAP site. Demonstrates three layers of stents – DES (purple arrow), 1st covered stent (blue arrow), 2nd covered stent (green arrow). EEL brake site (red arrow). A tear of covered stent (two yellow arrows). A communication between a vessel lumen and a perivascular and extravascular space filled with blood speckles (white arrows); B: Crescent shape intramural haematoma below the perforation site (white arrows) and two layers of covered stents (green arrows); C: Distal part of stented vessel shows well apposed and expanded covered stent.
6. Discussion
A coronary artery perforation (CAP) is a rare (0.2% - 0.6% [2-3]), but potentially fatal complication of PCI. CAP can be classified according to its severity using the Ellis classification [1]. Morbidity and mortality vary directly with Ellis classification: tamponade and mortality rates respectively range between 0.3% and 0.4% (Ellis class I) to 45.7% and 21.2% (Ellis class III), respectively [1, 4]. Most perforations are preventable. The most reproducible risk factors are: complex lesions, usage of atheroablative devices, oversizing of devices (balloons and stents), female gender, advanced age [4-5]. A proper lesion preparation is the key to avoiding of periprocedural complications and stent under expansion, which leads to the higher rate of acute stent thrombosis and stent restenosis. The algorithm for management of calcified coronary lesions suggests that an intravascular imaging should be performed to identify lesions with high calcium content. The lesions with multiple complex calcium imaging features should be modified by using advanced calcium modification techniques, such as mechanical atherectomy, laser atherectomy or intravascular lithoplasty [6]. If an artery cannot be properly prepared, stents should not be used.
Interventional cardiologist should be able to recognize CAP quickly and become familiar with available general and specific treatment options, such that its adverse impact can be minimised. The universal and type-specific coronary artery management algorithm suggests, that large vessel CAPs usually require covered stents [7]. But the first step after diagnosing the CAP is to stop extravasation with inflation of a balloon (1:1 balloon:vessel size) proximal to or at the site of perforation. Covered stents prevent blood leakage between stent struts and a high rate of success has been reported as the need for urgent surgical interventions has decreased since the introduction of covered stents. However, they have some limitations: the lack of elasticity, the deployment can be difficult and may fail in tortuous or calcified vessels. Moreover, these stents frequently require postdilatation to further seal the perforation and/or optimise the stent expansion and their design may render this step challenging. In addition, higher rates of stent restenosis and thrombosis have been reported compared with bare-metal and DES. The covered stents have higher rates of adverse events in long term follow up [4, 5, 8]. In cases like ours, a CAP sealing with covered stents might not be successful. If the patient’s condition is stable, performing an IVUS is useful in understanding the reasons behind the perforation site sealing failure, taking accurate measurements of a vessel wall, and ensure optimal final stenting result. The information provided by IVUS leads to the successful management of the CAP and reduces the risk of further adverse events.
7. Follow-up
In the ICU an urgent bed-side TTE showed mild pericardial effusion up to 11mm without signs of cardiac tamponade. Patient’s condition remained stable, no increasement of pericardial fluid was registered. A staged PCI to RCA and a successful TAVR was performed.
8. Conclusion
The majority of CAP instances can be suspected and avoided. In our case the lesion was not prepared properly prior stenting and the stent implantation at a high pressure leaded to the CAP at the distal stent edge. From the retrospective assessment, calcium modification with an intravascular lithotripsy balloon would have been an excel-ent approach in this case. However, we reacted immediately by inflating the balloon at the site of CAP to prevent the occurrence of tamponade. Despite the successful implant-ation of two covered stents, the extravasation persisted. The information provided by the IVUS imaging helped to understand the mechanism of persisting extravasation and leaded to the successful periprocedural complication management.
9. Learning Objectives
- To understand that the majority of perforations are preventable, proper lesion preparation is the key. If an artery cannot be properly prepared, stents should not be used.
- To understand that the perforation is an emergency – appropriate action in the first minute can prevent the occurrence of tamponade.
- To learn the first steps after getting the perforation:
- Recognize the perforations early
- Immediately occlude the perforation site with 1:1 size balloon
- Plan your treatment strategy
- Ask for help early
- To understand that the intravascular imaging can reveal reasons of treatment failure and to ensure correct covered stent expansion and apposition that reduces further adverse events.
References
- Ellis SG, Ajluni S, Arnold AZ, et al. Increased coronary perforation in the new device era. Incidence, classification, management, and out-come. Circulation 90 (1994): 2725-2730.
- Shimony A, Joseph L, Mottillo S, et al. Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analy-sis. Can J Cardiol 27 (2011): 843-850.
- Harnek J, James S, Lagerqvist B. Coronary artery perforation and tamponade — incidence, risk factors, predictors and outcomes from 12 years’ data of the SCAAR Registry. Circ J 84 (2019): 43-53.
- Hendry C, Fraser D, Eichhofer J, et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. Eurointervention 8 (2012): 79-86.
- Al-Lamee R, Ielasi A, Latib A, et al. Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforation. JACC Cardiovasc Interv 4 (2011): 87-95.
- De Maria GL, Scarsini R, Banning AP. Manag-ement of calcific coronary artery lesions: is it time to change our interventional therapeutic approach? JACC Cardiovasc Interv 12 (2019): 1465-1478.
- Abdalwahab A, Farag M, Brilakis ES, et al. Mana-gement of coronary artery perforation. Cardiovas-cular Revascularization Medicine 26 (2021): 55-60.
- Lansky AJ, Yang YM, Khan Y, et al. Treatment of coronary artery perforations complicating precut-aneous coronary intervention with a polytetra-fluoroethylene-covered stent graft. AmJ Cardiol 98 (2006): 370-374.
