A Case of MPO-ANCA Positive Systemic Lupus Erythematosus Developed into Acute Kidney Injury
Article Information
Satoshi Suzuki1, Kei Ogiwara2, Takuya Nishi1, Shota Sakuma1, Maiko Akira2, Masako Iwasaki2, Daisuke Honda2, Hisatsugu Takahara2, Keigo Ikeda1, Ken Yamaji3, Naoto Tamura3, Hisaki Rinno2, Shinji Morimoto1
1Department of Internal Medicine and Rheumatology, Juntendo University Urayasu Hospital, Chiba, Japan
2Department of Internal Medicine and Nephrology, Juntendo University Urayasu Hospital, Chiba, Japan
3Department of Internal Medicine and Rheumatology, Juntendo University School of Medicine, Tokyo, Japan
*Corresponding Author: Dr. Satoshi Suzuki, Department of Internal Medicine and Rheumatology, Juntendo University Urayasu Hospital, 2-1-1 Tomioka, Urayasu city, Chiba 279-0021, Japan
Received: 14 August 2019; Accepted: 03 September 2019; Published: 19 September 2019
Citation: Suzuki S, Ogiwara K, Nishi T, Sakuma S, Akira M, Iwasaki M, Honda D, Takahara H, Ikeda K, Yamaji K, Tamura N, Rinno H, Morimoto S. A Case of MPO-ANCA Positive Systemic Lupus Erythematosus Developed into Acute Kidney Injury. Archives of Clinical and Medical Case Reports 3 (2019): 560-566.
View / Download Pdf Share at FacebookAbstract
During the course of treatment for rheumatoid arthritis, anti-DNA antibody was detected, and systemic lupus erythematosus was diagnosed, who was subsequently treated with abatacept—and remission was achieved. Rebound of elevated anti-DNA antibody and urinary abnormalities were noted with MPO-ANCA positivity. Renal biopsy revealed lupus nephritis; followed by the development of rapidly progressive glomerulonephritis, which required apheresis (double filtration plasmapheresis) therapy, although no response was achieved. However, plasma exchange therapy was highly effective. These results suggest that MPO-ANCA-positive lupus nephritis can become severe and require intensive treatment in accordance with the treatment for severe vasculitis.
Keywords
Systemic lupus erythematosus; MPO-ANCA; Plasmapheresis; Renal biopsy; Acute kidney injury
Systemic lupus erythematosus articles, MPO-ANCA articles, Plasmapheresis articles, Renal biopsy articles, Acute kidney injury articles
Systemic lupus erythematosus articles Systemic lupus erythematosus Research articles Systemic lupus erythematosus review articles Systemic lupus erythematosus PubMed articles Systemic lupus erythematosus PubMed Central articles Systemic lupus erythematosus 2023 articles Systemic lupus erythematosus 2024 articles Systemic lupus erythematosus Scopus articles Systemic lupus erythematosus impact factor journals Systemic lupus erythematosus Scopus journals Systemic lupus erythematosus PubMed journals Systemic lupus erythematosus medical journals Systemic lupus erythematosus free journals Systemic lupus erythematosus best journals Systemic lupus erythematosus top journals Systemic lupus erythematosus free medical journals Systemic lupus erythematosus famous journals Systemic lupus erythematosus Google Scholar indexed journals MPO-ANCA articles MPO-ANCA Research articles MPO-ANCA review articles MPO-ANCA PubMed articles MPO-ANCA PubMed Central articles MPO-ANCA 2023 articles MPO-ANCA 2024 articles MPO-ANCA Scopus articles MPO-ANCA impact factor journals MPO-ANCA Scopus journals MPO-ANCA PubMed journals MPO-ANCA medical journals MPO-ANCA free journals MPO-ANCA best journals MPO-ANCA top journals MPO-ANCA free medical journals MPO-ANCA famous journals MPO-ANCA Google Scholar indexed journals Plasmapheresis articles Plasmapheresis Research articles Plasmapheresis review articles Plasmapheresis PubMed articles Plasmapheresis PubMed Central articles Plasmapheresis 2023 articles Plasmapheresis 2024 articles Plasmapheresis Scopus articles Plasmapheresis impact factor journals Plasmapheresis Scopus journals Plasmapheresis PubMed journals Plasmapheresis medical journals Plasmapheresis free journals Plasmapheresis best journals Plasmapheresis top journals Plasmapheresis free medical journals Plasmapheresis famous journals Plasmapheresis Google Scholar indexed journals Renal biopsy articles Renal biopsy Research articles Renal biopsy review articles Renal biopsy PubMed articles Renal biopsy PubMed Central articles Renal biopsy 2023 articles Renal biopsy 2024 articles Renal biopsy Scopus articles Renal biopsy impact factor journals Renal biopsy Scopus journals Renal biopsy PubMed journals Renal biopsy medical journals Renal biopsy free journals Renal biopsy best journals Renal biopsy top journals Renal biopsy free medical journals Renal biopsy famous journals Renal biopsy Google Scholar indexed journals Acute kidney injury articles Acute kidney injury Research articles Acute kidney injury review articles Acute kidney injury PubMed articles Acute kidney injury PubMed Central articles Acute kidney injury 2023 articles Acute kidney injury 2024 articles Acute kidney injury Scopus articles Acute kidney injury impact factor journals Acute kidney injury Scopus journals Acute kidney injury PubMed journals Acute kidney injury medical journals Acute kidney injury free journals Acute kidney injury best journals Acute kidney injury top journals Acute kidney injury free medical journals Acute kidney injury famous journals Acute kidney injury Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals kidney articles kidney Research articles kidney review articles kidney PubMed articles kidney PubMed Central articles kidney 2023 articles kidney 2024 articles kidney Scopus articles kidney impact factor journals kidney Scopus journals kidney PubMed journals kidney medical journals kidney free journals kidney best journals kidney top journals kidney free medical journals kidney famous journals kidney Google Scholar indexed journals lupus nephritis articles lupus nephritis Research articles lupus nephritis review articles lupus nephritis PubMed articles lupus nephritis PubMed Central articles lupus nephritis 2023 articles lupus nephritis 2024 articles lupus nephritis Scopus articles lupus nephritis impact factor journals lupus nephritis Scopus journals lupus nephritis PubMed journals lupus nephritis medical journals lupus nephritis free journals lupus nephritis best journals lupus nephritis top journals lupus nephritis free medical journals lupus nephritis famous journals lupus nephritis Google Scholar indexed journals infusion articles infusion Research articles infusion review articles infusion PubMed articles infusion PubMed Central articles infusion 2023 articles infusion 2024 articles infusion Scopus articles infusion impact factor journals infusion Scopus journals infusion PubMed journals infusion medical journals infusion free journals infusion best journals infusion top journals infusion free medical journals infusion famous journals infusion Google Scholar indexed journals Blood articles Blood Research articles Blood review articles Blood PubMed articles Blood PubMed Central articles Blood 2023 articles Blood 2024 articles Blood Scopus articles Blood impact factor journals Blood Scopus journals Blood PubMed journals Blood medical journals Blood free journals Blood best journals Blood top journals Blood free medical journals Blood famous journals Blood Google Scholar indexed journals
Article Details
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that can cause systemic damage to organs such as the lungs, kidneys, and skin. The pathological conditions and clinical presentations vary in patients, and therefore, close management by a rheumatologist is indispensable. One of the most serious complications associated with lupus nephritis is its progression to end-stage renal failure. Under the ISN/RPS 2003 revised classification system [1], lupus nephritis is classified as types ranging from type I to type VI and effective treatment for lupus nephritis is clearly classified by "2012 ACR recommendation" [2]. Although survival rates and end-stage renal failure rates have improved, cases of treatment resistance continue to be found.
2. Case Report
The patient was a 68-year-old female who presented with bilateral hand joint pain, onset of which was in the autumn of 2009. Although negative for anti-CCP antibody and rheumatoid factor, an increase in MMP3 levels with time was noted, and after she was diagnosed with rheumatoid arthritis in November 2009, treatment with 4 mg/w methotrexate (MTX) and 4 mg/d prednisolone (PSL) was initiated. Subsequently, MTX was increased to 6 mg/w, mizoribine (MZB) was included in her treatment regimen, and symptoms temporarily improved. Exacerbation of joint pain was noted from May 2011, requiring the patient to be admitted for further examination. Blood sample collected at the time of admission tested positive for antinuclear antibody, anti-DNA antibody, and anti-cardiolipin antibody and showed low count of white blood cells. The patient with concurrent arthritis was diagnosed with SLE based on the laboratory findings [3]. Treatment with MTX and MZB was discontinued, and the patient was started on 3 mg tacrolimus (TAC) with no improvement, and abatacept was introduced for arthritic symptoms. Remission was immediately achieved, and the patient was treated with abatacept alone until December 2016 when abatacept was discontinued and drug-free remission was achieved.
Change to positive anti-DNA antibody was recognized from September 2018, and in December 2018 revealed an increase in anti-DNA antibody from to 72 IU/mL. Urinalysis revealed 2 g proteinuria and macroscopic hematuria. The patient was admitted for the second time for further examination and treatment because SLE hyperactivity and the onset of lupus nephritis were suspected.
Renal biopsy was performed on hospital day 2 with the aim of scheduling treatment based on biopsy results. However, as of hospital day 2, serum Creatinine (Cre) increased from 1.32 to 1.85 mg/dL, treatment with PSL 50 mg (after renal biopsy on hospital day 2) was initiated at the rate of 1 mg/kg. Because serum Cre levels continued to rise (Cre 3.27 mg/dL), steroid semi-pulse therapy (mPSL 500 mg) was administered for 3 days from hospital day 6. Renal biopsy confirmed focal lupus nephritis (ISN/RPS Class III), which is considered to be a pathological condition of rapidly progressive glomerulonephritis (RPGN) based on lupus nephritis. Double filtration plasmapheresis (DFPP) and intravenous cyclophosphamide (CPA) pulse therapy were introduced on hospital day 9 (Cre 4.57 mg/dL). Despite the lack of increase in serum Cre, renal function did not improve. Blood sampling at admission had revealed positive results for MPO-anti-neutrophil cytoplasmic antibodies (ANCA) 139 U/mL and in conjunction with findings of peritubular capillary vasculitis by renal biopsy; the patient was treated for microscopic polyangiitis (MPA) with high disease activity. On hospital day 17, the reduction in blood platelet count to 55,000/μL and the introduction of rituximab for the possibility of cytopenia were correlated to a possible accompanying viral infection from the elapsed avoids; this led to the discontinuation of DFPP from hospital day 19 in favor of plasma exchange therapy (PE). Renal function gradually improved after the introduction of PE, which was administered intermittently for five times (Figure 1) and terminated on hospital day 35 when Cre reduced to 2.11 mg/dL. Hydroxychloroquine (HCQ) (200 mg/d) was initiated from hospital day 45, and 500 mg/d of mycophenolate mofetil was introduced from hospital day 56. The steroid dose was progressively reduced from hospital day 30, and the patient was discharged on hospital day 59 with favorable prognosis.
After the discharge, she continued receiving outpatient treatment, and low disease activity has been maintained without any deterioration in renal function.
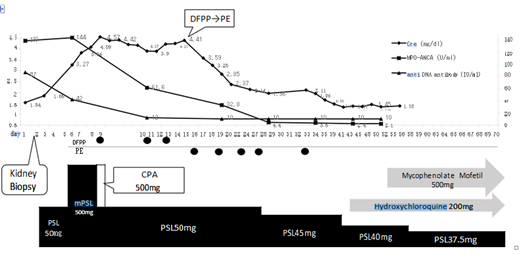
Figure 1: Clinical course.
3. Renal Biopsy Findings
A total of 10-15 glomeruli were collected. The mesangial matrix was partially visible, and imaging indicated the proliferation of mesangial cells in seven glomeruli. Additionally, segmental endocapillary proliferation was observed in three to four of the seven glomeruli that showed proliferative changes-three lesions appeared to be thrombus; glomerular fibrinoid thrombi were found in two glomeruli; and two glomeruli showed a cellular crescent. The paramesangium showed unclear periodic acid-methenamine-silver (PAM) staining, which was diffuse, global, or segmental, and did not clearly show deposition.
Mild and diffuse inflammatory cell infiltration was observed in the interstitium, with minor fibrosis in some margins. Small scattered mild atrophies were observed in some tubules with mild columns, and red blood cell fillings were observed in many partial tubular lumens. Moreover, tubulitis, proximal tubule vacuolization and possibly hemosiderin pigment were observed in some tubules; however, calcification was not observed. The vasculature showed mild–moderate wall thickening. Because partial inflammatory cell infiltration was observed in the peritubular capillaries, peritubular capillary vasculitis could not be ruled out. Immunohistochemical test did not reveal clear positive findings, including C1q, but electron microscopy showed a clear electron dense deposit in the mesangial region.
Based on the above findings, SLE was clinically diagnosed in the patient; active lesions comprised <50% of all observed glomeruli and corresponded to focal lupus nephritis [ISN/RPS Class III (A/C)] (Figure 2).
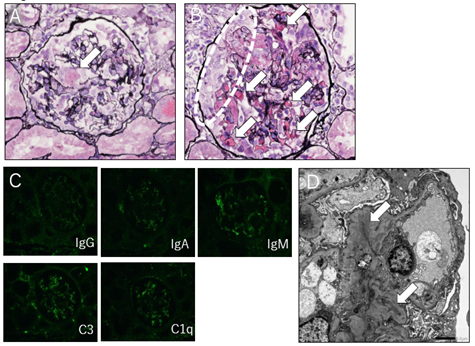
Figure 2: A. The patient’s renal specimen diagnosed as focal lupus nephritis [ISN/RPS Class III (A/C)]. Glomerular fibrinoid thrombi were found in two glomeruli (dotted enclosure). [Periodic acid-methenamine-silver (PAM) stain]
|
[Blood cell count] |
[Biochemistry] |
[Serology] |
MPO-ANCA 139 U/mL |
|
WBC 4900 /μL |
T-Bil 0.6 mg/dL |
CRP 0.8 mg/dL |
PR3-ANCA <1.0 U/mL |
|
neu 3689 /μL |
AST 21 IU/L |
ANA x320 |
anti GBM antibody <2.0 U/mL |
|
lym 828 /μL |
ALT 15 IU/L |
Homogeneous x320 |
|
|
RBC 368 *10^4/μL |
LDH 268 IU/L |
Speckled x320 |
[Urynalysis] |
|
Hb 11.0 g/dL |
γ-GTP 14 IU/L |
anti DNA antibody 72 IU/mL |
pH 6.0 |
|
Hct 33.5 % |
BUN 35 mg/dL |
anti RNP antibody (-) |
Protein 300mg/dL |
|
Plt 12.8 *10^4 /μL |
Cre 1.54 mg/dL eGFR 27 mL/min |
anti SS-A antibody (-) anti cardiolipin antibody (IgG) 13 IU/mL |
Ketone body(-) |
|
[Coagulation] |
UA 3.8 mg/dL |
CH50 59 /mL |
[Urinary sediment] |
|
PT 100 % |
CK 38 IU/L |
C3 84 mg/dL |
RBC >100 HPF |
|
(PT-INR 0.89) |
Na 142 mEq/L |
C4 17 mg/dL |
WBC 5-9 HPF |
|
APTT 21.8 sec |
K 4.0 mEq/L |
IgG 1053 mg/dL |
Hyaline cast(1+) |
|
D dimer 5.22 μg/mL |
Cl 107 mEq/L |
IgA 108 mg/dL |
Granular cast(1+) |
|
TP 6.2 g/dL |
IgM 112 mg/dL |
RBC cast (1+) |
|
|
Alb 3.5 g/dL |
MMP3 79.9 ng/mL |
WBC cast (2+) |
WBC White blood cell, RBC Red blood cell, Hb Hemoglobin, Hct Hematocrit, Plt Platelet, PT Prothrombin time, INR International normalized ratio, APTT Activated partial thromboplastin time, FDP Fibrinogen and fibrin degradation products, Bil Bilirubin, AST Aspartate transaminase, ALT Alanine transaminase, BUN Blood urea nitrogen, eGFR Estimated glemerular filtration rate, UA Uric acid, CK Creatinekinase ,TP Total protein, CRP C-reactive protein, ANA Antinuclear antibody, LAC Lupus anticoagulant , CH50 Total complement activity, Ig Immunoglobulin, MMP Matrix metalloproteinase ANCA antineutrophil cytoplasmic antibody, GBM Glomerular basement, HPF High power field
Table 1: Labolatory test findings.
4. Discussion
Although the patient was diagnosed with focal lupus nephritis [ISN/RPS Class III (A/C)] by renal biopsy, several findings-including slight deposition of fluorescent antibodies, MPO-ANCA-positivity, peritubular capillary vasculitis, and the fact that despite a rapid clinical course, there was no serological reduction in complement titer were not typical of lupus nephritis. Among secondary diseases that cause RPGN, MPA and granulomatosis with polyangiitis (GPA) have the "pauci-immune type," which pathologically shows no deposition of immunoglobulins or immune complexes, but in contrast, SLE generally exhibits an "immune complex type," in which extensive deposition of immunoglobulins and immune complexes is visible [4]. However, in the present case, deposition of immunoglobulins and immune complexes was weak for lupus nephritis, but did not fit the pauci-immune type, wherein fluorescent antibody is not deposited. One possible cause of such atypical findings may be the onset of high titer MPO-ANCA, despite it being a case of SLE.
Cases of MPO-ANCA-positive treatment-refractory lupus nephritis have been previously reported. Morimoto et al. reported that tacrolimus is effective against strongly positive MPO-ANCA treatment-refractory lupus nephritis [5, 6]. However, there are no reports of remission after changing the apheresis procedure from DFPP to PE as in the present case. Although DFPP is generally regarded as effective for the treatment of SLE, no clear efficacy was noted in the present case. In the present case, it is plausible that sufficient disease control could not be achieved only by removing anti-DNA antibodies and immune complexes, which is normally effective with DFPP. Considering the process of rapid improvement by switching apheresis to PE, it can be surmised that in the present case, removal of multiple inflammatory cytokines that pose a problem in highly active MPA, which are usually complicated with alveolar hemorrhage, and SLE associated with fatal pathologies, such as thrombotic microangiopathy and hemophagocytic syndrome, was critical for disease control. Additionally, rituximab, which is used for severe vasculitis, may be effective for the same reason, although it was not used in the present case because of cytomegalovirus infection.
Although MPO-ANCA is a representative disease marker for MPA, it is often detected in autoimmune diseases other than ANCA-associated vasculitis (AAV), and its significance and pathogenicity are unknown. The roles of ANCA in AAV include mobilization of various cytokines and cell adhesion factors on vascular endothelial cells, adhesion of neutrophils to endothelial cells and injury to cells by NETs release, and enhancement of C5a activity through alternative pathways [7]. The ANCA-positive rate in patients with SLE varies depending on the measurement method and is considered to be 20% by immunofluorescence or 9.3% by enzyme-linked immunosorbent assay commonly used in clinical practice [8-10]. While ANCA is associated with proliferative nephritis, it has also been associated with severity of the condition, such as in patients with ANCA-positive lupus nephritis [11]. However, as described above, manifestation of vascular inflammation and increase in severity do not necessarily occur in all ANCA-positive patients, and not all ANCA are pathogenic. Validated reports using epitope mapping have shown that only ANCA reactive with specific epitopes are considered pathogenic; however, uncertainties remain [12].
5. Conclusion
The clinical and pathological findings in the present case suggest that some cases of MPO-ANCA-positive lupus nephritis can become severe and require multidisciplinary?treatment in accordance with the treatment for severe vasculitis. While the type determination by kidney biopsy is important for initiating appropriate treatment for lupus nephritis, it may also be significant to measure MPO-ANCA.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Weening JJ, D’agati D, Schwartz M, et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. J Am Soc Nephrol 15 (2004): 241-250.
- Hahn BH, McMahon MA, Wilkinson A, at al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64 (2012): 797-808.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus, Arthritis Rheum 25 (1982): 1271-1277.
- Arimura Y, Muso E, Fujimoto S, et al. Evidence-based clinical practice guidelines for rapidly progressive glomerulonephritis 2014, Clinical and Experimental Nephrology 20 (2016): 322-341.
- Morimoto S, Watanabe T, Lee S, et al. Improvement of rapidly progressive lupus nephritis associated MPO-ANCA with tacrolimus. Mol Rheumatol 20 (2010): 291-294.
- Abe Y, Shima T, Izumi Y, et al. Successful Management of Lupus Nephritis with High Titers of Myeloperoxidase Anti-Neutrophil Cytoplasmic Antibodies Using Tacrolimus. Intern Med 54 (2015): 2929-2933.
- Jannette JC, Falk RJ, Gasim AH. Pathogenesis of ANCA Vasculitis, Curr Opin Nephrol Hypertens 20 (2011): 263-270.
- Pradhan VD, Badakere SS, Bichile LS, et al. Anti-neutrophil cytoplasmic antibodies (ANCA) in systemic lupus erythematosus: prevalence, clinical associations and correlation with other autoantibodies. J Assoc Physicians India 52 (2004): 533-537.
- Galeazzi M, Morozzi G, Sebastiani GD, et al. Anti-neutrophil cytoplasmic antibodies in 566 European patients with systemic lupus erythematosus: prevalence, clinical associations and correlation with other autoantibodies. European Concerted Action on the Immunogenetics of SLE. Clin Exp Rheumatol 16 (1998): 541-546.
- Chin HJ, Ahn C, Lim CS, at al. Clinical implications of antineutrophil cytoplasmic antibody test in lupus nephritis. Am J Nephrol 20 (2000): 57-63.
- Olson SW, Lee JJ, Poirier M, et al. Anti-Myeloperoxidase Antibodies Associate with Future Proliferative Lupus Nephritis. Autoimmune Disease 1872846 (2017): 11.
- Land J, Rutgers A, Kallenberg CGM. Anti-neutrophil cytoplasmic autoantibody pathogenicity revisited: pathogenic versus non-pathogenic anti-neutrophil cytoplasmic autoantibody, Nephrol Dial Transplant 29 (2014): 739-745.
