A Case of Excavated Pneumopathy after Bronchial Thermoplasty
Article Information
Steger M1, Migueres N1,2, Khayath N1, Marcot C1, Matau C1, Arboit F3, Ohana M4, De Blay F1,5
1Chest Disease Department, Strasbourg University Hospital, Strasbourg France
2UMR 7357, Laboratory of Engineering, Computer Science and Imagery, Strasbourg France
3Pulmonary Unit, Metz Hospital, Metz France
4Radiology Department, Strasbourg University Hospital, Strasbourg France
5EA 3070, Federation of Translational Medicine, Strasbourg University, Strasbourg France
*Corresponding Author: Steger M, Chest Disease Department, Nouvel Hôpital Civil, University Hospital of Strasbourg, 1 Place de l’Hôpital, F- 67000 Strasbourg, France.
Received: 15 August 2022; Accepted: 02 September 2022; Published: 16 September 2022
Citation: Steger M, Migueres N, Khayath N, Marcot C, Matau C, Arboit F, Ohana M, De Blay F. A Case of Excavated Pneumopathy after Bronchial Thermoplasty. Archives of Clinical and Medical Case Reports 6 (2022): 639-642.
View / Download Pdf Share at FacebookAbstract
Bronchial thermoplasty (BT) is a bronchoscopic treatment for patients with severe asthma that remains uncontrolled despite optimal medical therapy. Several adverse effects and acute radiological abnormalities have been described, but excavated pneumopathy has never been reported as a complication of BT. We describe the development of excavated pneumopathy after the last BT procedure. A 60-year-old woman with severe uncontrolled asthma, despite maximal medical treatment, underwent BT. After the third procedure, she developed an exacerbation of asthma with respiratory deterioration after a reduction in systemic corticosteroid treatment. Chest computer tomography revealed nodular excavated opacities in contact with the bronchial tree, limited to the upper lobes and the lingula. This case suggested that intense thermal stimulation of the bronchial mucosa may lead to tissue fragility and alveolar lung lesions.
Keywords
Asthma; Bronchial Thermoplasty; Excavated Pneumopathy
Asthma articles; Bronchial Thermoplasty articles; Excavated Pneumopathy articles
Asthma articles Asthma Research articles Asthma review articles Asthma PubMed articles Asthma PubMed Central articles Asthma 2023 articles Asthma 2024 articles Asthma Scopus articles Asthma impact factor journals Asthma Scopus journals Asthma PubMed journals Asthma medical journals Asthma free journals Asthma best journals Asthma top journals Asthma free medical journals Asthma famous journals Asthma Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Bronchial Thermoplasty articles Bronchial Thermoplasty Research articles Bronchial Thermoplasty review articles Bronchial Thermoplasty PubMed articles Bronchial Thermoplasty PubMed Central articles Bronchial Thermoplasty 2023 articles Bronchial Thermoplasty 2024 articles Bronchial Thermoplasty Scopus articles Bronchial Thermoplasty impact factor journals Bronchial Thermoplasty Scopus journals Bronchial Thermoplasty PubMed journals Bronchial Thermoplasty medical journals Bronchial Thermoplasty free journals Bronchial Thermoplasty best journals Bronchial Thermoplasty top journals Bronchial Thermoplasty free medical journals Bronchial Thermoplasty famous journals Bronchial Thermoplasty Google Scholar indexed journals Thermoplasty articles Thermoplasty Research articles Thermoplasty review articles Thermoplasty PubMed articles Thermoplasty PubMed Central articles Thermoplasty 2023 articles Thermoplasty 2024 articles Thermoplasty Scopus articles Thermoplasty impact factor journals Thermoplasty Scopus journals Thermoplasty PubMed journals Thermoplasty medical journals Thermoplasty free journals Thermoplasty best journals Thermoplasty top journals Thermoplasty free medical journals Thermoplasty famous journals Thermoplasty Google Scholar indexed journals Immunotherapy articles Immunotherapy Research articles Immunotherapy review articles Immunotherapy PubMed articles Immunotherapy PubMed Central articles Immunotherapy 2023 articles Immunotherapy 2024 articles Immunotherapy Scopus articles Immunotherapy impact factor journals Immunotherapy Scopus journals Immunotherapy PubMed journals Immunotherapy medical journals Immunotherapy free journals Immunotherapy best journals Immunotherapy top journals Immunotherapy free medical journals Immunotherapy famous journals Immunotherapy Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Breast Cancer articles Breast Cancer Research articles Breast Cancer review articles Breast Cancer PubMed articles Breast Cancer PubMed Central articles Breast Cancer 2023 articles Breast Cancer 2024 articles Breast Cancer Scopus articles Breast Cancer impact factor journals Breast Cancer Scopus journals Breast Cancer PubMed journals Breast Cancer medical journals Breast Cancer free journals Breast Cancer best journals Breast Cancer top journals Breast Cancer free medical journals Breast Cancer famous journals Breast Cancer Google Scholar indexed journals Excavated Pneumopathy articles Excavated Pneumopathy Research articles Excavated Pneumopathy review articles Excavated Pneumopathy PubMed articles Excavated Pneumopathy PubMed Central articles Excavated Pneumopathy 2023 articles Excavated Pneumopathy 2024 articles Excavated Pneumopathy Scopus articles Excavated Pneumopathy impact factor journals Excavated Pneumopathy Scopus journals Excavated Pneumopathy PubMed journals Excavated Pneumopathy medical journals Excavated Pneumopathy free journals Excavated Pneumopathy best journals Excavated Pneumopathy top journals Excavated Pneumopathy free medical journals Excavated Pneumopathy famous journals Excavated Pneumopathy Google Scholar indexed journals Nephropathy articles Nephropathy Research articles Nephropathy review articles Nephropathy PubMed articles Nephropathy PubMed Central articles Nephropathy 2023 articles Nephropathy 2024 articles Nephropathy Scopus articles Nephropathy impact factor journals Nephropathy Scopus journals Nephropathy PubMed journals Nephropathy medical journals Nephropathy free journals Nephropathy best journals Nephropathy top journals Nephropathy free medical journals Nephropathy famous journals Nephropathy Google Scholar indexed journals
Article Details
Abbreviations:
BT- Bronchial Thermoplasty; CS- Corticosteroid; CT- Computer Tomography; ACT- Asthma Control Test
1. Introduction
Bronchial thermoplasty (BT) is a bronchoscopic treatment for patients with severe asthma that remains uncontrolled, despite optimal medical therapy. Through the operating channel of the bronchoscope, a catheter with a basket design emits thermal energy produced by a radiofrequency generator (Alair™ Bronchial Thermoplasty System). The thermal energy is directed at segmental and subsegmental airways. This system delivers a constant temperature of 65°C in 10-second pulses at each treatment site. Sites range from small 3-mm bronchi to lobar bronchi and treatments are delivered over three sessions at 3-week intervals. This treatment directly reduces airway smooth muscles [1], and thus, relieves bronchoconstriction in asthma attacks. During intervention, or a few days after, significant respiratory adverse effects have been described, but disappear within a few days [2-4]. It is well known that different patterns of acute radiological abnormalities (including bronchial dilatations) occur after BTs, but they lack clinical impact in nearly all cases. In this case report, we describe an excavated pneumopathy that appeared after the last BT procedure.
2. Case Presentation
A 60-year-old Caucasian woman, who was a nurse and marathon runner, presented with severe uncontrolled asthma despite maximal medical treatment (Asthma Control Test (ACT) score 16). She was referred to our center for BT. Her medical history included chronic rhinosinusitis with nasal polyps, treated with a left middle meatotomy; dysfunctional dysphonia which required speech therapy; sleep apnea syndrome, treated with continuous positive airway pressure; hepatitis C and a SARS Cov2 infection in March 2020 without complication. She was a 7 pack/year ex-smoker (quit smoking at age 23 years). Atopic asthma was diagnosed in childhood. Then, asthma recurred at age 50 years, and at that time, skin prick tests showed negative responses to aeroallergens. In 2017, a pneumologist referred her to our department for severe asthma. We found that her total IgE level was 98 kU/L without any sensitization to specific aeroallergens. The blood eosinophil count was 120/µL, but the fraction of exhaled nitric oxide was 60 ppb, and an induced sputum analysis showed a mixed granulocytic pattern (i.e., neutrophils 68%, eosinophils 13%). She was not eligible for biotherapy, due to the absence of elevated blood eosinophil counts and negative skin prick tests. She displayed uncontrolled severe asthma, despite high-dose inhaled therapy with budesonide (160 µg), formoterol fumarate (4.5 µg, two puffs, three times daily) and tiotropium (18 µg, two puffs daily). In addition, she required several treatments of nebulized terbutaline and ipratropium per week, frequent systemic corticosteroid (CS) bursts (at least three days of 40 mg prednisolone every two months), and three intravenous antibiotic treatments per year. Non-enhanced chest computed tomography (CT) demonstrated moderate, diffuse, bronchial wall thickening with minimal air trapping. There was no bronchiectasis. According to standard perioperative procedures, 50 mg of prednisolone/day was administered orally, three days prior, on the day of BT, and on the day after BT. She was hospitalized the day before BT for 3 days to monitor vital parameters, pulmonary function, and chest x-ray examinations. The first two sessions for the right and left lower lobe were performed at 3 weeks intervals (total numbers of activations were 119 and 83 respectively). There were no major complications, except mild asthma exacerbation with dyspnea and dry cough. Although these symptoms did not affect the vital or functional respiratory parameters, oral CS therapy was continued for approximately 1 week after each BT session. On March 25, 2020, the third BT procedure was performed, with 214 activations, in the left and right upper lobe bronchi with minimal bleeding. There were no endoscopic abnormalities in the airway. Before the BT, the forced expiratory volume in 1 second (FEV1) was 2.54 L (108% of predicted) and the day after the BT, it was 2.32 L (99% of predicted). Immediately after the procedure, the patient experienced a new asthma exacerbation that required the continuation of CS treatment at discharge from the hospital. The chest radiography showed no change. About ten days later, due to incomplete improvement, a new course of CS was prescribed. However, respiratory deterioration occurred when the dose was reduced. At four weeks after the third BT, the patient presented with increasing dry cough, dyspnea, and persistent left chest pain, which projected to the apex, despite oral corticosteroid therapy and nebulized terbutaline-ipratropium treatment. A chest x-ray revealed a culminal consolidation. Thus, antibiotic therapy (ceftriaxone) was initiated, and the next day, she was admitted to the hospital. There was no febrile syndrome or signs of respiratory distress, but she presented bilateral expiratory wheezing with mMRC-grade4 dyspnea and left apico-thoracic pain. These symptoms did not improve with level I analgesics. An analysis of an arterial blood sample in ambient air showed the following results: pH: 7.5, pO2: 98 mmHg, and pCO2: 28 mmHg. CT pulmonary angiography ruled out a diagnosis of pulmonary embolism but showed bilateral upper lobar and lingual parenchymal abnormalities (Figure 1) with nodular excavations.
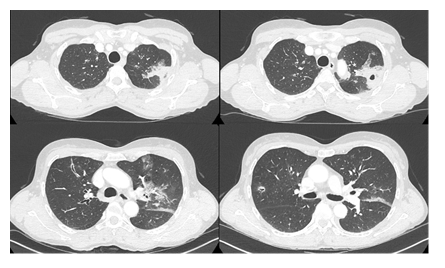
Figure 1: Chest CT acquired on April 21, one month after the third BT procedure. Bilateral abnormalities were present with increased bronchial wall thickening, peribronchial infiltration, mucoid impactions and nodular excavations in direct contact with the bronchial tree. These abnormalities remain limited to the upper lobes and the lingula. Around the left upper lobar apicodorsal lesions, there were alveolar consolidations with scattered ground glass opacities.
The serum C-reactive peptide level was 19 mg/L, with no increase in white blood cells, particularly eosinophils. The echocardiogram showed normal results, and the PCR test showed a negative result for the SARS Cov2 virus. Despite three days of treatment with antibiotics and oral CS, no improvement was observed, and the patient was transferred to our department on April 23. The total serum IgE level was 137 kU/L, and blood inflammatory markers were within normal limits. Aspergillus serology and antigenemia assessments showed negative results. An auto-immune assessment showed negative results. Bronchial fibroscopy showed normal results. A broncho-alveolar lavage performed in the culmen showed normal cytology (71% macrophages, 17% lymphocytes, 5% neutrophils, 1% eosinophils, 6% bronchial cells) and no bacteriological or parasitological infection. Gradually, the patient improved, and the peak flow value increased from 300 to 400 L/min. A follow-up chest CT performed 15 days later, demonstrated a reduction in pulmonary excavations (Figure 2).
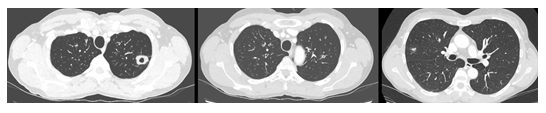
Figure 2: Chest CT acquired on May 3, demonstrating a decrease in the overall extension of alveolar consolidations and a remaining nodular excavation.
Ceftriaxone treatment was stopped (after 14 days of treatment). CS therapy was reduced during hospitalization, and the reduction was continued at home. After 13 days of hospitalization, she returned home and received decreasing doses of oral CS for two months. The asthma improved and was controlled. She returned to work and performed sports again. A follow-up chest CT (Figure 3) showed a significant improvement.
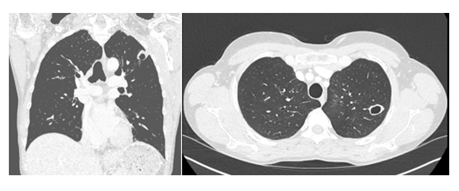
Figure 3: Follow-up chest CT performed 6 months after the last BT procedure. The perilesional ground glass opacities in the left upper lobe have completely disappeared, and the right upper lobe lesions appeared stable. The excavated lesion in the posteroapical upper left lobe remains present, with direct bronchial communication.
3. Discussion
Previously, several international trials in patients with mild to severe asthma demonstrated that BT could effectively reduce asthma exacerbations, decrease emergency department visits, and improve quality of life, with an acceptable rate of adverse events in the short-term [2-4]. The most common respiratory adverse effects, including a worsening cough, wheezing, respiratory infections, chest pain, and distress, generally occur in the initial six weeks post-BT [4,5]. In recent years, with the increasingly common use of BT, some unexpected, but notable complications have been reported, including lung abscess [6], heat-induced necrosis [7], recurrent lung atelectasis from fibrin plugs [8], pneumothorax and pulmonary cyst [9], or Aspergillus fumigatus and Nocardia spp. infections [10]. However, this study was the first to report excavated pneumopathy outside the context of an infection.
Indeed, it has been suggested that intense thermal stimulation of the bronchial mucosa with the administration of high-dose systemic steroids to control asthma attacks around the time of the BT procedure could lead to tissue fragility and might induce an infection [10]. However, we did not find any positive markers of infection. This result was consistent with findings from Debray et al [11]. They described acute asymptomatic abnormalities visible on a chest CT after a BT. Those abnormalities included ground-glass opacities or peribronchial consolidations, which spontaneously disappeared within a month. The authors hypothesized that those findings likely reflected alveolar inflammation and edema, due to heat shock. We could not confirm that hypothesis, because no biopsy was performed in our case.
Our patient presented pulmonary lesions similar to the abnormalities found after radiofrequency ablation of pulmonary neoplasms [12, 13]. In that scenario, a large temperature increase causes tissue coagulation and can destroy microcirculation, which induces peribronchial consolidations; then, scars might form during the healing process. Similar to our findings, at 1 month after a radiofrequency ablation, cavitation is a common finding. This feature was described as a reparative process that progressively decreased, with more or less scarring [13].
It remains unclear why our patient developed excavated pneumopathy. One might suspect that a dysfunction of the BT catheter might cause excessive energy delivery. However, no abnormality was observed during the three procedures.
Our case study findings raised the question of whether the number of radiofrequency activations affected adverse effects. Previously, Yamamoto et al reported that increasing the number of activations during a BT induced a reduction in FEV1 from baseline (greater when treating the upper lobes), without association with serious adverse events [14]. Nevertheless, in this case, the number of radiofrequency activations was higher than the typical number during the third session [15]. As a result, the increased number of activations seems to be the only explanation of the exacerbation of asthma and the excavated pneumopathy.
In conclusion, the distribution and the evolution of lesions, identified by chest CT with a slow healing, suggest that this excavated pneumopathy was the consequence of an acute intense thermal stimulation. An increased radiofrequency activations number could raise the risk of complications after BT.
Conflict of Interests
The authors declare no conflict of interest.
References
- D’Hooghe JNS, Ten Hacken NHT, Weersink EJM, et al. Emerging understanding of the mechanism of action of Bronchial Thermoplasty in asthma. Pharmacol Ther 181 (2018): 101-107.
- Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 181 (2010): 116-124.
- Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 176 (2007): 1185-1191.
- Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med 356 (2007): 1327-1337.
- Nong Y, Lin JT, Su N, et al. Analysis of short-term respiratory adverse events in 183 bronchial thermoplasty procedures. Zhonghua Jie He He Hu Xi Za Zhi = Chinese journal of tuberculosis and respiratory diseases 40 (2017): 176-181.
- Balu A, Ryan D, Niven R. Lung abscess as a complication of bronchial thermoplasty. J Asthma 52 (2015): 740-742.
- Menzella F, Lusuardi M, Galeone C, et al. Heat-induced necrosis after bronchial thermoplasty: a new concern. Allergy, Asthma Clin Immunol 14 (2018): 1-5.
- Facciolongo N, Menzella F, Lusuardi M, et al. Recurrent lung atelectasis from fibrin plugs as a very early complication of bronchial thermoplasty: a case report. Multidiscip Respir Med 10 (2015): 1-4.
- Funatsu A, Kobayashi K, Iikura M, et al. A case of pulmonary cyst and pneumothorax after bronchial thermoplasty. Respirology case reports 6 (2018): e00286.
- Matsubayashi S, Likura M, Numata T, et al. A case of Aspergillus and Nocardia infections after bronchial thermoplasty. Respirol Case Rep 7 (2018): e00392.
- Debray MP, Dombret MC, Pretolani M, et al. Early computed tomography modifications following bronchial thermoplasty in patients with severe asthma. Eur Respir J 49 (2017): 1601565.
- D’Hooghe JNS, van den Berk IAH, Annema JT, et al. Acute radiological abnormalities after bronchial thermoplasty: a prospective cohort trial. Respiration 94 (2017): 258-262.
- Abtin FG, Eradat J, Gutierrez AJ, et al. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics 32 (2012): 947-969.
- Yamamoto S, Iikura M, Kakuwa T, et al. Can the number of radiofrequency activations predict serious adverse events after bronchial thermoplasty? A retrospective case-control study Pulm Ther 5 (2019): 221-233.
- Bonta PI, Chanez P, Annema JT, et al. Bronchial Thermoplasty in Severe Asthma: Best Practice Recommendations from an Expert Panel. Respiration 95 (2018): 289-300.
