A 5-Year-Old Boy with Equinus Walking: Clinical and Biochemical Improvement of Becker’s Muscular Dystrophy with a Chinese Patent Medicine, Liu Wei Di Huang Wan
Article Information
Andrew J Louie*
Providence Health, Vancouver BC
*Corresponding Author: Andrew J Louie, Providence Health, Vancouver BC.
Received: 08 February 2023; Accepted: 09 June 2023; Published: 15 June 2023
Citation:
Andrew J. Louie. A 5-Year-Old Boy with Equinus Walking: Clinical and Biochemical Improvement of Becker’s Muscular Dystrophy with a Chinese Patent Medicine, Liu Wei Di Huang Wan. Journal of Pediatrics, Perinatology and Child Health. 7 (2023): 111-116.
View / Download Pdf Share at FacebookAbstract
A 5-year-old male presented with trouble walking. He had hypertrophic calves with positive Gower’s sign. CK was elevated at 12,000. Becker’s Muscular Dystrophy was diagnosed based on molecular DNA analysis of the dystrophin gene. As there was no known treatment, therapeutic trial of a Chinese herbal pill called Liu Wei Di Huang Wan was used. This is based on a 1000-year-old Chinese pediatrician’s formula for use in the 6 delays of child development in China. The patient improved clinically with regaining his walking skills and biochemical reduction of CK by 80%. Follow-up over a 15-year period showed periods of increased CK levels when the patient was not taking the pills or when suspected counterfeit pills were taken, Cardiomyopathy developed at age 20. Reduction of CK was indicative of biochemical efficacy. Further studies need to be done on this method of treatment.
Keywords
Becker’s Muscular Dystrophy; Creatine kinase; Liu Wei Di Huang Wan; Cardiomyopathy
Becker?s Muscular Dystrophy articles; Creatine kinase articles; Liu Wei Di Huang Wan articles; Cardiomyopathy articles
Article Details
1. Introduction
Becker’s Muscular Dystrophy (BMD) [1] is an inherited X- linked recessive disease of muscles. It is similar but less severe than Duchenne Muscular Dystrophy (DMD), with no known treatment. The gene is passed along the X chromosome.
Becker’s is similarly to Duchenne muscular dystrophy except it is rarer and less severe. DMD has an incidence of 1/3500 live births while BMD has an incidence of approximately 1/20,000 live births [2]. Thus, Canada with a population of 38 million and birth rate of 380,000 per year would have 19 new Becker’s muscular dystrophy cases per year. The European Union with a population of 446 million and 1% birth rate would have about 223 new cases of BMD per year. The world with a birth rate of 130 million per year would have about 7000 new cases of BMD per year. The prevalence of BMD is 17-27 cases per million population. Thus, Canada would have a prevalence of 650 to 1075 cases.
BMD is X-linked recessive and is passed from mother to son. It is a disease of muscle in which there is insufficient dystrophin produced in the muscle cells resulting in instability in the structure of the muscle cell membrane and deletions in the dystrophin gene Xp21 (X chromosome, short arm p, region 2, band 1) [3]. Abnormal but functional dystrophin may be produced in contrast to pathology in Duchenne muscular dystrophy in which frame-shift mutation essentially leads to failure to produce dystrophin. Dystrophin levels in BMD are generally 30-80% of normal while DMD, the levels are less than 5%.
Cardiac arrythmia and cardiomyopathy [4,5,6] with congestive heart failure can present in males between ages 20-40 years and explains why BMD have a shorter lifespan.
The mean age of onset is 11 years with the age range of 2 to 21 years. The mean age at which patients became non ambulatory was 27 years with a range of 12 to 30 years. Death resulted from respiratory or cardiac failure at a mean age of 42 with the age range of 23-63 years [6].
Diagnosis is by in the pelvic and legs with equinus walking, poor balance, and frequent falls. Biochemical diagnosis is by measuring and finding elevated levels of creatine kinase (CK) up to 50 times normal. CK levels can fall with time and with loss of ambulation and muscle mass [1].
Until now, there is no known treatment besides supportive care with ankle foot splints and treatment of respiratory failure with nocturnal home ventilation [7,8] and cardiac failure which are complications of the illness. Patients should have baseline EKG and echocardiograph at age 10 and be reviewed every one to two years [6]. They should be monitored for cardiac arrythmia and dilated or hypertrophic cardiomyopathy which are potentially treatable [5,6].
The prognosis for Becker’s muscular dystrophy is better than Duchenne muscular dystrophy in which patients present earlier and be wheelchair bound by age 10 and deceased by age 20. However, severe forms of Becker muscular dystrophy present at an earlier age and can be similarly to Duchenne muscular dystrophy.
2. Clinical case, Treatment and Follow-up
A mother brought her son aged five years, and three months into my office. She complained that her son had an abnormal gait. He was born normally and was able to walk at 14 months. However, at age four and a half, he began having trouble with his gait. Examination showed that he was walking on his toes. He was referred to a pediatric orthopedic surgeon at the age of five years, ten months. The surgeon found that he had hypertrophic calves bilaterally with a weakly positive Gowers’ sign. He had external rotation, abducted position of his legs, and walked with a stiff-legged gait. He had equinus contractures bilaterally. His reflexes at the knees were brisk at about ¾. There were a few beats of clonus. His upper extremities had a completely different muscle tone than his lower extremities. CK was ordered and found to be elevated at 12,000 units. He was referred to a pediatric neurologist and neuromuscular clinic. Molecular DNA analysis showed a deletion involving exons 45 to 48 in the dystrophin gene. The diagnosis was Becker’s muscular dystrophy. There was a family history of the mother’s brother in China developing trouble walking at age 18.
Table 1: Composition and Function of Ingredients in Liu Wei Di Huang Wan from Lan Zhou Foci Pharmaceutical Factory.
The mother had been told that there was no known treatment aside from ankle foot splints for his muscle contractures. I had taken an acupuncture course and hadbeen reading about Chinese patent medicines. I had read of Liu Wei Di Huang Wan or six flavour pills [9]. This patent medicine was developed during the Song Dynasty (960-1279) by a pediatric physician for children who experienced any of the “Five Delays”: delay in standing up, delay in walking, delay in growth of hair on the head, delay in the development of teeth and delay in speech development. In more modern times, these pills are used by the elderly for diseases of old age and yin deficiency. The adult dose is 8-10 pills, three times a day, taken with warm water. The 6 herbal ingredients with percentage compositions are Table 1 with the Chinese name, Latin Name, TCM function, and pharmacologic effects of the herbs. Since the child had walked normally before and now was walking abnormally or had regressed in his walking. I suggested to the mother that he should take 4 pills, twice a day to see if they would help her son’s condition. He returned about a month later and his walking seemed to be getting better. He was able to squat ¾ way. Four months later his walking appeared to be normal, and he was able to squat and stand on one leg with good balance. At age 7 he was able to jump, stand on his toes, and heel-toe walk. At age 7½ he was able to walk for greater than one hour, play soccer, ride a bike, and swim. At age 10½ he was playing soccer and at age 12, basketball.
Figure 1 shows the variations in CK levels over the past 15 years. At age 7, his CK was re-checked, and he was much improved at 3500. At age seven and a half, his CK levels were elevated, and the mother suspected that the pills he was taking were counterfeit. He was advised to take a Beijing brand of pills and his CK dropped to 5000. A month later it was up to 28,000 and the brand of medications was changed.
At age ten years and ten months, he had not been taking his pills for six months and he was advised to take 12 pills, twice a day. At age 11 and seven months he was not taking his medications and CK was elevated to 23,000. At age 12 years and ten months his CK was also elevated at 26,000. His mother stated that he had not been taking pills for a year. At age 13 his CK was 6500. His mother said he was taking Reishi pills for one month and his legs felt 20-30% less tired. Between ages 14-17 he did not return to our office. He was not taking pills and was not able to squat at age 17. His CPK was elevated to 10,000. He was advised to take his six flavour pills for life as he had a chronic condition. At age 18, his CK level was 2600. At age 19 and four months he had not been taking pills and his CK level was elevated to 16,000. On Lan Zhou brand of pills his CK was down to 2000. At age 20½ years he was on Beijing brand of pills and his CK was 6000. He was advised to change back to the Lan Zhou formulation which brought his CK down to 2500.
At age 20½ his pulse was abnormal being tachycardic at 148 per minute. He was referred to a cardiologist and cardiomyopathy was suspected as his ejection fraction was reduced to 55%. On ACE inhibitors and beta blockers his pulse slowed to 76 per minute.
At age 29 he is working full-time but has obvious muscle atrophy with a BMI of 16. He can walk well but no longer run due to decreased strength in his legs. He can climb stairs but he needs to hold on to handrails to prevent falls.
Becker’s muscular dystrophy can be mild, moderate, or severe [10]. Mild cases present at a later age with less trouble walking. Severe cases present in early childhood as the present case and the course is expected to be more progressive with less ambulation by age 10 or 12. In the present case, the patient has done well with the six flavour pills with regression of equinus gait and significant reduction biochemically in CK levels with the correct medication and rebound in CK with respect to the counterfeit pills or failure to take his medications.
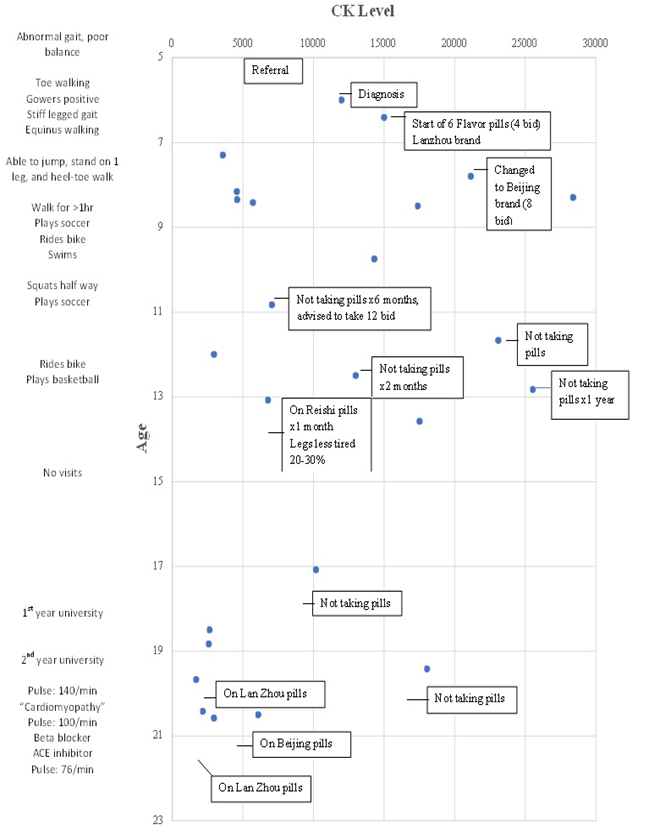
Figure 1: CK levels charted over time of a male patient with Becker's Muscular Dystrophy (deletion in exons 45-48 in the dystrophin gene). Symptoms presented at age 4½ years, diagnosis at age 5¾ years, and treated with 6 Flavor Chinese Patent medicine pills.
3. Discussion
The number one cause of death in BMD is cardiomyopathy [11]. Most are symptom free, but a high percentage have subclinical cardiomyopathy [12]. Our patient had deletions in exons 45 to 48, and deletions in exons 48 were associated with cardiac disease [13]. Melacini [13] concluded that a specific segment of the intron (noncoding sequence) located between exons (coding sequences) 48 and 49 is involved with cardiomyopathy.
Patients with BMD should be monitored with ECG and echocardiography every 2 years and treated with ACE inhibitors when left ventricular ejection fraction is less than 55% [11] and β blockers when cardiomyopathy develops [11]. Heart transplant can be considered for end stage heart failure [14].
Studies of various pharmaceuticals (steroids, cyclosporine, pentoxifylline) and nutritional interventions (coenzyme, Q10, creatine, taurine, arginine) have been reviewed [15]. Most used in muscular dystrophy are steroids which have anti-inflammatory effects and improve muscle strength and function in the short term (6 months – 2 years) [16].
More recently, Tadalafil, an inhibitor of phosphodiesterase 5, has been shown to improve muscle blood flow in Becker muscular dystrophy [17].
Type I facioscapulohumeral muscular dystrophy is the third most common muscular dystrophy with a prevalence of 1/15,000 individuals and has no known effective treatments in western medical therapy. Recently a clinical case using a Chinese herbal formula Bu Zhong Yi Qi has been shown to be therapeutically beneficial in a female age 15 with symptoms since age 9 [18]. The formula is believed to regulate inflammation and immune response, improve tolerance to oxidative stress in muscle cells, restore mitochondrial function and balance metabolic processes [18].
In the mouse model of muscular dystrophy, Liu Wei Di Huang Wan has been reported to be effective in improving muscular activity [19]. A parent reported his child with Duchenne muscular dystrophy had treatment starting at age 7 with 10 pills a day of six-flavour pills and physical improvement [20]. In our case of severe Becker’s muscular dystrophy, there was a rapid improvement in clinical symptoms.
Improved symptoms with reduction in CK levels and variations of levels of CK over time depending upon whether the patient was or was not taking his medication are indicative of therapeutic effect. The Liu Wei Di Huang Wan is not a cure but can somehow biochemically suppress breakdown of muscle tissue which leads to elevated levels of CK. The reduction in CK suggests slower breakdown of muscle tissue and allows the disability to improve from toe-walking to normal walking. Further studies should be done with a larger case series of Becker muscular dystrophy and with Duchenne muscular dystrophy as monitoring treatment with CK levels is easily done to check for therapeutic effect. The effects of different dosages also need to be studied as the pharmaco-kinetics of the medication complex is not known.
Since Bu Zhong Yi Qi has been beneficial in a case of facioscapulohumeral muscular dystrophy, it could be tried and compared to Liu Wei Di Huang Wan in BMD. Because of the seriousness of the illness and no previously known treatments, a randomized controlled clinical trial using this medication with placebo would be unethical since untreated patients would be severely disadvantaged.
The mechanism or action of the six flavour pills in Becker’s muscular dystrophy is speculative and needs to be studied. However, the pharmacologic effects of the individual herbs are known as shown in Table 1. The individual herbs are antioxidants, anti-inflammatory, anti-apoptosis, neuroprotective, immunomodulatory and inhibit NF-kappa B pathway [22-27]. The sum action of the herbs may be greater than the individual parts.
The cost of the treatment is inexpensive as a bottle of 200 pills costs about $4 to $5 and is readily available in Chinese herbal shops in Canada and worldwide. The dosage for the child used in this study was four pills, twice a day or $5 a month. At eight pills, twice a day (2.5 bottles per month), the cost is $12.5. An adult dose of eight pills, three times a day or 12 pills, twice a day, would cost about $18 per month. The lot number and date of production with CK monitoring should be done to verify therapeutic effect and monitor to eliminate counterfeit and bogus pills [28].
4. Clinical Pearls
- Duchenne and Becker’s muscular dystrophy are genetic X-Linked disorders leading to young males losing strength and balance in their legs. They presents with difficulty walking and climbing.
- Elevated creatine kinase levels are found in blood.
- Liu Wei Di Huang Wan or six flavour pills have been shown to reduce CK levels and clinically improve the gait and walking in a case of Becker’s muscular dystrophy.
- Patients should be monitored and treated for arrythmias and cardiomyopathy.
- Other cases should be treated with these pills to help this otherwise incurable disease.
Availability of Data and Materials:
All data supporting the findings are contained within the manuscript.
Consent for Publication:
Written informed consent was obtained from the patient of this case report.
Ethics Approval and Consent to Participate:
Ethical approval to report this case was not required.
Declaration of Competing Interests:
The author declares that he has no competing interests.
Funding:
None
References
- Emery AEH, Skinner R. Clinical studies in benign (Becker type) X-linked muscular dystrophy. Clinical Genetics10 (1976): 189-201.
- Engel AG. Muscular Dystrophies. Cecil Textbook of Medicine. W.B. Saunders Company 20 (1996): 2161-2163.
- Matsumura K, Campbell KP. Dystrophin- Glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle and Nerve 17 (1994): 2-15.
- Romfh A, McNally EM. Cardiac Assessment in Duchenne and Becker Muscular Dystrophies. Current Heart Failure Reports 7 (2010): 212-218.
- Finsterer J, Stöllberger C. Cardiac involvement in Becker muscular dystrophy. Canadian Journal of Cardiology 24 (2008): 786-792.
- Sultan A, Fayaz M. Prevalence of Cardiomyopathy in Duchenne and Becker’s Muscular Dystrophy. J Ayub Med Coll Abbottabad 20 (2008): 7-13.
- Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscular Disorders 12 (2002): 926-929.
- Jeppesen J, Green A, Steffensen BF, et al. The Duchenne muscular dystrophy population in Denmark, 1977-2001: prevalence, incidence and survival in relation to the introduction of ventilator use. Neuromuscular Disorders 13 (2003): 804-812.
- Naeser MA. Outline Guide to Chinese Herbal Patent Medicines in pill Form. Boston Chinese Medicine 2 (1990): 291-292.
- Bushby KMD, Gardner-Medwin D. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. I: Natural history. Journal of Neurology 240 (1993): 98-104.
- Ho R, Nguyen ML, Mather P. Cardiomyopathy in becker muscular dystrophy:Overview. World Journal of Cardiology 8 (2016): 356-361.
- Steare SE, Dubowitz V, Benatar A. Subclinical Cardiomyopathy in Becker Muscular-Dystrophy. British Heart Journal 68 (1992): 304-308.
- Paola M, Marina Fanin BS, Gian Antonio Danieli BS, et al. Cardiac Involvement in Becker Muscular Dystrophy. Journal of the American College of Cardiology 22 (1993): 1927-1934.
- Roland Wu S, Gupta S, Browm RN, et al. Clinical Outcomes after Cardiac Transplantation in Muscular Dystrophy Patients. The Journal of Heart and Lung Transplantation 29 (2010): 432-438.
- Radley HG, De Luca A, Lynch GS, et al. Duchenne muscular dystrophy: Focus on pharmaceutical and nutritional interventions. The International Journal of Biochemistry and Cell Biology 39 (2007): 469-477.
- Manzur AY, Kuntzer T, Pike M, et al. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database of Systematic Reviews (2008): 1-71.
- Martin EA, Barresi R, Bryne BJ, et al. Tadalafil Alleviates Muscle Ischemia in Patients with Becker Muscular Dystrophy. Science Translational Medicine 4 (2012): 1-19.
- Li H, Huang H, Long W, et al. Herbal Medicine Significantly Improved Muscle Function in a Patient with Type 1 Facioscapulohumeral Muscular Dystrophy: A Case Report. Explore (New York, N.Y.) 17 (2020): 247-251.
- Chen SS, Wang DC, Chen TJ, et al. Administration of Chinese Herbal Medicines Facilitates the Locomotor Activity in Dystrophin-Deficient Mice. American Journal of Chinese Medicine 29 (2001): 281-292.
- Liu Wei Di Huang Wan – 360 pills. Retrieved from http://www.depbuy.com/p/227122-B000VIWLKS/Liu-Wei-Di-Huang-Wan
- Urtizberea JA, Fan QS, Vroom E, et al. Looking under every rock: Duchenne muscular dystrophy and traditional Chinese medicine. Neuromuscular Disorders 13 (2003): 705-707.
- Zhang Ru-Xue, Li Mao-Xing, Jia Zheng-Ping. Rehmannia Glutinosa: Review of Botany, Chemistry and Pharmacology. Journal of Ethnopharmacology 117 (2008): 199-214.
- Kim S, Shin S, Hyun B, et al. Immunomodulatory Effects of Dioscoreae Rhizome Against Inflammation through Suppressed Production of Cytokines Via Inhibition of the NF-κB Pathway. Immune Network 12 (2021): 181-188.
- Gao X, Liu Y, An Z, et al. Active Components and Pharmacological Effects of Cornus Officinalis?: Literature Review. Frontiers in Pharmacology 12 (2021): 633447.
- Feng L, Liu TT, Huo XK, et al. Alisma Genus: Phytochemical Constituents, Biosynthesis, and Biological Activities. Phytotherapy Research 35 (2021): 1872-1886.
- Nie A, Chao Y, Zhnag X, et al. Phytochemistry and Pharmacological Activities of Wolfiporia cocos (F.A. Wolf) Ryvarden and Gilb. Frontiers in Pharmacology 11 (2020): 505249.
- Wang Z, He C, Peng Y, et al. P.Origins, Phytochemistry, Pharmacology, Analytical Methods and Safety of Cortex Moutan (Paeonia suffruticosa Andrew): A Systematic Review. Molecules (Basel, Switzerland) 22 (2017): 946.
- Taylor M. Chinese Patent Medicine: A Beginner’s Guide. Chapter 2: Counterfeiting and Mountain Bandit Factories Global Eyes International Press (1998): 33-43.
